Abstract
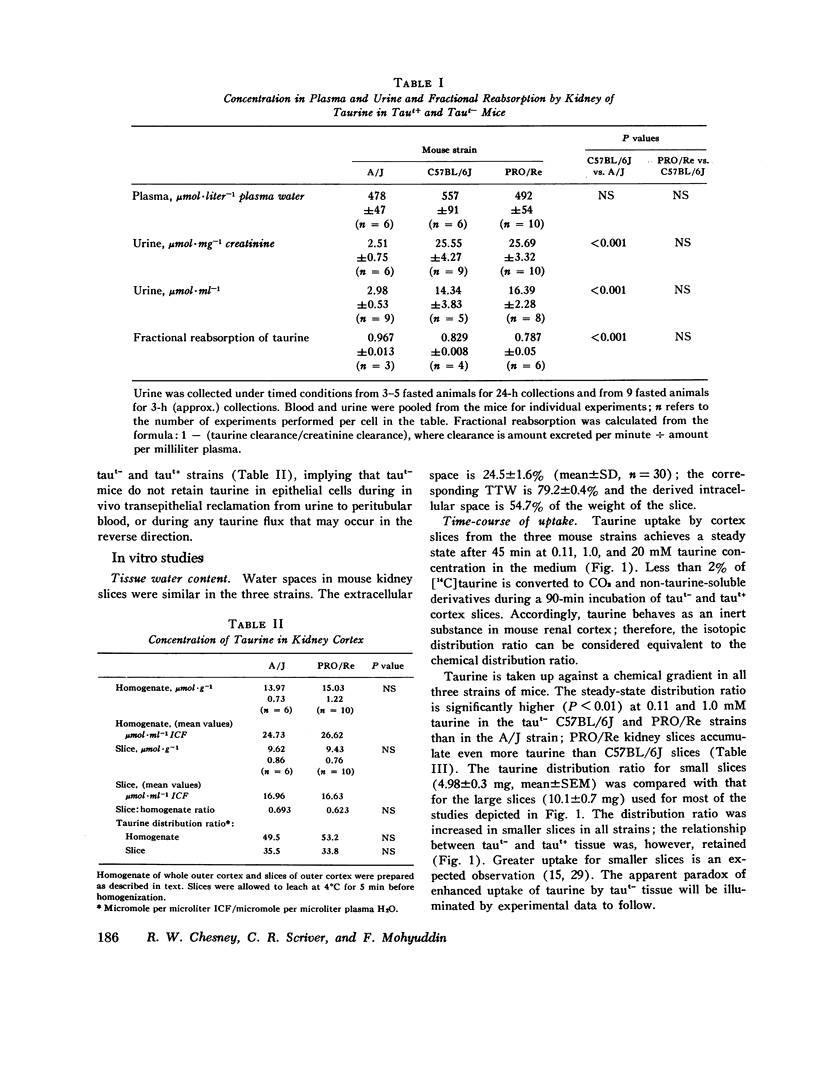
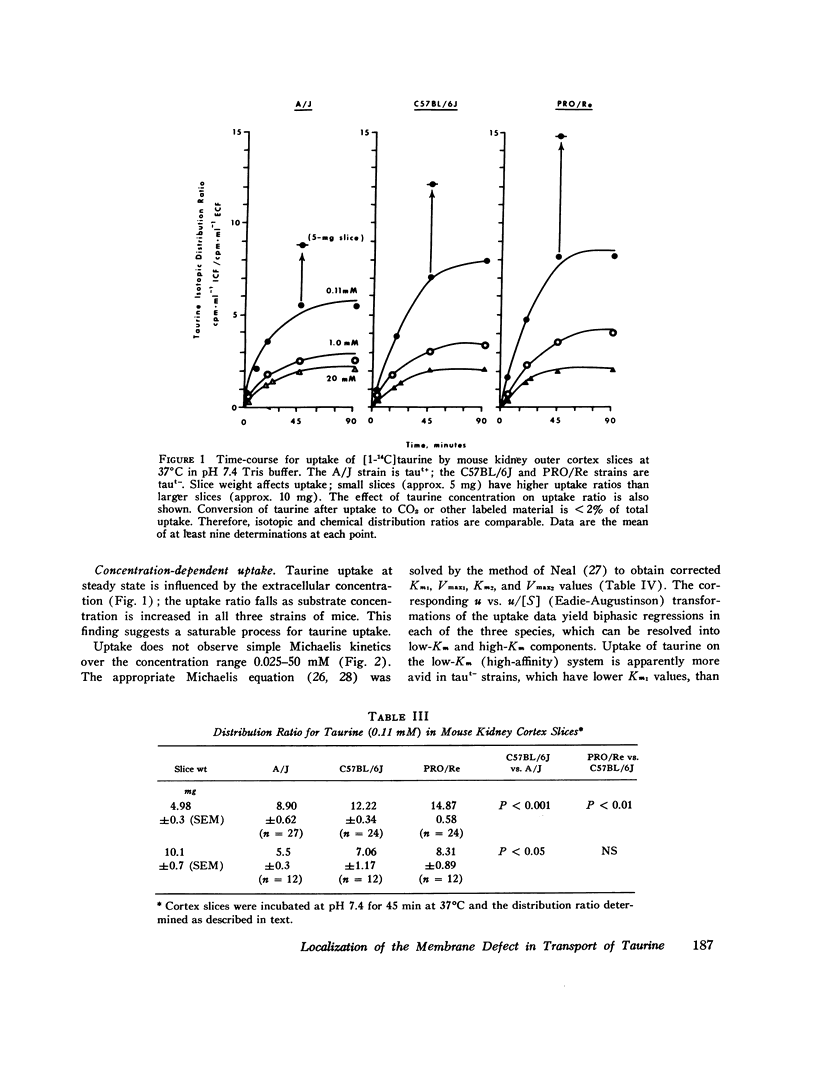
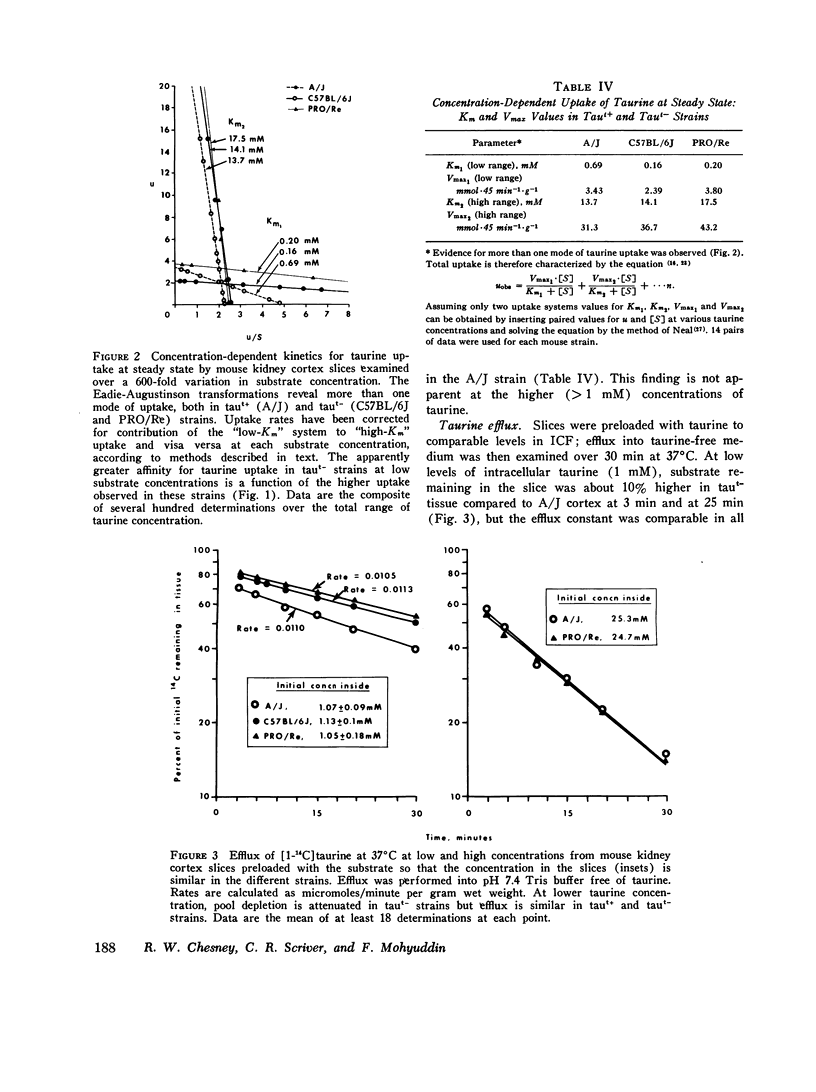
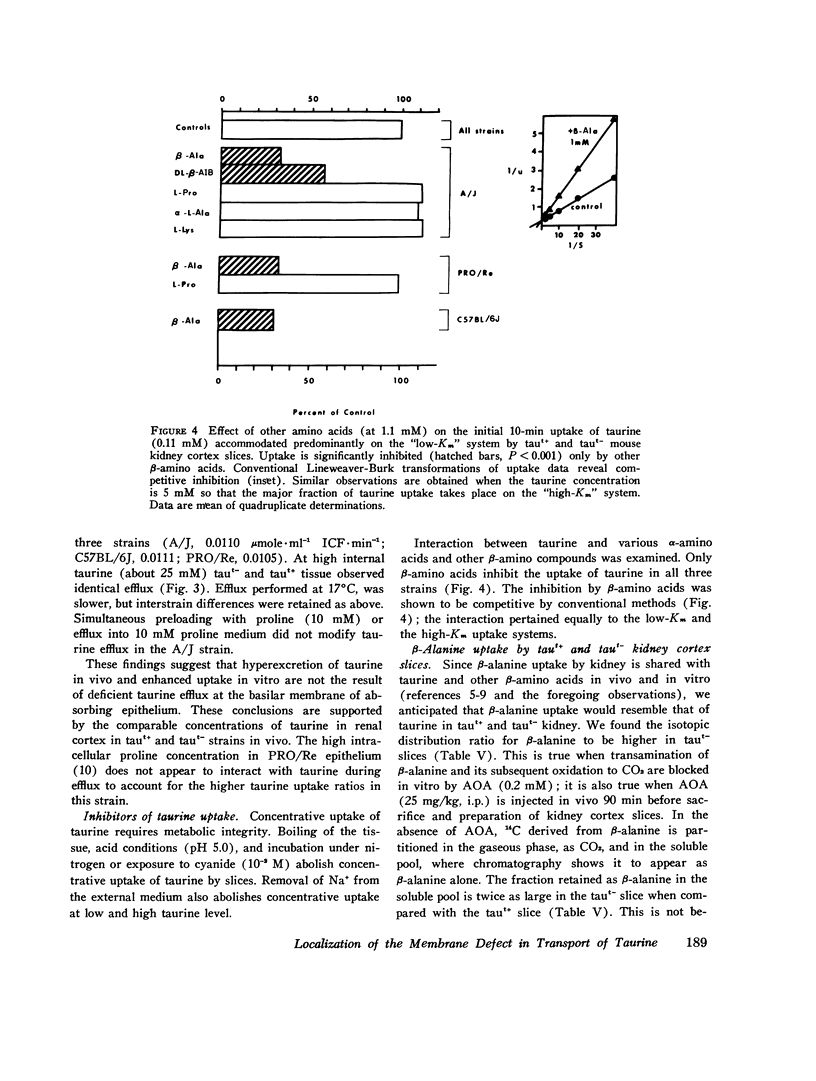
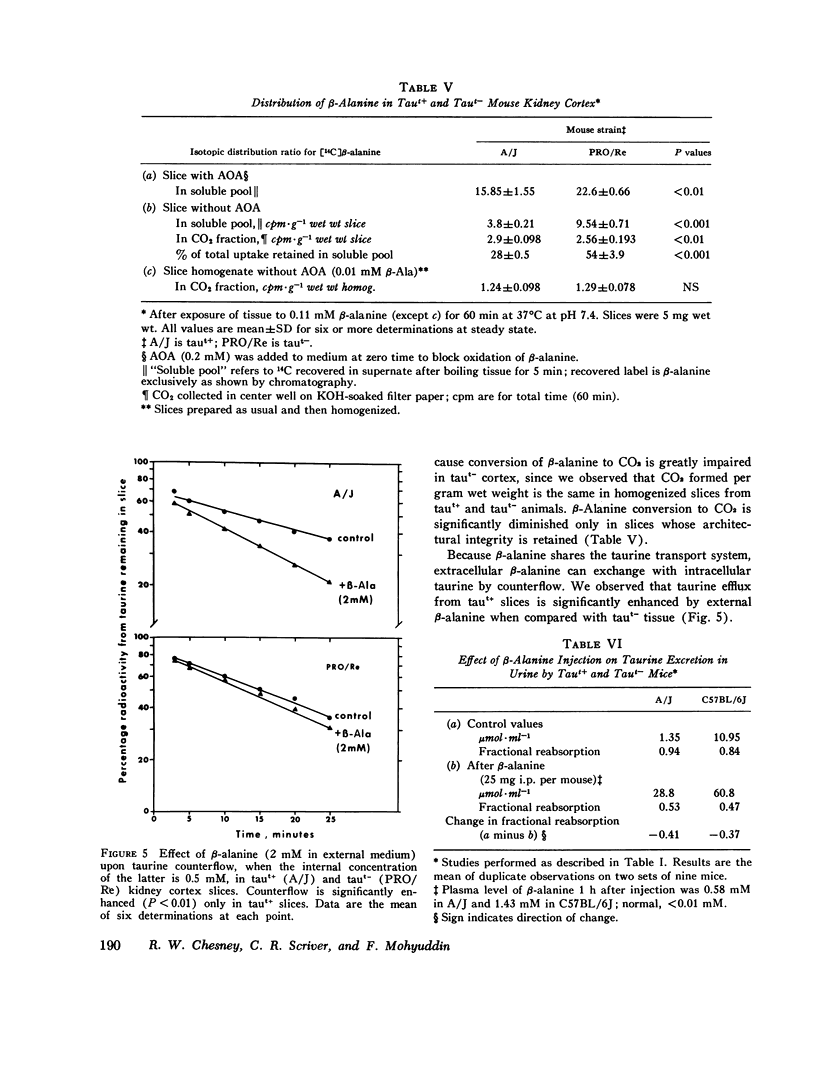
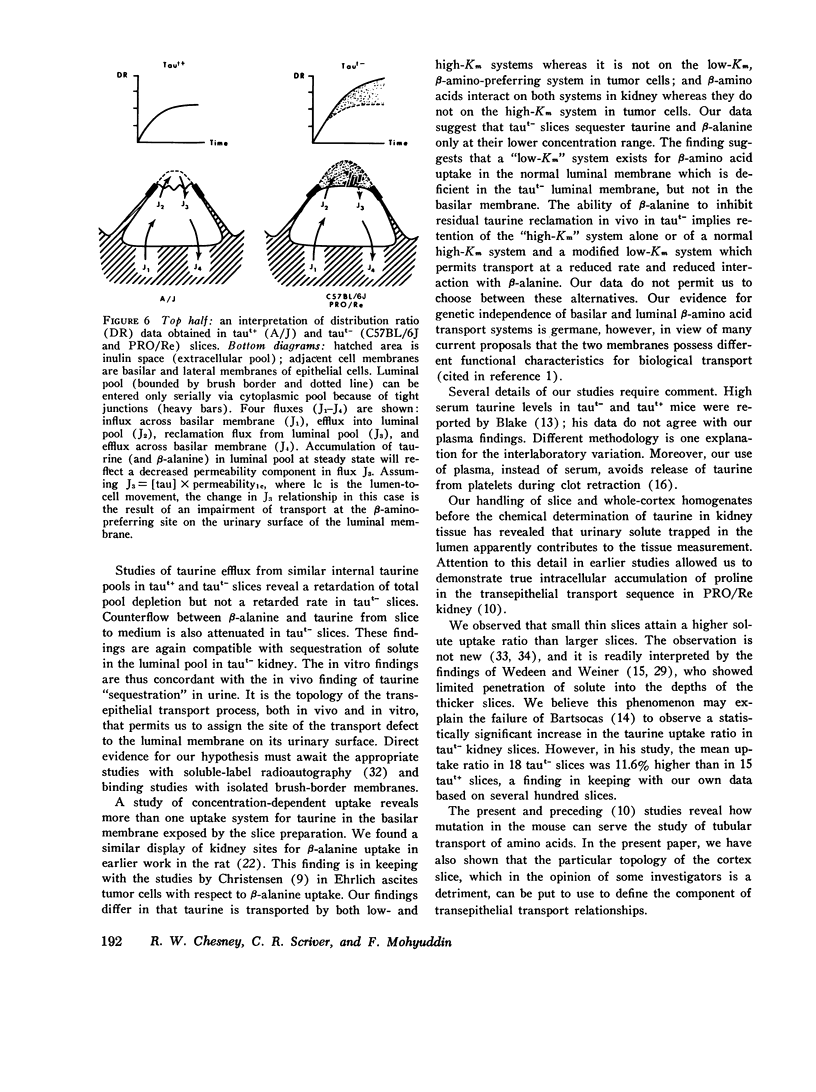
We investigated the mechanism of taurinuria in three inbred strains of mice: A/J, a normal taurine excretor (taut+); and two hypertaurinuric (taut-) strains, C57BL/6J and PRO/Re. Plasma taurine is comparable in the three strains (approximately 0.5 mM), but taurinuria is 10-fold greater in taut- animals. Fractional reabsorption of taurine is 0.967 +/- 0.013 (mean +/- SD) in A/J); and 0.839 +/- 0.08 and 0.787 +/- 0.05 in C57BL/6J and PRO/Re, respectively. Taurine concentration in renal cortex intracellular fluid (free of urine contamination) is similar in the three strains. Taurine reabsorption is inhibited by beta-alanine, in taut+ and taut- strains. These in vivo findings reveal residual taurine transport activity in the taut- phenotype and no evidence for impaired efflux at basilar membranes as the cause of impaired taurine reabsorption. Cortex slices provide information about uptake of amino acids at the antiluminal membrane. Taurine behaves as an inert metabolite in mouse kidney cortex slices. Taurine uptake by slices is active and, at less than 1 mM, is greater than normal in taut- slices. Concentration-dependent uptake studies reveal more than one taurine carrier in taut+ and taut- strains. The apparent Km values for uptake below 1 mM are different in taut- and taut+ slices (approximately 0.2 mM and approximately 0.7 mM, respectively); the apparent Km values above 1 mM taurine are similar in taut+ and taut- slices. Efflux from slices in all strains in the same (0.0105-0.0113 mumol-min-1-g-1 wet wt), but taut- tissue retains about 10% more radioactivity over the period of efflux. beta-Alanine is actively metabolized in mouse kidney. Its uptake in the presence of blocked transamination, is greater; its intracellular oxidation is attenuated; and its exchange with intracellular taurine is diminished in taut- slices. These findings indicate impaired beta-amino acid permeation on a low-Km uptake system at the luminal membrane in the taut- phenotype. beta-Amino acids are not reclaimed efficiently either from the innermost luminal pool in cortex slices or from the ultrafiltrate in the tubule lumen in vivo. The former leads to high uptake ratios in vitro, the latter to high clearance rates in vivo. In vitro and in vivo data are thus concordant. This is the first time that a hereditary defect in amino acid transport has been assigned to a specific membrane surface in mammalian kidney.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake R. L. Animal model for hyperprolinaemia: deficiency of mouse proline oxidase activity. Biochem J. 1972 Oct;129(4):987–989. doi: 10.1042/bj1290987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R. L., Grillo R. V., Russell E. S. Increased taurine excretion in hereditary hyperprolinemia of the mouse. Life Sci. 1974 Apr 1;14(7):1285–1290. doi: 10.1016/0024-3205(74)90437-8. [DOI] [PubMed] [Google Scholar]
- Blake R. L., Russell E. S. Hyperprolinemia and prolinuria in a new inbred strain of mice, PRO-Re. Science. 1972 May 19;176(4036):809–811. doi: 10.1126/science.176.4036.809. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN H. N. RELATIONS IN THE TRANSPORT OF BETA-ALANINE AND THE ALPHA-AMINO ACIDS IN THE EHRLICH CELL. J Biol Chem. 1964 Oct;239:3584–3589. [PubMed] [Google Scholar]
- Dent C. E. A study of the behaviour of some sixty amino-acids and other ninhydrin-reacting substances on phenol-;collidine' filter-paper chromatograms, with notes as to the occurrence of some of them in biological fluids. Biochem J. 1948;43(2):169–180. [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M. Tight and leaky junctions of epithelia: a perspective on kisses in the dark. Fed Proc. 1974 Nov;33(11):2220–2224. [PubMed] [Google Scholar]
- GILBERT J. B., KU Y., ROGERS L. L., WILLIAMS R. J. The increase in urinary taurine after intraperitoneal administration of amino acids to the mouse. J Biol Chem. 1960 Apr;235:1055–1060. [PubMed] [Google Scholar]
- HARRIS H., SEARLE A. G. Urinary amino-acids in mice of different genotypes. Ann Eugen. 1953 Feb;17(3):165–167. doi: 10.1111/j.1469-1809.1953.tb02544.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen J. G., Smith L. H. Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev. 1968 Apr;48(2):424–511. doi: 10.1152/physrev.1968.48.2.424. [DOI] [PubMed] [Google Scholar]
- Mohyuddin F., Scriver C. R. Amino acid transport in mammalian kidney: Multiple systems for imino acids and glycine in rat kidney. Am J Physiol. 1970 Jul;219(1):1–8. doi: 10.1152/ajplegacy.1970.219.1.1. [DOI] [PubMed] [Google Scholar]
- Neal J. L. Analysis of Michaelis kinetics for two independent, saturable membrane transport functions. J Theor Biol. 1972 Apr;35(1):113–118. doi: 10.1016/0022-5193(72)90196-8. [DOI] [PubMed] [Google Scholar]
- ROSENBERG L. E., BERMAN M., SEGAL S. Studies of the kinetics of amino acid transport, incorporation into portein and oxidation in kidney-cortex slices. Biochim Biophys Acta. 1963 Jun 4;71:664–675. doi: 10.1016/0006-3002(63)91140-5. [DOI] [PubMed] [Google Scholar]
- ROSENBERG L. E., BLAIR A., SEGAL S. Transport of amino acids by slices of rat-kidney cortex. Biochim Biophys Acta. 1961 Dec 23;54:479–488. doi: 10.1016/0006-3002(61)90088-9. [DOI] [PubMed] [Google Scholar]
- ROSENBERG L. E., DOWNING S. J., SEGAL S. Extracellular space estimation in rat kidney slices using C saccharides and phlorizin. Am J Physiol. 1962 Apr;202:800–804. doi: 10.1152/ajplegacy.1962.202.4.800. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., Hechtman P. Human genetics of membrane transport with emphasis on amino acids. Adv Hum Genet. 1970;1:211–274. doi: 10.1007/978-1-4684-0958-1_4. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., Lamm P., Clow C. L. Plasma amino acids: screening, quantitation, and interpretation. Am J Clin Nutr. 1971 Jul;24(7):876–890. doi: 10.1093/ajcn/24.7.876. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., McInnes R. R., Mohyuddin F. Role of epithelial architecture and intracellular metabolism in proline uptake and transtubular reclamation in PRO/re mouse kidney. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1431–1435. doi: 10.1073/pnas.72.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriver C. R., Mohyuddin F. Amino acid transport in kidney. Heterogeneity of alpha-aminoisobutyric uptake. J Biol Chem. 1968 Jun 25;243(12):3207–3213. [PubMed] [Google Scholar]
- Scriver C. R., Pueschel S., Davies E. Hyper-beta-alaninemia associated with beta-aminoaciduria and gamma-aminobutyricaciduaia, somnolence and seizures. N Engl J Med. 1966 Mar 24;274(12):635–643. doi: 10.1056/NEJM196603242741201. [DOI] [PubMed] [Google Scholar]
- Segal S., Rea C., Smith I. Separate transport systems for sugars and amino acids in developing rat kidney cortex. Proc Natl Acad Sci U S A. 1971 Feb;68(2):372–376. doi: 10.1073/pnas.68.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I., Segal S. The influence of size of rat kidney cortex slices on the accumulation of amino acids. Biochim Biophys Acta. 1968 Sep 17;163(2):281–283. doi: 10.1016/0005-2736(68)90111-9. [DOI] [PubMed] [Google Scholar]
- TENENHOUSE A., QUASTEL J. H. Amino acid accumulation in Ehrlich ascites carcinoma cells. Can J Biochem Physiol. 1960 Nov;38:1311–1326. [PubMed] [Google Scholar]
- Webber W. A. The influence of size and shape of kidney tissue from newborn and mature rats on the uptake of amino acid. Can J Physiol Pharmacol. 1970 Feb;48(2):152–154. doi: 10.1139/y70-025. [DOI] [PubMed] [Google Scholar]
- Wedeen R. P., Weiner B. The distribution of p-aminohippuric acid in rat kidney slices. I. Tubular localization. Kidney Int. 1973 Apr;3(4):205–213. doi: 10.1038/ki.1973.33. [DOI] [PubMed] [Google Scholar]
- Wedeen R. P., Weiner B. The distribution of p-aminohippuric acid in rat kidney slices. II. Depth of uptake. Kidney Int. 1973 Apr;3(4):214–221. doi: 10.1038/ki.1973.34. [DOI] [PubMed] [Google Scholar]
- Wilson O. H., Scriver C. R. Specificity of transport of neutral and basic amino acids in rat kidney. Am J Physiol. 1967 Jul;213(1):185–190. doi: 10.1152/ajplegacy.1967.213.1.185. [DOI] [PubMed] [Google Scholar]


