Abstract
HIV-1 replication in the central nervous system (CNS) is typically limited by the availability of target cells. HIV-1 variants that are transmitted and dominate the early stages of infection almost exclusively use the CCR5 coreceptor and are well adapted to entering, and thus infecting, cells expressing high CD4 densities similar to those found on CD4+ T cells. While the “immune privileged” CNS is largely devoid of CD4+ T cells, macrophage and microglia are abundant throughout the CNS. These cells likely express CD4 densities that are too low to facilitate efficient entry or allow sustained replication by most HIV-1 isolates. Examination of CNS viral populations reveals that late in disease the CNS of some individuals contains HIV-1 lineages that have evolved the ability to enter cells expressing low levels of CD4 and are well-adapted to entering macrophages. These macrophage-tropic (M-tropic) viruses are able to maintain sustained replication in the CNS for many generations, and their presence is associated with severe neurocognitive impairment. Whether conditions such as pleocytosis are necessary for macrophage-tropic viruses to emerge in the CNS is unknown, and extensive examinations of macrophage-tropic variants have not revealed a genetic signature of this phenotype. It is clear, however, that macrophage tropism is rare among HIV-1 isolates and is not transmitted, but is important due to its pathogenic effects on hosts. Prior to the evolution of macrophage-tropic variants, the viruses that are predominately infecting T cells (R5 T cell-tropic) may infect macrophages at a low level and inefficiently, but this could contribute to the reservoir.
Keywords: Macrophage tropism, HIV-1, Pathogenesis, Host range variants, NeuroAIDS, CNS
Introductory summary
HIV-1 pathogenesis has been a major focus of research for the last 30 years. The central feature of HIV-1 disease is the loss of CD4+ T cells leading to immunodeficiency and the appearance of opportunistic infections and virus-driven cancers. In addition, untreated HIV-1 infection can lead to HIV-associated dementia (HAD) in a significant proportion of subjects. Viral replication in and destruction of CD4+ T cells is only part of the story of viral pathogenesis. To maintain replication in the face of the host immune system, HIV-1 has the capacity to undergo rapid evolution that results in the outgrowth of immune escape mutants, escaping the selective pressure of both humoral and cellular immune responses. This capacity for rapid evolution underlies the ability to generate drug-resistant variants in the presence of incomplete suppression of replication. However, these are not the only places where the effects of viral evolution can be seen.
The rapid evolution of HIV-1 also generates host range variants, i.e., variants of the virus that have evolved to replicate in new cell types. In addition to the typical virus, which uses CD4 and CCR5 to enter activated T cells, there are two well-recognized variants. One variant switches from using CCR5 as the coreceptor to using CXCR4 for entry, in addition to CD4, and these viruses have been termed X4 viruses. X4 viruses likely represent an expansion of viral replication into naive CD4+ T cells, which express high levels of CXCR4 and little to no CCR5 (Zamarchi et al. 2002). The second variant retains the requirement for both CD4 and CCR5 but evolves to enter cells that have lower levels of CD4. The obvious target cell for this variant is the macrophage, which has significantly lower levels of CD4 than CD4+ T cells, and these variants have been termed macrophage-tropic (or M-tropic) viruses. The appearance of X4 variants has been associated with a more rapid loss of CD4+ T cells (Connor et al. 1997; Koot et al. 1993), although the question of cause or effect persists (Arrildt et al. 2012). Viral replication in both naive CD4+ T cells and macrophages may be slower than in activated memory CD4+ T cells, thus allowing some level of immune surveillance prior to immunodeficiency. Alternatively, the virus may be more likely to encounter activated memory CD4+ T cells with their high levels of CD4 and CCR5, thus driving the selective pressure for replication in this cell type until it declines in number with advanced disease. This review will focus on macrophage-tropic variants, their evolution, and their role in pathogenesis. As interest in pathogenesis morphs into questions about viral persistence and latency on therapy, a different set of issues arise about the role of macrophage-tropic viruses.
Nomenclature
“Words have meaning and names have power.” Cervantes
There is an unfortunate twist in the history of HIV-1 research that has led to great confusion about the nature of macrophage-tropic HIV-1. Initial attempts to grow HIV-1 in CD4+ T cell lines (transformed cells typically isolated from lymphomas or leukemias) showed that only a fraction of viral isolates were capable of infecting these cells and quite naturally the viruses that could were called T cell-tropic. Of all the isolates not able to infect these Tcell lines, some of them could efficiently infect macrophages and the entire group of “non-T cell-tropic” viruses have come to be called macrophage-tropic. The subsequent identification of CCR5 and CXCR4 as coreceptors revealed an important flaw in this dichotomous designation, and as a field we are now struggling to redefine these viruses and integrate how the new definitions impact our understanding of HIV-1 pathogenesis and persistence.
Specifically, we now know that most transformed CD4+ T cell lines do not express CCR5 and therefore the “T cell-tropic” viruses are now appropriately called X4 viruses for their use of CXCR4. The confusion comes with the “macrophage-tropic” viruses, which we now know are a collection of two distinct types of CCR5-using viruses. Although a small minority of the CCR5-using viruses can enter cells with low levels of CD4 and infect macrophages efficiently, the vast majority of these viruses require high levels of CD4 to enter cells and only infect macrophages poorly. Thus, it is a serious conceptual mistake to refer to all of them as macrophage-tropic. We have developed the following designations to describe the host range of most HIV-1. (1) R5 T cell-tropic: these variants use CCR5 and require high levels of CD4 to enter cells, and represent the vast majority of HIV-1, including transmitted/founder viruses. (2) X4 T cell-tropic: T cell-tropic variants that have switched to use the CXCR4 coreceptor. (3) Macrophage-tropic: variants that still use CCR5 but can enter macrophages and other cells expressing low levels of CD4. We review the evidence for these designations below, but want to point out that it is essential to view HIV-1 as these three different types of host range variants—the normal R5 T cell-tropic virus and the two host range variants, X4 T cell-tropic viruses and macrophage-tropic viruses—to place HIV-1 transmission, evolution, pathogenesis, and persistence in their proper context.
There are two (at least) types of HIV-1 that do not fit neatly into our basic nomenclature. First, some T cell-tropic viruses evolve the ability use CXCR4, while retaining the ability to use CCR5. It is unknown whether these dual-tropic variants use both coreceptors in vivo, but the extent to which the virus favors one co-receptor or the other largely predicts its sensitivity to coreceptor antagonists in cell culture assays (Toma et al. 2010). Second, some macrophages express low levels of CXCR4 (Lee et al. 1999), raising the possibility that CXCR4-using viruses could evolve macrophage tropism. CXCR4-using viruses have been isolated from the macrophage-rich central nervous system (CNS) (Mefford et al. 2008; Yi et al. 2003), but it is unknown whether these variants are macrophage-tropic as defined by the ability to enter using low levels of CD4. One of these variants was shown to infect macrophages differentiated in culture (Yi et al. 2003), but differentiation methods are known to strongly influence expression of CXCR4 and CD4 (Lee et al. 1999), making it difficult to know if this variant would be likely to infect macrophages in vivo. At a minimum, putative X4 macrophage-tropic viruses are exceedingly rare, indicating that either CXCR4-using viruses cannot adapt to replicating in macrophages or do so infrequently.
The nomenclature that we have adopted indicates the cell type in which viruses are replicating and adapting. This is similar to the “lymphocyte-R5” nomenclature that has been proposed by Goodenow and Collman (2006). A limitation of both approaches is that “R5 T cell-tropic” viruses (or “lymphocyte-R5” viruses) have not been shown to be better adapted at replicating in T cells than are “macrophage-tropic” variants. In order to avoid any implication that one type of virus is better able to infect T cells than another, some researchers (Peters et al. 2006) simply divide viruses into “macrophage-tropic” and “non-macrophage-tropic”. The disadvantage of this approach is that it provides no information about where the vast majority of HIV-1 (i.e., “non-macrophage-tropic” HIV-1) replicates. We have chosen to distinguish viruses based on the cells in which they are replicating, and we acknowledge that we have an incomplete understanding of the relative replication rates of different viruses in T cells and T cell subsets.
Note that tropism is often assessed based on the ability of a HIV-1 variant to enter a specific host cell, but replication blocks that arise after entry may also influence cellular tropism. HIV-1 entry is determined by the HIV-1 Env protein. The Env precursor protein is cleaved into two subunits by a host furin-like protease. The resulting subunits, gp120 and gp41, stay non-covalently associated as heterodimers and are organized as trimers on the surface of the virion, with gp41 being a transmembrane protein anchoring the trimer in the viral envelope/membrane. Entry into the host cell is initiated when the CD4 binding site of gp120 binds a CD4 molecule on the surface of the host cell, which results in conformational changes that expose the coreceptor binding site on gp120. Binding either the CCR5 or CXCR4 coreceptor causes the extracellular domain of gp41 to form a six-helix bundle and generate a fusion pore between the viral and host membrane that allows the capsid to enter the host cell (reviewed by Wilen et al. 2012). Thus, functional or genetic analyses of Env proteins are effective tools for assessing the ability of an HIV-1 variant to enter a target cell. However, other factors may also influence cellular tropism after entry. For example, the concentration of dNTPs in macrophages is approximately 100-fold lower than that of activated CD4+ T cells and efficient replication in macrophages requires that reverse transcriptase be able to catalyze DNA synthesis when the dNTP concentration is low (Diamond et al. 2004). We would argue that the viral proteins that act against intracellular innate host responses evolved to do so in T cells, but it is possible that these viral proteins also adapt to carry out similar functions, or the long terminal repeats (LTRs) adapt to different host transcription factors, in the environment of different cell types.
HIV-1 in the blood is R5 T cell-tropic
There are three types of evidence indicating that the vast majority of HIV-1 is R5 T cell-tropic. The first type of evidence comes from studies showing that T cells are the most commonly infected cells in the blood. This is illustrated by the fact that HIV-1 proviral DNA and replication competent viruses can be readily recovered from CD4+ T cells isolated from HIV+ subjects both on (Brenchley et al. 2004; Chun et al. 1997; Finzi et al. 1997; Ho et al. 2013; Wong et al. 1997; Yukl et al. 2013) and off antiretroviral therapy (Brinchmann et al. 1991; Psallidopoulos et al. 1989; Schnittman et al. 1989). The only other cell type in the blood reported to be infected is monocytes. A number of studies report the detection of HIV-1 DNA in monocytes (Wang et al. 2013; Xu et al. 2008; Zhu et al. 2002) and the isolation of replication competent virus (Lambotte et al. 2000; Sonza et al. 2001; Wang et al. 2013) from monocytes, but T cells are clearly infected with much greater frequency than monocytes (Fulcher et al. 2004; Lambotte et al. 2000; Spivak et al. 2011) and some investigators have failed to find viral DNA in monocytes above levels that could be accounted for by T-cell contamination (Spivak et al. 2011).
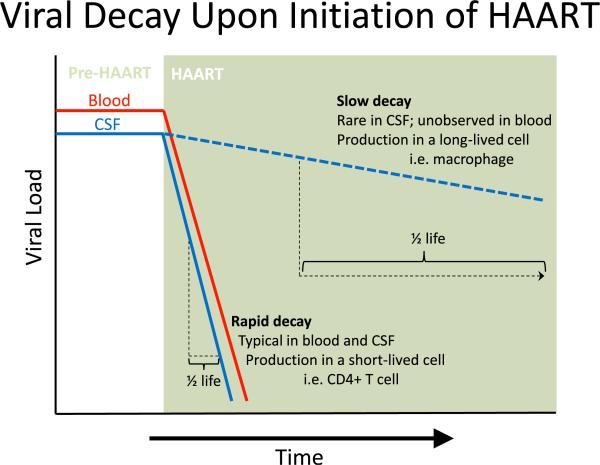
The second line of evidence that CD4+ T cells are the primary targets of HIV-1 in vivo comes from the observation that initiating highly active antiretroviral therapy (HAART) results in exponential decay of HIV-1 in the blood (Ho et al. 1995; Perelson et al. 1996) (Fig. 1). Since antiretroviral therapy blocks infection of new cells, viral decay reflects the death of infected cells and can be used to calculate the half-life of HIV-1-infected cells. Exponential viral decay after the initiation of antiretroviral therapy indicates that most viruses are produced by short-lived cells such as infected Tcells (Ho et al. 1995; Perelson et al. 1996; Simon and Ho 2003; Wei et al. 1995).
Fig. 1.
Successful HAART blocks new infection but does not affect cells already infected with HIV-1. Therefore, the remaining viral production (and viral load) is directly related to the lifespan of the cells infected prior to initiating HAART. In essentially all patients infected with HIV-1, viral load (VL) decays rapidly upon initiation of HAART with a half-life of approximately 1 to 2 weeks in the blood (indicated by the solid red line) and cerebrospinal fluid (CSF; solid blue line), which is consistent with virus produced from infected CD4+ T cells in both compartments. In rare cases, patients will have the same rapid decay of VL in the blood but will have a much slower decay in the CSF (dashed blue line). This indicates that in these patients viruses are being produced in CD4+ T cells in the blood but are being produced by a longer-lived cell, likely perivascular macrophages or microglia, in the CSF.
The final type of evidence comes from in vitro analyses showing that most HIV-1 isolates are well adapted to replicating in CD4+ T cells and poorly adapted to replicating in macrophages, the rationale being that an HIV-1 lineage will be well-adapted to the cell type in which it typically replicates. Multiple studies have shown that infectious molecular clones (Li et al. 2010; Ochsenbauer et al. 2012; Salazar-Gonzalez et al. 2009) and env gene clones (Isaacman-Beck et al. 2009) generated from transmitted/founder viruses encode surface Env proteins that do not allow efficient infection of monocyte-derived macrophages (MDM). A limitation of this approach is that MDMs are highly variable in their susceptibility to infection by HIV-1 both across donors and from different time points (Joseph et al. 2014; Naif et al. 1998; Sonza et al. 1995), which may cause a virus to appear well adapted to infecting MDMs in one experiment and poorly adapted at another.
There is another, more reproducible approach to examining whether viruses are adapted to entering macrophages by examining whether viruses are adapted to entering cells with similar CD4 receptor levels compared to macrophages. Monocyte-derived macrophages and CD4+ T cells express similar numbers of CD4 molecules (Joseph et al. 2014; Lee et al. 1999), but their larger surface area causes MDMs to have a 20-fold lower CD4 density than do CD4+ T cells (Joseph et al. 2014). Using this approach, we (Joseph et al. 2014; Ping et al. 2013) and others (Alexander et al. 2010; Parrish et al. 2012; Peters et al. 2006) have found that blood-derived Env proteins from all stages of infection require high densities of CD4 to facilitate entry, similar to those levels expressed on CD4+ T cells (Joseph et al. 2014). In vitro assays have also revealed that the vast majority of blood-derived Env proteins require CCR5 (Alexander et al. 2010; Isaacman-Beck et al. 2009; Keele et al. 2008; Parrish et al. 2012; Wilen et al. 2011), but it is unclear whether they differ from CNS-derived, macrophage-tropic Env proteins. We previously observed no difference in how paired macrophage-tropic and T cell-tropic Env proteins from five patients interact with CCR5 (Joseph et al. 2014). In contrast, another study suggested that macrophage-tropic viruses are more “CCR5-dependent” (Salimi et al. 2013); however, their analysis may have confounded CCR5 usage with CD4 usage.
Because most blood-derived viruses are well adapted to entering CD4+ T cells and use the CCR5 coreceptor, most HIV-1 isolates are R5 T cell-tropic. This situation changes with the appearance of X4 T cell-tropic and/or dual-tropic viruses appearing in the blood of approximately one half of subjects infected with subtype B HIV-1 as they progress to late in disease (Berger et al. 1999).
While T cells are the most commonly infected cells in the blood and most viruses in the blood are R5 T cell-tropic, there is evidence that tissue macrophages may represent important viral reservoirs and may be detected in the blood under some circumstances. It has long been noted that after initiating antiretroviral therapy, the plasma viral load initially decays very rapidly but then enters a period of slow decay that corresponds to the release of virus from longer-lived cells (Perelson et al. 1997). One potential source of this plasma virus is infected macrophages in tissue. In some patients, CNS macrophages are known to be productively infected by HIV-1 (see below). It is still unknown how often HIV-1 infects macrophages in different tissues and what conditions favor those events, but some studies suggest that coinfections may promote HIV-1 infection of macrophages in tissue. An analysis of macrophages in lymphoid tissue found that macrophages were infected with HIV-1 in subjects with opportunistic infections (OIs), but not in subjects lacking OIs (Orenstein et al. 1997). Further, a study of subjects coinfected with HIV-1 and Mycobacterium tuberculosis found that both macrophages and T cells contributed to virus in pleural fluid (Lawn et al. 2001). However, when the slow decay virus was examined using the heteroduplex tracking assay to assess its genetic complexity, it was found to be the same as the virus in the blood plasma indicating that this component of virus, at least in the subjects examined, does not represent a distinct population (Ince et al. 2009).
The CNS is an “immune privileged” site containing a unique mix of target cells
The CNS has long been viewed as an “immune privileged” site where T cells are rare and antigens do not induce a strong adaptive immune response. The foundation of this concept can be traced as far back as 1921 when it was observed that a rat sarcoma tumor grew well if transplanted into the mouse brain but failed to grow when transplanted outside of the CNS (Shirai 1921). Subsequent studies were able to show that growth of the tumor in the CNS was possible because the CNS shielded it from immune surveillance (reviewed by Galea et al. 2007).
How the immune system achieves this “privilege” is generally attributed to the blood–brain barrier (BBB) and the blood–cerebrospinal fluid barrier (BCSFB), which restrict the movement of cells and other materials from the peripheral blood into the CNS (Ousman and Kubes 2012). The BBB lines blood vessels in the brain and consists of endothelial cells expressing tight junctions. In the average adult, this barrier has a large surface area of between 12 and 18 m2 (Nag and Begley 2005), presenting many potential points of weakness where substances in the blood might gain direct access to the brain parenchyma. Alternatively, substances can cross the BCSFB at the choroid plexus. Ependymal cells within the choroid plexus secrete CSF by processing the peripheral blood (Brown et al. 2004) and the resulting CSF flows into the brain ventricular system and circulates through the subarachnoid spaces surrounding the brain. Substances in the CSF can enter the brain parenchyma and spinal cord by crossing ciliated ependymal cells that line the ventricles and subarachnoid spaces (Del Bigio 2010). Thus, there are different barriers separating the blood from the CNS and multiple ways for substances to breach those barriers and reach the brain parenchyma.
Immune privilege functions to maintain the proper conditions for neuronal and glial signaling (Verkhratsky et al. 1998) and protect the delicate cells of the CNS. The efficiency of these barriers is well illustrated by the observation that the concentration of T cells and HIV-1 in the cerebrospinal fluid (CSF) is typically less than 1 % of that found in the blood. These barriers also appear to alter the ratio of cells. For example, neutrophils, the most common leukocytes in the blood, are rarely observed in the CSF, and the ratio of CD4+ to CD8+ T cells is higher in the CSF than in the blood (Ransohoff et al. 2003). Thus, the BBB and BCSFB considerably limit movement from the blood into the CNS and also select for specific cells.
There are a number of ways for T cells may to enter the CNS (Ransohoff et al. 2003). An analysis of fluorescently labeled T cells injected into the peripheral blood of mice showed that 2 h after being injected, the cells could be observed entering the CNS through both the choroid plexus and meninges (Carrithers et al. 2002). This is supported by the observation that T cells are clustered in the choroid plexus and meninges of human autopsy tissue (Kivisakk et al. 2003). Another study found that CD4+ T cells that have been primed to attack myelinated nerves primarily enter the CNS at the fifth lumbar cord (Arima et al. 2012). Interestingly, this study also found that this point of entry could be blocked by reducing the expression of the chemokine CCL20 (MIP3A) (Arima et al. 2012), and other studies have shown that inflammatory cytokines such as tumor necrosis factor α (TNFα), interleukin (IL)-1β, and IL-6 reduce the integrity of the BBB (deVries et al. 1996). Thus, permeability of the BBB varies both by anatomical region and immunologic state.
HIV-1 in the CNS can be either R5 T cell-tropic or R5 macrophage-tropic
The CNS contains three groups of CD4+ immune cells that are likely to be infected by HIV-1: CD4+ T cells, macrophages, and microglia. The CNS also contains CD4− astrocytes which have been proposed to be infected by HIV-1. Unlike CD4-mediated infection of T cells, macrophages, and microglia, infection of astrocytes would require a CD4-independent pathway. Here we review the evidence that HIV-1 infects these cells in vivo.
CD4+ T cells
CD4+ Tcells are the most commonly HIV-infected cells in the human body overall (see above) and are major targets of HIV-1 infection in the CNS. The CSF of a healthy individual contains as many as 300,000 CD4+ T cells in a total volume of approximately 150 ml (Ransohoff and Engelhardt 2012), the majority of which are CD4+ CD45RA− CD27+ CD69+ activated central memory T cells (Kivisakk et al. 2002, 2003). Given that CD4+ memory T cells are the primary target of HIV-1 in the blood (Brenchley et al. 2004; Douek et al. 2002; Sleasman et al. 1996), the T-cell population in the CSF is likely to be highly susceptible to infection by HIV-1.
Direct identification of HIV-infected CD4+ T cells in the CNS is challenging given that CD4+ T cells are primarily confined to the CSF, where they are present at low concentration. Similarly, the concentration of CD4+ T cells in the brain parenchyma is likely to be very low. Thus, identifying HIV-infected CD4+ Tcells in the CNS requires less direct methods, such as assessing whether viruses replicating in the CNS are well adapted to entering T cells and not adapted to entering macrophages or microglia. In an analysis of eight subjects diagnosed with HAD, we observed three subjects with CSF viral populations that were genetically distinct compared to virus in the blood (i.e., compartmentalized) (Schnell et al. 2011), poorly adapted to entering macrophages (Schnell et al. 2011) and other low CD4 cells (Schnell et al. 2011) and decayed rapidly when the subject initiated therapy (Schnell et al. 2009). The observation that some viral populations in the CSF decay rapidly after initiating therapy and are poorly adapted to entering macrophage/microglia indicates that the virus was replicating in CD4+ T cells at the time of sampling and had been replicating in CD4+ T cells for many generations. Whether CNS inflammation and pleocytosis are necessary to support ongoing replication in CD4+ T cells in the CNS is unknown.
Microglia
Examinations of microglia and macrophages in the CNS reveal that they are frequently infected by HIV-1 despite expressing low levels of CD4 (Dick et al. 1997; Wang et al. 2002), levels that are well below those expressed on CD4+ T cells in the blood (Dick et al. 1997). Assessing the relative susceptibility of these two types of myeloid cells to HIV-1 infection is complicated by the fact that they express many of the same surface markers. Prior to activation, these two cell types are easily distinguished based on morphological differences, but activation by infection, trauma, etc. causes microglia to take on macrophage-like characteristics (reviewed by Kettenmann et al. 2011). Despite these similarities, the glucose transporter 5 protein (GLUT5) is expressed on both resting and activated microglia, but not on macrophages (Horikoshi et al. 2003; Maher et al. 1994; Vannucci et al. 1997), and has been used to stain microglia in healthy (Kitamura et al. 2010) and damaged brains (He and Crews 2008; Kitamura et al. 2010; Sasaki et al. 2004). Similarly, antibodies to the mannose receptor stain macrophages, but not microglia, in inflamed and normal human (Fabriek et al. 2005) and mouse brains (Galea et al. 2005). Given how difficult it is to distinguish these cells by morphology and/or immunohistochemistry, they are often identified based on their localization within the CNS.
Microglia are the resident macrophages in the CNS. They are the only myeloid cells located within the CNS parenchyma (i.e., the brain tissue proper, which does not include the CSF spaces, blood vessels, or meningeal coverings) (Ransohoff and Cardona 2010) and they represent 5–20 % of the adult brain cells (Polazzi and Monti 2010). In their non-activated or “resting” state, the surface of microglia are made up of many branched processes that are continuously surveying the brain, but activation causes them to transition to an amoeboid, macrophage-like appearance (Kettenmann et al. 2011). Recent studies have shown that unlike most myeloid cells, microglia are not derived from hematopoietic stem cells in the bone marrow, but rather they are derived from precursors that colonize the CNS during embryonic development (reviewed by Perdiguero et al. 2013). Microglia share many functions with macrophages such as phagocytosis, secretion of proinflammatory cytokines, and antigen presentation (Polazzi and Monti 2010), but they also have protective roles that promote proper brain function and development (Tremblay et al. 2011).
HIV-1 has been shown to infect microglia both in vivo and in vitro. At autopsy, infected microglia can be identified by labeling brain tissue for HIV-1 RNA (Stoler et al. 1986; Wiley et al. 1986) and/or protein (Cosenza et al. 2002; Neuenjacob et al. 1993; Wiley et al. 1986). However, these analyses are typically performed on subjects who died with severe neurocognitive impairment and HIV-1 infection of microglia may be less common earlier in disease. HIV-1 has also been shown to infect microglia in culture (Albright et al. 2000; Ioannidis et al. 1995; McCarthy et al. 1998; Watkins et al. 1990), but it is unclear how culturing these cells influences their susceptibility to infection. Together, these studies provide strong evidence that microglia are susceptible to HIV-1 infection but provide little information about the frequency of microglial infection in subjects that do not have severe neurocognitive impairment.
It is clear that the viruses replicating in the CNS can make an evolutionary transition from replicating in T cells to replicating in macrophages or microglia. This transition can be assessed by determining whether viruses replicating in the CNS are well adapted to entering cells expressing low levels of CD4 and whether viral RNA load decays slowly in the CSF after initiation of antiretroviral therapy. One caveat of this approach is that microglia and macrophages are both long-lived cells with low surface densities of CD4. As a result, replication in and adaptation to either cell type would likely result in viral populations that decay slowly and are well adapted to entering cells expressing low levels of CD4. In our analysis of eight subjects diagnosed with HAD, we observed five subjects with CSF viral populations that were genetically distinct from virus in the blood (i.e., compartmentalized) (Joseph and Kincer unpublished data; Schnell et al. 2011), well adapted to entering macrophages and/or other low CD4 cells (Schnell et al. 2011) and decayed slowly after initiation of therapy (Joseph and Kincer unpublished data; Schnell et al. 2009). This suggests that the CSF lineages had been replicating in long-lived, low CD4 cells in the CNS (either microglia or macrophage) for many generations to allow evolution of this host range variant.
It is important to make a distinction about two different circumstances under which cells with low levels of CD4 may become infected. There is not an absolute block to infection of CD4-low cells by R5 T cell-tropic viruses. These viruses will infect MDMs in culture but at very low efficiency compared to M-tropic viruses (K. Arrildt unpublished observation). Thus, there could be low levels of infection of macrophages in vivo throughout infection. Such levels may be enhanced by juxtaposition of an infected T cell to a macrophage (cell–cell transmission). However, this type of infection is distinct from the situation where the virus actually evolves to infect cells with low levels of CD4, i.e., macrophage tropism, signaling a change in the predominant cell type that is being infected locally.
Choroid plexus, meningeal, and perivascular macrophages
There are three types of CNS macrophages found outside of the brain parenchyma: perivascular macrophages, choroid plexus macrophages, and meningeal macrophages (Ransohoff and Cardona 2010). These bone-marrow-derived macrophages are named for anatomical region in which they reside. Perivascular macrophages reside in the perivascular (Virchow–Robin) space of cerebral vessels and are likely to be exposed to HIV-1 that crosses the BBB. In contrast, choroid plexus macrophages are located in the stroma of the choroid plexus on the peripheral side of the BCSFB. Thus, choroid plexus macrophages are likely exposed to HIV-1 in the blood being used to form CSF and meningeal macrophages would be exposed to HIV-1 that reaches the CSF. It is unclear whether HIV-1 migrates readily between these macrophage populations or whether they represent independent sites of viral replication. These questions have been addressed by experiments infecting macaques with highly neurotropic SIV. One study identified genetically distinct viral lineages in the meninges and brain parenchyma of animals with normal disease progression, but failed to detect compartmentalization in animals that progressed rapidly (Matsuda et al. 2013). This indicates that viruses can replicate independently at different anatomical sites or mix freely throughout the CNS, and it provides suggestive evidence that these viral states are associated with different outcomes.
Most of what we know about the susceptibility of CNS macrophages to HIV-1 is based on immunohistochemistry of autopsy tissue, which most often identify infection of perivascular macrophages (Cosenza et al. 2002; Fischer-Smith et al. 2001). Their position in the perivascular (Virchow–Robin) space of cerebral vessels may increase their exposure to HIV-1 and increase their probability of being infected. This is consistent with the observation that perivascular macrophages are productively infected more often than microglia both in humans with HAD (Fischer-Smith et al. 2001) and in macaques infected with SIV (Williams et al. 2001). Similarly there is some evidence of HIV-1 infection in the meninges, although it is often localized to the perivascular region (Lamers et al. 2011), suggesting that perivascular macrophages are more often infected than other meningeal cells. Two studies observed that 50 % of HIV+ subjects had infected choroid plexus macrophages (Falangola et al. 1995; Petito et al. 1999), but another study failed to find infected cells in the choroid plexus (Zhou et al. 2008). Further, we (Joseph et al. 2014; Schnell et al. 2011) and others (Gorry et al. 2001, 2002; Koyanagi et al. 1987; Li et al. 1999; Peters et al. 2004) have shown that some CNS-derived HIV-1 Env proteins can enter monocyte-derived macrophage in culture, though it is unclear how closely cultured MDMs represent macrophage in vivo. Together, these findings suggest that HIV-1 infects different types of CNS macrophages in vivo and that HIV-1 can adapt to replication in these cells.
Astrocytes
Astrocytes are the most abundant cell type in the brain (Nedergaard et al. 2003) and they express little or no CD4 (Harouse et al. 1989; Liu et al. 2004); also, there is no obvious functional reason for this cell type to express CD4, so its absence could be expected based on first principles. However, several groups of researchers have identified the presence of viral DNA in astrocytes, especially in autopsy samples taken from subjects with HIV-associated neurological disease (Nuovo and Alfieri 1996; Bagasra et al. 1996; Takahashi et al. 1996). These initial observations were confirmed in subsequent studies that showed in some areas of the brain up to 10–20 % of astrocytes have viral DNA (Churchill et al. 2009), although in a non-productive infection (Churchill et al. 2006; Gorry et al. 2003; Thompson et al. 2004). These observations represent a challenge to put in the context of a virus that requires the presence of CD4 for entry, including the M-tropic variants (Joseph et al. 2014). One possible explanation may lie in the recently appreciated capacity of astrocytes to engage in phagocytosis in the context of removal of neuronal debris (Cahoy et al. 2008; Chung et al. 2013; Tasdemir-Yilmaz and Freeman 2014). If these pathways were also active in the context of cell death during CNS infection, then evidence of HIV-1 in astrocytes could be coming from cell debris of infected CD4+ cells.
The evolution of macrophage tropism in the CNS
There is clear evidence that HIV-1 replication in the CNS is associated with neurocognitive impairment. However, our understanding of this relationship has been limited by the fact that HIV-infected cells can only be directly observed in autopsy tissue and may be difficult to detect if they are latently infected, producing low levels of virus, and/or buried deep in the tissue. Despite these limitations, a study of autopsy samples from 39 HIV+ subjects both on and off antiretroviral treatment found that most had HIV-associated encephalitis (HIVE) as characterized by features including multinucleated giant cells, microglial nodules, astrocytosis, and myelin pallor (Cherner et al. 2002). Of those subjects with neurocognitive impairment in their last 18 months of life, almost all had HIVE (95 %) at autopsy (Cherner et al. 2002), and other studies have shown that neurocognitive impairment is more common in HIV+ subjects than in HIV− subjects (Antinori et al. 2007). Thus, HIV-1 infected individuals often have elevated CNS damage and neurocognitive impairment.
Macrophages and microglia are the predominant CD4-expressing cells in the CNS and are known to be infected by HIV-1 (see above). Further, we have shown that between 40 % and 70 % of subjects with HAD have macrophage-tropic HIV-1 in their CNS (Schnell et al. 2011; Joseph and Kincer unpublished data). This strongly suggests that infection of macrophages and/or microglia contributes to neurocognitive impairment, making it essential to understand how and when macrophage-tropic variants evolve and to develop methods for identifying these variants.
As soon as 8 days after transmission, HIV-1 RNA can be isolated from the CSF (Valcour et al. 2012), but it is unclear whether the CNS is initially colonized by free virus or by infected cells that subsequently release virus into the CNS/ CSF. In a subset of infected individuals, there is minimal CNS viral burden early (Spudich et al. 2011; Sturdevant et al. under review); thus, HIV-1 is likely entering the CSF/CNS at low levels via incomplete partitioning of virus at the BBB, or background levels of trafficking of immune cells, including small numbers of infected CD4+ T cells. Often, HIV-1 viral RNA loads within the CNS are elevated during primary infection, and this can be associated with higher levels of CSF pleocytosis (Sturdevant et al. under review). Thus, the CNS viral burden early may result from the release of virus from increased numbers of infected CD4+ T cells trafficking from the periphery into the CNS in response to local HIV-1 replication in the CNS or another inflammatory condition.
In the CSF of a subset of HIV-infected subjects, it is possible to detect compartmentalized CSF viral populations that are undergoing independent replication (Dunfee et al. 2006; Harrington et al. 2009; Ohagen et al. 2003; Pilcher et al. 2001; Schnell et al. 2009). These CSF viral populations are genetically distinct from virus in the periphery. Studies using both heteroduplex track assays and single genome amplification have shown that compartmentalized CSF populations can be detected during primary infection (Harrington et al. 2009; Schnell et al. 2010; Sturdevant et al. under review), suggesting that viruses can begin replicating in the CNS very early and in the absence of overt neurological symptoms. CNS viral populations detected early are often clonally amplified populations, observed in the CNS as early as 2 to 6 months post-infection (Sturdevant et al. under review), but can also be characterized by more genetically diverse variants, indicative of persistent replication beyond a single clonal amplification event (Schnell et al. 2010; Sturdevant et al. under review). Additionally, in both vertical (Sturdevant et al. 2012) and horizontal transmission (Sturdevant et al. under review), CNS compartmentalization can also be established via the early sequestration of one of multiple transmitted variants in the CNS shortly after transmission, with replication of a second transmitted variant occurring predominantly in the periphery. We have shown that viruses in the CSF during primary infection are unable to efficiently infect cells expressing low levels of CD4 (Sturdevant et al. under review) and so are not adapted to infect macrophages/microglia. Little is known about how viral populations in the CSF transition from being R5 T cell-tropic during primary infection to being macrophage-tropic in some subjects late in disease.
Many groups have used env sequences from HIV-1 genomes isolated from macrophages or from the CNS of patients with HIV-associated neurocognitive disorders to locate the genetic determinants of macrophage tropism. Although these studies have had some success in identifying genetic determinants that work in patient-matched viruses, these same genetic changes do not occur in all or even most other macrophage-tropic env genes (Table 1), and a previous study found that the genetic determinants of macrophage-tropism can be complex and/or do not translate between patients (Rossi et al. 2008). Because of this complexity, comparisons of much larger sets of patient-matched macrophage- and R5 T cell-tropic env sequences will need to be compiled with differences tested as functional determinants before we are able to develop a full understanding of the evolutionary pathway(s) that create the macrophage-tropic phenotype.
Table 1.
Mutations proposed to confer macrophage tropism are not observed in a well-validated collection of macrophage-tropic clones
| Env amino acid changes thought to convey macrophage tropism | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Tropism | 153 | 167 | 238b | 240 | 283 | 308 | 317 | 326 | 373 | 386 | 396 |
| Consensus (sub. B) | Tcell | E | D | P | T | T | H | F | I | M | N* | N* |
| Proposed mutation | Macrophage | Ga | Na | Kb | Kb | Nc,d | Pe | Ld | (M→I)b | Kf | Xf,g | Xh |
| 4013 | T cell | – | – | L | R | – | P | – | – | – | – | K |
| 4013 | Macrophage | – | – | – | – | – | – | I | – | – | – | – |
| 4051 | T cell | – | – | – | – | – | P | – | – | T | – | – |
| 4051 | Macrophage | – | – | L | K | – | P | – | – | T | T | – |
| 4059 | T cell | – | N | E | K | – | N | W | – | – | – | T |
| 4059 | Macrophage | – | N | E | K | – | S | W | – | – | – | T |
| 5002 | T cell | – | – | – | – | I | – | – | T | – | – | – |
| 5002 | Macrophage | D | – | – | – | – | – | – | – | – | – | – |
| 7115 | T cell | – | – | – | K | – | – | – | – | – | – | – |
| 7115 | Macrophage | – | – | – | K | – | – | – | – | – | – | – |
Eradicating HIV-1 reservoirs in the CNS
HIV-1 infection of long-lived macrophage, microglia, and astrocytes may present a major barrier to eradication strategies. One such strategy for eradicating latent viral reservoirs is to “shock and kill” them by inducing expression of latent proviruses so that HIV-infected cells will be killed by virus production or eliminated by the immune system (Archin and Margolis 2014). The most promising method of inducing this reservoir is the use of histone deacetylase inhibitors (HDACi) (reviewed by Archin and Margolis 2014). Expression of HIV-1 proviruses in latently infected cells is inhibited by deacetylation of histones on the HIV-1 LTR (reviewed by Wightman et al. 2012). The addition of HDACi facilitates acetylation of histones and expression of the proviral genome. A recent study found that addition of the HDACi vorinostat increased HIV-1 expression approximately 5-fold in resting CD4+ T cells isolated from the blood of HIV+ subjects who were virologically suppressed by antiretrovirals (Archin et al. 2012). This finding suggests that HDACi may be able to stimulate the latent reservoir which could subsequently be targeted by “kill” strategies.
If a “shock and kill” strategy is developed that can effectively induce latent reservoirs in the periphery, it is still unclear whether the same strategy would be effective at eliminating latent cells in the CNS. The first barrier will be the development of therapeutics that can reach the CNS. Studies examining the ability of vorinostat to treat Huntington's disease (Hockly et al. 2003) and cancer (Palmieri et al. 2009) suggest it is able to reach the CNS in mice, but it is unclear whether it could reach to human CNS at concentrations necessary to induce HIV-1 reservoirs. In contrast, the HDACi belinostat penetrates the CNS very poorly in macaques (Warren et al. 2008). A more fundamental question is whether HDACi are able to induce HIV-1 expression in CNS cells. Vorinistat has been shown to stimulate host gene expression in astrocytes (Nuutinen et al. 2010), but testing of HDACi for induction of HIV-1 expression in HIV-infected macrophage, microglia, or astrocytes has not been reported. A final concern is whether the immune system would be capable of eliminating HIV-infected cells from the CNS after induction. This may be a problem given the low concentration of T cells in the CNS. Further, efforts to target T cells to that compartment could damage the CNS.
Conclusions
A new assay that provides a more quantitative measurement of the ability of HIV-1 to enter cells with low levels of CD4 has now provided a clearer view of macrophage-tropic variants. This new view is forcing a reassessment of the role of macrophage-tropic HIV-1 variants in transmission and disease progression. The molecular determinants of macrophage tropism are as yet poorly understood. This more rigorous definition of macrophage-tropic HIV-1 will allow an assessment of the role these viruses play in the persistence of HIV-1 during suppressive antiviral therapy.
Acknowledgments
We thank all of those who contributed in the collection of these samples and all of the subjects who provided the samples. We are especially grateful to our UNC CFAR colleagues and especially the UNC CFAR Clinical Core led by Dr. Joseph Eron. In addition, Drs. Richard Price and Serena Spudich have been essential collaborators on many of our studies of HIV-1 in the CNS/CSF.
This work was funded by awards from the National Institutes of Health R37 AI44667 and P01 MH094177 to R.S. K.T.A. was supported by T32 AI07419. We also received support from the UNC Center for AIDS Research (P30 AI50410) and the Lineberger Comprehensive Cancer Center (P30 CA16086).
Footnotes
Conflict of interest statement The authors declare that they have no conflict of interest.
Contributor Information
Sarah B. Joseph, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
Kathryn T. Arrildt, Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
Christa B. Sturdevant, Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA
Ronald Swanstrom, Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; Department of Biochemistry and Biophysics, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; UNC Center for AIDS Research, University of North Carolina at Chapel Hill, School of Medicine, Chapel Hill, NC 27599, USA.
References
- Albright AV, Shieh JTC, O'Connor MJ, Gonzalez-Scarano F. Characterization of cultured microglia that can be infected by HIV-1. J Neurovirology. 2000;6:S53–S60. [PubMed] [Google Scholar]
- Alexander M, Lynch R, Mulenga J, Allen S, Derdeyn CA, Hunter E. Donor and recipient envs from heterosexual human immunodeficiency virus subtype C transmission pairs require high receptor levels for entry. J Virol. 2010;84:4100–4104. doi: 10.1128/JVI.02068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Margolis DM. Emerging strategies to deplete the HIV reservoir. Curr Opin Infect Dis. 2014;27:29–35. doi: 10.1097/QCO.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–U1650. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima Y, Harada M, Kamimura D, Park JH, Kawano F, Yull FE, Kawamoto T, Iwakura Y, Betz UAK, Marquez G, Blackwell TS, Ohira Y, Hirano T, Murakami M. Regional neural activation defines a gateway for autoreactive T cells to cross the blood–brain barrier. Cell. 2012;148:447–457. doi: 10.1016/j.cell.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Arrildt K, Joseph SB, Swanstrom R. The HIV-1 ENV protein: a coat of many colors. Curr HIV/AIDS Rep. 2012;9:52–63. doi: 10.1007/s11904-011-0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: Identification by the combination of in situ polymerase chain reaction and immunohistochemistry. Aids. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, Kuruppu J, Yazdani J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol. 2004;78:1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinchmann JE, Albert J, Vartdal F. Few infected CD4+ Tcells but a high proportion of replication-competent provirus copies in asymptomatic human immunodeficiency virus type 1 infection. J Virol. 1991;65:2019–2023. doi: 10.1128/jvi.65.4.2019-2023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Davies SL, Seake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957–970. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. Journal of Neuroscience. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrithers MD, Visintin I, Viret C, Janeway CA. Role of genetic background in P selectin-dependent immune surveillance of the central nervous system. J Neuroimmunol. 2002;129:51–57. doi: 10.1016/s0165-5728(02)00172-8. [DOI] [PubMed] [Google Scholar]
- Cashin K, Roche M, Sterjovski J, Ellett A, Gray LR, Cunningham AL, Ramsland PA, Churchill MJ, Gorry PR. Alternative coreceptor requirements for efficient CCR5- and CXCR4-mediated HIV-1 entry into macrophages. J Virol. 2011;85:10699–709. doi: 10.1128/JVI.05510-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Masliah E, Ellis RJ, Marcotte TD, Moore DJ, Grant I, Heaton RK. Neurocognitive dysfunction predicts postmortem findings of HIV encephalitis. Neurology. 2002;59:1563–1567. doi: 10.1212/01.wnl.0000034175.11956.79. [DOI] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JAM, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen CF, Smith SJ, Barres BA. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DFJ, Thompson KA, Gabuzda D, McArthur JC, Pardo CA, Wesselingh SL. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol. 2006;12:146–152. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza MA, Zhao ML, Si QS, Lee SC. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 2002;12:442–455. doi: 10.1111/j.1750-3639.2002.tb00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR. Ependymal cells: biology and pathology. Acta Neuropathol. 2010;119:55–73. doi: 10.1007/s00401-009-0624-y. [DOI] [PubMed] [Google Scholar]
- deVries HE, BlomRoosemalen MCM, vanOosten M, deBoer AG, vanBerkel TJC, Breimer DD, Kuiper J. The influence of cytokines on the integrity of the blood–brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- Diamond TL, Roshal M, Jamburuthugoda VK, Reynolds HM, Merriam AR, Lee KY, Balakrishnan M, Bambara RA, Planelles V, Dewhurst S, Kim B. Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J Biol Chem. 2004;279:51545–51553. doi: 10.1074/jbc.M408573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AD, Pell M, Brew BJ, Foulcher E, Sedgwick JD. Direct ex vivo flow cytometric analysis of human microglial cell CD4 expression: examination of central nervous system biopsy specimens from HIV-seropositive patients and patients with other neurological disease. AIDS. 1997;11:1699–1708. doi: 10.1097/00002030-199714000-00006. [DOI] [PubMed] [Google Scholar]
- Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kuntsman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. HIV preferentially infects HIV-specific CD4(+) T cells. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- Duenas-Decamp MJ, Peters P, Burton D, Clapham PR. Natural resistance of human immunodeficiency virus type 1 to the CD4bs antibody b12 conferred by a glycan and an arginine residue close to the CD4 binding loop. J Virol. 2008;82:5807–5814. doi: 10.1128/JVI.02585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duenas-Decamp MJ, Peters PJ, Burton D, Clapham PR. Determinants flanking the CD4 binding loop modulate macrophage tropism of human immunodeficiency virus type 1 R5 envelopes. J Virol. 2009;83:2575–2583. doi: 10.1128/JVI.02133-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunfee RL, Thomas ER, Gorry PR, Wang J, Taylor J, Kunstman K, Wolinsky SM, Gabuzda D. The HIV Env variant N283 enhances macrophage tropism and is associated with brain infection and dementia. Proc Natl Acad Sci U S A. 2006;103:15160–15165. doi: 10.1073/pnas.0605513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunfee RL, Thomas ER, Wang JB, Kunstman K, Wolinsky SM, Gabuzda D. Loss of the N-linked glycosylation site at position 386 in the HIV envelope V4 region enhances macrophage tropism and is associated with dementia. Virology. 2007;367:222–234. doi: 10.1016/j.virol.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabriek BO, Van Haastert ES, Galea I, Polfliet MMJ, Dopp ED, Van den Heuvel MM, Van den Berg TK, De Groot CJA, Van der Valk P, Dijkstra CD. CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation. Glia. 2005;51:297–305. doi: 10.1002/glia.20208. [DOI] [PubMed] [Google Scholar]
- Falangola MF, Hanly A, Galvaocastro B, Petito CK. HIV-infection of human choroid-plexus—a possible mechanism of viral entry into the CNS. J Neuropathol Exp Neurol. 1995;54:497–503. doi: 10.1097/00005072-199507000-00003. [DOI] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L'Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- Fulcher JA, Hwangbo Y, Zioni R, Nickle D, Lin XD, Heath L, Mullins JI, Corey L, Zhu TF. Compartmentalization of human immuno-deficiency virus type 1 between blood monocytes and CD4(+) T cells during infection. J Virol. 2004;78:7883–7893. doi: 10.1128/JVI.78.15.7883-7893.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea I, Palin K, Newman TA, Van Rooijen N, Perry VH, Boche D. Mannose receptor expression specifically reveals perivascular macrophages in normal, injured, and diseased mouse brain. Glia. 2005;49:375–384. doi: 10.1002/glia.20124. [DOI] [PubMed] [Google Scholar]
- Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Goodenow MM, Collman RG. HIV-1 coreceptor preference is distinct from target cell tropism: a dual-parameter nomenclature to define viral phenotypes. J Leukocyte Biol. 2006;80:965–972. doi: 10.1189/jlb.0306148. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Bristol G, Zack JA, Ritola K, Swanstrom R, Birch CJ, Bell JE, Bannert N, Crawford K, Wang H, Schols D, De Clercq E, Kunstman K, Wolinsky SM, Gabuzda D. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J Virol. 2001;75:10073–10089. doi: 10.1128/JVI.75.21.10073-10089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry PR, Taylor J, Holm GH, Mehle A, Morgan T, Cayabyab M, Farzan M, Wang H, Bell JE, Kunstman K, Moore JP, Wolinsky SM, Gabuzda D. Increased CCR5 affinity and reduced CCR5/CD4 dependence of a neurovirulent primary human immunodeficiency virus type 1 isolate. J Virol. 2002;76:6277–6292. doi: 10.1128/JVI.76.12.6277-6292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Wesselingh SL, Purcell DFJ. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1:463–473. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- Harouse JM, Kunsch C, Hartle HT, Laughlin MA, Hoxie JA, Wigdahl B, Gonzalezscarano F. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol. 1989;63:2527–2533. doi: 10.1128/jvi.63.6.2527-2533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington PR, Schnell G, Letendre SL, Ritola K, Robertson K, Hall C, Burch CL, Jabara CB, Moore DT, Ellis RJ, Price RW, Swanstrom R. Cross-sectional characterization of HIV-1 env compartmentalization in cerebrospinal fluid over the full disease course. AIDS. 2009;23:907–915. doi: 10.1097/QAD.0b013e3283299129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DIS, Lai J, Blankson JN, Siliciano JD, Siliciano RF. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockly E, Richon VM, Woodman B, Smith DL, Zhou XB, Rosa E, Sathasivam K, Ghazi-Noori S, Mahal A, Lowden PAS, Steffan JS, Marsh JL, Thompson LM, Lewis CM, Marks PA, Bates GP. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington's disease. Proc Natl Acad Sci U S A. 2003;100:2041–2046. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi Y, Sasaki A, Taguchi N, Maeda M, Tsukagoshi H, Sato K, Yamaguchi H. Human GLUT5 immunolabeling is useful for evaluating microglial status in neuropathological study using paraffin sections. Acta Neuropathol. 2003;105:157–162. doi: 10.1007/s00401-002-0627-4. [DOI] [PubMed] [Google Scholar]
- Ince WL, Harrington PR, Schnell GL, Patel-Chhabra M, Burch CL, Menezes P, Price RW, Eron JJ, Swanstrom RI. Major coexisting human immunodeficiency virus type 1 env gene subpopulations in the peripheral blood are produced by cells with similar turnover rates and show little evidence of genetic compartmentalization. J Virol. 2009;83:4068–4080. doi: 10.1128/JVI.02486-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JPA, Reichlin S, Skolnik PR. Long-term productive human immunodeficiency virus-1 infection in human infant microglia. Am J Pathol. 1995;147:1200–1206. [PMC free article] [PubMed] [Google Scholar]
- Isaacman-Beck J, Hermann EA, Yi YJ, Ratcliffe SJ, Mulenga J, Allen S, Hunter E, Derdeyn CA, Collman RG. Heterosexual transmission of human immunodeficiency virus type 1 subtype C: macrophage tropism, alternative coreceptor use, and the molecular anatomy of CCR5 utilization. J Virol. 2009;83:8208–8220. doi: 10.1128/JVI.00296-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SB, Arrildt KT, Swanstrom AE, Schnell G, Lee B, Hoxie JA, Swanstrom R. Quantification of entry phenotypes of macrophage-tropic HIV-1 across a wide range of CD4 densities. J Virol. 2014;88:1858–1869. doi: 10.1128/JVI.02477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun CX, Grayson T, Wang SY, Li H, Wei XP, Jiang CL, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. Identification and characterisation of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch U-K, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Takeichi T, Wang EL, Tokunaga I, Ishigami A, S-i K. Microglial and astrocytic changes in the striatum of methamphetamine abusers. Legal Med. 2010;12:57–62. doi: 10.1016/j.legalmed.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Kivisakk P, Trebst C, Liu Z, Tucky BH, Sorensen TL, Rudick RA, Mack M, Ransohoff RM. T-cells in the cerebrospinal fluid express a similar repertoire of inflammatory chemokine receptors in the absence or presence of CNS inflammation: implications for CNS trafficking. Clin Exp Immunol. 2002;129:510–518. doi: 10.1046/j.1365-2249.2002.01947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivisakk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T, Wu LJ, Baekkevold ES, Lassmann H, Staugaitis SM, Campbell JJ, Ransohoff RM. Human cerebrospinal fluid central memory CD4(+) T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koot M, Keet IPM, Vos AHV, Degoede REY, Roos MTL, Coutinho RA, Miedema F, Schellekens PTA, Tersmette M. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen ISY. Dual infection of the central-nervous-system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Lambotte O, Taoufik Y, de Goer MG, Wallon C, Goujard C, Delfraissy JF. Detection of infectious HIV in circulating monocytes from patients on prolonged highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2000;23:114–119. doi: 10.1097/00126334-200002010-00002. [DOI] [PubMed] [Google Scholar]
- Lamers SL, Gray RR, Salemi M, Huysentruyt LC, McGrath MS. HIV-1 phylogenetic analysis shows HIV-1 transits through the meninges to brain and peripheral tissues. Infect Genet Evol. 2011;11:31–37. doi: 10.1016/j.meegid.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn SD, Pisell TL, Hirsch CS, Wu M, Butera ST, Toossi Z. Anatomically compartmentalized human immunodeficiency virus replication in HLA-DR(+) cells and CD14(+) macrophages at the site of pleural tuberculosis coinfection. J Infect Dis. 2001;184:1127–1133. doi: 10.1086/323649. [DOI] [PubMed] [Google Scholar]
- Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1999;96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Juarez J, Alali M, Dwyer D, Collman R, Cunningham A, Naif HM. Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J Virol. 1999;73:9741–9755. doi: 10.1128/jvi.73.12.9741-9755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Bar KJ, Wang S, Decker JM, Chen Y, Sun C, Salazar-Gonzalez JF, Salazar MG, Learn GH, Morgan CJ, Schumacher JE, Hraber P, Giorgi EE, Bhattacharya T, Korber BT, Perelson AS, Eron JJ, Cohen MS, Hicks CB, Haynes BF, Markowitz M, Keele BF, Hahn BH, Shaw GM. High multiplicity infection by HIV-1 in men who have sex with men. PLoS Pathog. 2010;6:e1000890. doi: 10.1371/journal.ppat.1000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu H, Kim BO, Gattone VH, Li JL, Nath A, Blum J, He JJ. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol. 2004;78:4120–4133. doi: 10.1128/JVI.78.8.4120-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher F, Vannucci SJ, Simpson IA. Glucose transporter proteins in brain. FASEB J. 1994;8:1003–1011. doi: 10.1096/fasebj.8.13.7926364. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Brown CR, Foley B, Goeken R, Whitted S, Dang Q, Wu F, Plishka R, Buckler-White A, Hirscha VM. Laser capture microdissection assessment of virus compartmentalization in the central nervous systems of macaques infected with neurovirulent simian immunodeficiency virus. J Virol. 2013;87:8896–8908. doi: 10.1128/JVI.00874-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy M, He J, Wood C. HIV-1 strain-associated variability in infection of primary neuroglia. J Neurovirol. 1998;4:80–89. doi: 10.3109/13550289809113484. [DOI] [PubMed] [Google Scholar]
- Mefford ME, Gorry PR, Kunstman K, Wolinsky SM, Gabuzda D. Bioinformatic prediction programs underestimate the frequency of CXCR4 usage by R5X4 HIV type 1 in brain and other tissues. Aids Res Hum Retroviruses. 2008;24:1215–1220. doi: 10.1089/aid.2008.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musich T, Peters PJ, Duenas-Decamp MJ, Gonzalez-Perez MP, Robinson J, Zolla-Pazner S, Ball JK, Luzuriaga K, Clapham PR. A conserved determinant in the V1 loop of HIV-1 modulates the V3 loop to prime low CD4 use and macrophage infection. J Virol. 2011;85:2397–2405. doi: 10.1128/JVI.02187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S, Begley DJ. Blood–brain barrier, exchange of metabolites and gases. In: Kalimo H, editor. Pathology and genetics: cerebrovascular diseases. Neuropath; Basel: 2005. pp. 22–29. [Google Scholar]
- Naif HM, Li S, Alali M, Sloane A, Wu LJ, Kelly M, Lynch G, Lloyd A, Cunningham AL. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J Virol. 1998;72:830–836. doi: 10.1128/jvi.72.1.830-836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Neuenjacob E, Arendt G, Wendtland B, Jacob B, Schneeweis M, Wechsler W. Frequency and topographical distribution of CD68-positive macrophages and HIV-1 core proteins in HIV-associated brain lesions. Clin Neuropathol. 1993;12:315–324. [PubMed] [Google Scholar]
- Nuovo GJ, Alfieri ML. AIDS dementia is associated with massive, activated HIV-1 infection and concomitant expression of several cytokines. Mol Med. 1996;2:358–366. [PMC free article] [PubMed] [Google Scholar]
- Nuutinen T, Suuronen T, Kauppinen A, Salminen A. Valproic acid stimulates clusterin expression in human astrocytes: implications for Alzheimer's disease. Neurosci Lett. 2010;475:64–68. doi: 10.1016/j.neulet.2010.03.041. [DOI] [PubMed] [Google Scholar]
- Ochsenbauer C, Edmonds TG, Ding HT, Keele BF, Decker J, Salazar MG, Salazar-Gonzalez JF, Shattock R, Haynes BF, Shaw GM, Hahn BH, Kappes JC. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. J Virol. 2012;86:2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohagen A, Devitt A, Kunstman KJ, Gorry PR, Rose PP, Korber B, Taylor J, Levy R, Murphy RL, Wolinsky SM, Gabuzda D. Genetic and functional analysis of full-length human immunodeficiency virus type 1 env genes derived from brain and blood of patients with AIDS. J Virol. 2003;77:12336–12345. doi: 10.1128/JVI.77.22.12336-12345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein JM, Fox C, Wahl SM. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nat Neurosci. 2012;15:1096–1101. doi: 10.1038/nn.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y, Liu L, Zhang Y, Yuan L, Liu Z, Yang S, Wei F, Qiao L, Chen D. Discordant patterns of tissue-specific genetic characteristics in the HIV-1 env gene from HIV-associated neurocognitive disorder (HAND) and non-HAND patients. J Neurovirol. 2014;20:332–340. doi: 10.1007/s13365-014-0247-5. [DOI] [PubMed] [Google Scholar]
- Palmieri D, Lockman PR, Thomas FC, Hua E, Herring J, Hargrave E, Johnson M, Flores N, Qian YZ, Vega-Valle E, Taskar KS, Rudraraju V, Mittapalli RK, Gaasch JA, Bohn KA, Thorsheim HR, Liewehr DJ, Davis S, Reilly JF, Walker R, Bronder JL, Feigenbaum L, Steinberg SM, Camphausen K, Meltzer PS, Richon VM, Smith QR, Steeg PS. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin Cancer Res. 2009;15:6148–6157. doi: 10.1158/1078-0432.CCR-09-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish NF, Wilen CB, Banks LB, Iyer SS, Pfaff JM, Salazar-Gonzalez JF, Salazar MG, Decker JM, Parrish EH, Berg A, Hopper J, Hora B, Kumar A, Mahlokozera T, Yuan S, Coleman C, Vermeulen M, Ding H, Ochsenbauer C, Tilton JC, Permar SR, Kappes JC, Betts MR, Busch MP, Gao F, Montefiori D, Haynes BF, Shaw GM, Hahn BH, Doms RW. Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin α4β7. PLoS Pathog. 2012;8:e1002686. doi: 10.1371/journal.ppat.1002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero EG, Schulz C, Geissmann F. Development and homeostasis of “resident” myeloid cells: the case of the microglia. Glia. 2013;61:112–120. doi: 10.1002/glia.22393. [DOI] [PubMed] [Google Scholar]
- Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell lifespan, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- Perelson AS, Essunger P, Cao YZ, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho DD. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- Peters PJ, Bhattacharya J, Hibbitts S, Dittmar MT, Simmons G, Bell J, Simmonds P, Clapham PR. Biological analysis of human immunodeficiency virus type 1 R5 envelopes amplified from brain and lymph node tissues of AIDS patients with neuropathology reveals two distinct tropism phenotypes and identifies envelopes in the brain that confer an enhanced tropism and fusigenicity for macrophages. J Virol. 2004;78:6915–6926. doi: 10.1128/JVI.78.13.6915-6926.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters PJ, Sullivan WM, Duenas-Decamp MJ, Bhattacharya J, Ankghuambom C, Brown R, Luzuriaga K, Bell J, Simmonds P, Ball J, Clapham PR. Non-macrophage-tropic human immunodeficiency virus type 1 R5 envelopes predominate in blood, lymph nodes, and semen: implications for transmission and pathogenesis. J Virol. 2006;80:6324–6332. doi: 10.1128/JVI.02328-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Chen HX, Mastri AR, Torres-Munoz J, Roberts B, Wood C. HIV infection of choroid plexus in AIDS and asymptomatic HIV-infected patients suggests that the choroid plexus may be a reservoir of productive infection. J Neurovirol. 1999;5:670–677. doi: 10.3109/13550289909021295. [DOI] [PubMed] [Google Scholar]
- Pilcher CD, Shugars DC, Fiscus SA, Miller WC, Menezes P, Giner J, Dean B, Robertson K, Hart CE, Lennox JL, Eron JJ, Jr, Hicks CB. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS. 2001;15:837–45. doi: 10.1097/00002030-200105040-00004. [DOI] [PubMed] [Google Scholar]
- Ping LH, Joseph SB, Anderson JA, Abrahams MR, Salazar-Gonzalez JF, Kincer LP, Treurnicht FK, Arney L, Ojeda S, Zhang M, Keys J, Potter EL, Chu H, Moore P, Salazar MG, Iyer S, Jabara C, Kirchherr J, Mapanje C, Ngandu N, Seoighe C, Hoffman I, Gao F, Tang Y, Labranche C, Lee B, Saville A, Vermeulen M, Fiscus S, Morris L, Karim SA, Haynes BF, Shaw GM, Korber BT, Hahn BH, Cohen MS, Montefiori D, Williamson C, Swanstrom R. Comparison of viral Env proteins from acute and chronic infections with subtype C human immunodeficiency virus type 1 identifies differences in glycosylation and CCR5 utilization and suggests a new strategy for immunogen design. J Virol. 2013;87:7218–7233. doi: 10.1128/JVI.03577-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polazzi E, Monti B. Microglia and neuroprotection: from in vitro studies to therapeutic applications. Prog Neurobiol. 2010;92:293–315. doi: 10.1016/j.pneurobio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Psallidopoulos MC, Schnittman SM, Thompson LM, Baseler M, Fauci AS, Lane HC, Salzman NP. Integrated proviral human immunodeficiency virus type 1 is present in CD4+ peripheral blood lymphocytes in healthy seropositive individuals. J Virol. 1989;63:4626–4631. doi: 10.1128/jvi.63.11.4626-4631.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–81. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- Rossi F, Querido B, Nimmagadda M, Cocklin S, Navas-Martin S, Martin-Garcia J. The V1–V3 region of a brain-derived HIV-1 envelope glycoprotein determines macrophage tropism, low CD4 dependence, increased fusogenicity and altered sensitivity to entry inhibitors. Retrovirology. 2008;5:89. doi: 10.1186/1742-4690-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, Decker JM, Wang SY, Baalwa J, Kraus MH, Parrish NF, Shaw KS, Guffey MB, Bar KJ, Davis KL, Ochsenbauer-Jambor C, Kappes JC, Saag MS, Cohen MS, Mulenga J, Derdeyn CA, Allen S, Hunter E, Markowitz M, Hraber P, Perelson AS, Bhattacharya T, Haynes BF, Korber BT, Hahn BH, Shaw GM. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi H, Roche M, Webb N, Gray LR, Chikere K, Sterjovski J, Ellett A, Wesselingh SL, Ramsland PA, Lee B, Churchill MJ, Gorry PR. Macrophage-tropic HIV-1 variants from brain demonstrate alterations in the way gp120 engages both CD4 and CCR5. J Leukocyte Biol. 2013;93:113–126. doi: 10.1189/jlb.0612308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Yamaguchi H, Horikoshi Y, Tanaka G, Nakazato Y. Expression of glucose transporter 5 by microglia in human gliomas. Neuropathol Appl Neurobiol. 2004;30:447–455. doi: 10.1111/j.1365-2990.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- Schnell G, Spudich S, Harrington P, Price RW, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 originates from long-lived cells in some subjects with HIV-1-associated dementia. PLoS Pathog. 2009;5:e1000395. doi: 10.1371/journal.ppat.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalization and clonal amplification of HIV-1 variants in the cerebrospinal fluid during primary infection. J Virol. 2010;84:2395–2407. doi: 10.1128/JVI.01863-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R. HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog. 2011;7:e1002286. doi: 10.1371/journal.ppat.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittman SM, Psallidopoulos MC, Lane HC, Thompson L, Baseler M, Massari F, Fox CH, Salzman NP, Fauci AS. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989;245:305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- Shirai Y. On the transplantation of the rat sarcoma in adult heterogeneous animals. Jpn Med World. 1921;1:14–15. [Google Scholar]
- Simon V, Ho DD. HIV-1 dynamics in vivo: implications for therapy. Nat Rev Microbiol. 2003;1:181–190. doi: 10.1038/nrmicro772. [DOI] [PubMed] [Google Scholar]
- Sleasman JW, Aleixo LF, Morton A, SkodaSmith S, Goodenow MM. CD4+ memory T cells are the predominant population of HIV-1-infected lymphocytes in neonates and children. AIDS. 1996;10:1477–1484. doi: 10.1097/00002030-199611000-00004. [DOI] [PubMed] [Google Scholar]
- Sonza S, Maerz A, Uren S, Violo A, Hunter S, Boyle W, Crowe S. Susceptibility of human monocytes to HIV type 1 infection in vitro is not dependent on their level of CD4 expression. Aids Res Hum Retroviruses. 1995;11:769–776. doi: 10.1089/aid.1995.11.769. [DOI] [PubMed] [Google Scholar]
- Sonza S, Mutimer HP, Oelrichs R, Jardine D, Harvey K, Dunne A, Purcell DF, Birch C, Crowe SM. Monocytes harbour replication-competent, non-latent HIV-1 in patients on highly active antiretroviral therapy. AIDS. 2001;15:17–22. doi: 10.1097/00002030-200101050-00005. [DOI] [PubMed] [Google Scholar]
- Spivak AM, Salgado M, Rabi SA, O'Connell KA, Blankson JN. Circulating monocytes are not a major reservoir of HIV-1 in elite suppressors. J Virol. 2011;85:10399–403. doi: 10.1128/JVI.05409-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich S, Gisslen M, Hagberg L, Lee E, Liegler T, Brew B, Fuchs D, Tambussi G, Cinque P, Hecht FM, Price RW. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebro-spinal fluid viral burden. J Infect Dis. 2011;204:753–60. doi: 10.1093/infdis/jir387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler MH, Eskin TA, Benn S, Angerer RC, Angerer LM. Human T-cell lymphotropic virus type III infection of the central nervous system. A preliminary in situ analysis. JAMA. 1986;256:2360–2364. [PubMed] [Google Scholar]
- Sturdevant CB, Dow A, Jabara CB, Joseph SB, Schnell G, Takamune N, Mallewa M, Heyderman RS, Van Rie A, Swanstrom R. Central nervous system compartmentalization of HIV-1 subtype C variants early and late in infection in young children. PLoS Pathog. 2012;8:e1003094. doi: 10.1371/journal.ppat.1003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT, Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- Tasdemir-Yilmaz OE, Freeman MR. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Gene Dev. 2014;28:20–33. doi: 10.1101/gad.229518.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ER, Dunfee RL, Stanton J, Bogdan D, Taylor J, Kunstman K, Bell JE, Wolinsky SM, Gabuzda D. Macrophage entry mediated by HIV Envs from brain and lymphoid tissues is determined by the capacity to use low CD4 levels and overall efficiency of fusion. Virology. 2007;360:105–119. doi: 10.1016/j.virol.2006.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KA, Churchill MJ, Gorry PR, Sterjovski J, Oelrichs RB, Wesselingh SL, McLean CA. Astrocyte specific viral strains in HIV dementia. Ann Neurol. 2004;56:873–877. doi: 10.1002/ana.20304. [DOI] [PubMed] [Google Scholar]
- Toma J, Whitcomb JM, Petropoulos CJ, Huang W. Dual-tropic HIV type 1 isolates vary dramatically in their utilization of CCR5 and CXCR4 coreceptors. Aids. 2010;24:2181–2186. doi: 10.1097/QAD.0b013e32833c543f. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S, van Griensven F, de Souza M, Kim J, Ananworanich J. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- Wang JB, Crawford K, Yuan ML, Wang H, Gorry PR, Gabuzda D. Regulation of CC chemokine receptor 5 and CD4 expression and human immunodeficiency virus type 1 replication in human macrophages and microglia by T helper type 2 cytokines. J Infect Dis. 2002;185:885–897. doi: 10.1086/339522. [DOI] [PubMed] [Google Scholar]
- Wang T, Xu YN, Zhu HY, Andrus T, Ivanov SB, Pan C, Dolores J, Dann GC, Zhou M, Forte D, Yang ZH, Holte S, Corey L, Zhu TF. Successful isolation of infectious and high titer human monocyte-derived HIV-1 from two subjects with discontinued therapy. Plos One. 2013;8:e65071. doi: 10.1371/journal.pone.0065071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren KE, McCully C, Dvinge H, Tjornelund J, Sehested M, Lichenstein HS, Balis FM. Plasma and cerebrospinal fluid pharmacokinetics of the histone deacetylase inhibitor, belinostat (PXD101), in non-human primates. Cancer Chemother Pharmacol. 2008;62:433–437. doi: 10.1007/s00280-007-0622-5. [DOI] [PubMed] [Google Scholar]
- Watkins BA, Dorn HH, Kelly WB, Armstrong RC, Potts BJ, Michaels F, Kufta CV, Duboisdalcq M. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science. 1990;249:549–553. doi: 10.1126/science.2200125. [DOI] [PubMed] [Google Scholar]
- Wei XP, Ghosh SK, Taylor ME, Johnson VA, Emini EA, Deutsch P, Lifson JD, Bonhoeffer S, Nowak MA, Hahn BH, Saag MS, Shaw GM. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- Wightman F, Ellenberg P, Churchill M, Lewin SR. HDAC inhibitors in HIV. Immunol Cell Biol. 2012;90:47–54. doi: 10.1038/icb.2011.95. [DOI] [PubMed] [Google Scholar]
- Wilen CB, Parrish NF, Pfaff JM, Decker JM, Henning EA, Haim H, Petersen JE, Wojcechowskyj JA, Sodroski J, Haynes BF, Montefiori DC, Tilton JC, Shaw GM, Hahn BH, Doms RW. Phenotypic and immunologic comparison of clade B transmitted/founder and chronic HIV-1 envelope glycoproteins. J Virol. 2011;85:8514–8527. doi: 10.1128/JVI.00736-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilen CB, Tilton JC, Doms RW. HIV: Cell binding and Entry. In: Bushman FD, Nabel GJ, Swanstrom R, editors. HIV: from biology to prevention to treatment. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MBA. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- Xu YN, Zhu HY, Wilcox CK, van't Wout A, Andrus T, Llewellyn N, Stamatatos L, Mullins JI, Corey L, Zhu TF. Blood monocytes harbor HIV type 1 strains with diversified phenotypes including macrophage-specific CCR5 virus. J Infect Dis. 2008;197:309–318. doi: 10.1086/524847. [DOI] [PubMed] [Google Scholar]
- Yi YJ, Chen W, Frank I, Cutilli J, Singh A, Starr-Spires L, Sulcove J, Kolson DL, Collman RG. An unusual syncytia-inducing human immunodeficiency virus type 1 primary isolate from the central nervous system that is restricted to CXCR4, replicates efficiently in macrophages, and induces neuronal apoptosis. J Neurovirol. 2003;9:432–441. doi: 10.1080/13550280390218706. [DOI] [PubMed] [Google Scholar]
- Yukl SA, Shergill AK, Ho T, Killian M, Girling V, Epling L, Li PL, Wong LK, Crouch P, Deeks SG, Havlir DV, McQuaid K, Sinclair E, Wong JK. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: implications for viral persistence. J Infect Dis. 2013;208:1212–1220. doi: 10.1093/infdis/jit308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarchi R, Allavena P, Borsetti A, Stievano L, Tosello V, Marcato N, Esposito G, Roni V, Paganin C, Bianchi G, Titti F, Verani P, Gerosa G, Amadori A. Expression and functional activity of CXCR-4 and CCR-5 chemokine receptors in human thymocytes. Clin Exp Immunol. 2002;127:321–330. doi: 10.1046/j.1365-2249.2002.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ng T, Yuksel A, Wang B, Dwyer DE, Saksena NK. Absence of HIV infection in the choroid plexus of two patients who died rapidly with HIV-associated dementia. Aids Res Hum Retroviruses. 2008;24:839–843. doi: 10.1089/aid.2007.0147. [DOI] [PubMed] [Google Scholar]
- Zhu TF, Muthui D, Holte S, Nickle D, Feng F, Brodie S, Hwangbo Y, Mullins JI, Corey L. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol. 2002;76:707–716. doi: 10.1128/JVI.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]