During 2002–2013, neither CD4 count at presentation to care nor CD4 count at treatment initiation increased significantly in sub-Saharan Africa. Barriers to human immunodeficiency virus diagnosis and linkage to care continue to challenge the feasibility of guidelines recommending early treatment initiation.
Keywords: HIV/AIDS, sub-Saharan Africa, antiretroviral therapy, linkage to care, meta-analysis
Abstract
Background. Both population- and individual-level benefits of antiretroviral therapy (ART) for human immunodeficiency virus (HIV) are contingent on early diagnosis and initiation of therapy. We estimated trends in disease status at presentation to care and at ART initiation in sub-Saharan Africa.
Methods. We searched PubMed for studies published January 2002–December 2013 that reported CD4 cell count at presentation or ART initiation among adults in sub-Saharan Africa. We abstracted study sample size, year(s), and mean CD4 count. A random-effects meta-regression model was used to obtain pooled estimates during each year of the observation period.
Results. We identified 56 articles reporting CD4 count at presentation (N = 295 455) and 71 articles reporting CD4 count at ART initiation (N = 549 702). The mean estimated CD4 count in 2002 was 251 cells/µL at presentation and 152 cells/µL at ART initiation. During 2002–2013, neither CD4 count at presentation (β = 5.8 cells/year; 95% confidence interval [CI], −10.7 to 22.4 cells/year), nor CD4 count at ART initiation (β = −1.1 cells/year; 95% CI, −8.4 to 6.2 cells/year) increased significantly. Excluding studies of opportunistic infections or prevention of mother-to-child transmission did not alter our findings. Among studies conducted in South Africa (N = 14), CD4 count at presentation increased by 39.9 cells/year (95% CI, 9.2–70.2 cells/year; P = .02), but CD4 count at ART initiation did not change.
Conclusions. CD4 counts at presentation to care and at ART initiation in sub-Saharan Africa have not increased over the past decade. Barriers to presentation, diagnosis, and linkage to HIV care remain major challenges that require attention to optimize population-level benefits of ART.
(See the Editorial Commentary by Ford, Mills, and Egger on pages 1128–30.)
The number of human immunodeficiency virus (HIV)-infected persons taking antiretroviral therapy (ART) in sub-Saharan Africa increased from tens of thousands in 2000 to >10 million by the end of 2012 [1]. HIV treatment has prolonged survival for infected persons and reduced secondary transmission to uninfected persons [2–6], resulting in large-scale improvements in population health: HIV incidence in some countries has begun to decline [1, 5], and life expectancy for those on ART is approaching that of HIV-uninfected persons [2, 7]. These developments have emboldened the public health community to call for an “AIDS-free generation” [8].
Achieving the goal of zero new HIV infections, however, will largely depend on the success of a much broader ART rollout [9]. As of 2012, only 60% of HIV-infected persons eligible for ART in sub-Saharan Africa had initiated therapy [1]. Successful implementation of new World Health Organization (WHO) guidelines—which recommend ART initiation for those with a CD4 count ≤500 cells/μL and for HIV-infected members of discordant couples regardless of CD4 status [10, 11]—will require a multifaceted approach to diagnose and initiate ART for individuals early in the course of infection. Yet a complex array of factors undermine uptake of HIV testing, presentation to HIV care, and desire to initiate ART, including poverty [12], geographic and transportation-related barriers [13, 14], and stigma associated with HIV infection [15], among others. Therefore, we conducted a meta-analysis to assess progress in HIV care delivery by estimating temporal trends in CD4 count at the time of presentation to care and at ART initiation.
METHODS
Data Sources and Searches
We followed the Meta-analysis of Observational Studies in Epidemiology guidelines for study procedures [16]. We systematically searched PubMed with the goal of identifying published journal articles including reports of CD4 count at the time of presentation to care and/or at ART initiation among HIV-infected adults in sub-Saharan Africa during January 2002–December 2013 (Supplementary Appendix Table 1). There were no language restrictions. We chose 2002 as the beginning year for our analysis because it was the first year of published WHO guidelines for ART. An initial search was conducted in October 2013 and updated in December 2013 (to identify articles published in the intervening 2 months and to preferentially include cohort studies focusing on the cascade of HIV care). All citations were imported into EndNote (version X6, Thomson Reuters, New York, New York).
Study Selection and Data Extraction
Studies had to meet each of the following inclusion criteria: (1) based on data obtained from HIV-infected adults living in a sub-Saharan African country; (2) reported a summary measure of CD4 count at presentation to care and/or at ART initiation; and (3) data were collected during 2002–2013. For studies of ART initiation, we did not include data on study participants who were eligible for ART but had not initiated treatment. We excluded studies that did not include HIV-infected adults, were not conducted at least partly in a sub-Saharan African country, did not report original CD4 count data, or featured a design with a prespecified CD4 count range for inclusion.
We first screened titles and abstracts to identify relevant articles for inclusion. Two authors (M. J. S., C. K. N.) screened the first 250 abstracts in duplicate, in groups of 50. After agreement between the 2 authors exceeded Cohen κ >0.90 for 2 successive groups of 50 abstracts, a single reviewer (C. K. N.) screened the remaining abstracts. We reviewed full-text articles for all abstracts that passed initial screening and included any that met the inclusion criteria described above. Bibliographies of systematic reviews and large cohort studies were screened to identify additional articles not identified by the systematic evidence search.
To avoid including duplicate data (ie, data from a single cohort published in multiple articles), we identified all instances in which there was temporal overlap in data published from the same study site. We included in our analysis the article with the largest average sample size per year. We made specific requests to authors of large cohorts to supply data disaggregated by year when they were not available in the published articles. Whenever possible, we disaggregated data by year and included, without temporal overlap, data from multiple studies at the same study site. We used Microsoft Excel (version 14.1.0 for Mac, Microsoft Corporation, Redmond, Washington) to record data of interest for each article.
Data Synthesis and Analysis
For each included study, we abstracted the mean or median CD4 count, and disaggregated these figures by year and country where possible. When both mean and median were presented, we used the median CD4 for our analysis. When studies presented mean or median CD4 count stratified by participant subcategories (eg, sex), we calculated a weighted summary measure. For studies that presented the number and proportion of participants in CD4 count strata, we calculated a weighted mean CD4 using the method described previously by Lesko et al [17]. For CD4 strata with a lower bound ≥200 cells/µL but no upper bound, we set an upper bound of 850 cells/µL. We excluded studies in which >50% of the sample was in a single category with a lower bound <200 cells/µL and no upper bound. (For example, a study by Abaasa et al [18] was excluded because 78% of study participants were reported to have a CD4 count ≥50 cells/µL with no upper bound reported.)
We assigned the median year of the study period as the imputed study year. If the last year of data collection was not reported in the article, we assigned the first year of data collection as the imputed year. When both the first and last years of data collection were not reported in the article, we assigned 2 years before the publication year as the imputed year. The robustness of these imputations was probed in the sensitivity analyses described below.
For our primary analyses, we fitted 2 random-effects meta-regression models for CD4 count at presentation to care and CD4 count at initiation of ART, modeling both variables as a linear function of the imputed year. To account for potentially nonlinear trends resulting from updated 2009 WHO guidelines, which recommended ART for all HIV-infected persons with a CD4 count <350 cells/µL [19], we created a linear spline of the imputed year with a knot at 2009. We also assessed for the presence of a curvilinear relationship in both models by including a quadratic year term. In subanalyses, we assessed for potential confounding by indication for ART by alternately excluding studies that were primarily focused on opportunistic infections and studies primarily focused on prevention of mother-to-child transmission (PMTCT). Last, we fitted country-specific meta-regression models for countries where ≥10 studies had been identified (South Africa and Uganda).
Because attributing multiple years of data to a single imputed year could potentially mask temporal trends within studies, we conducted 2 additional sensitivity analyses. First, we restricted estimation to studies reporting data that spanned ≤3 years, reasoning that short-term trends would be less likely to be masked if data spanning longer periods of time were excluded from analysis. Second, for studies that reported CD4 count data disaggregated by year, we refit the meta-regression models with the data disaggregated by year (ie, multiyear studies were represented as multiple rows in the dataset). To account for within-study clustering, we used the bootstrap method to calculate 95% confidence intervals (CIs); we generated 2000 random samples with replacement of the regression estimates, clustered by the study from which they were drawn. We performed all analyses with Stata software version 13.0 (StataCorp LP, College Station, Texas).
RESULTS
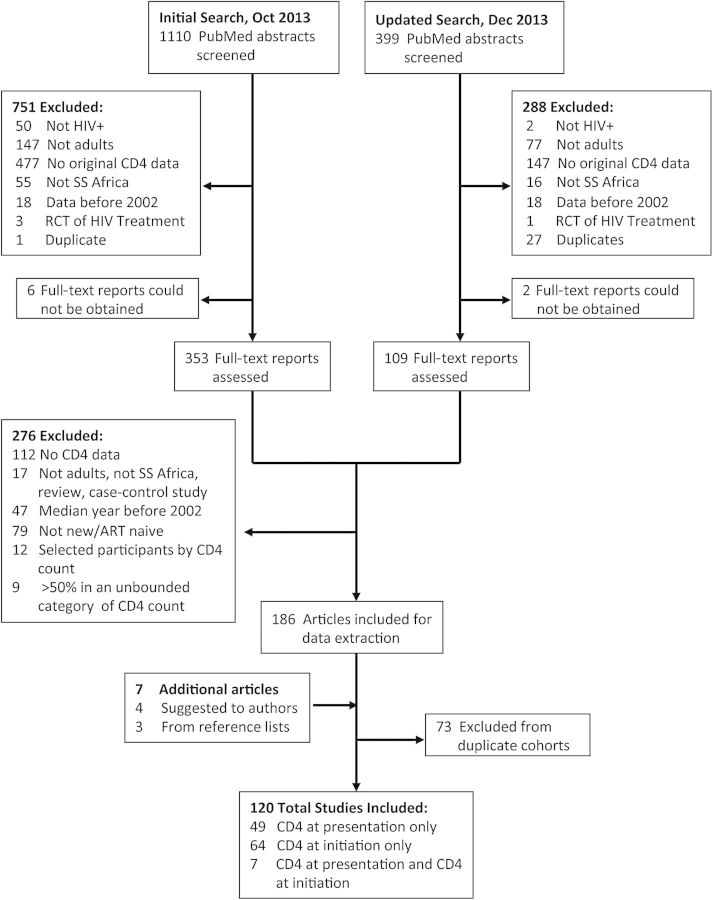
The evidence search returned a total of 1509 abstracts, of which 1039 were excluded. We reviewed the full text of 470 journal articles (Figure 1). Of these, 8 manuscripts could not be obtained and another 276 were excluded on the basis of study design. Seven manuscripts were added to the sample, as they were known by the authors or identified in reference lists of relevant review articles. In total, after excluding 73 articles with overlapping data (Supplementary Appendix Table 2), our analytic sample included data from 120 journal articles (Supplementary Appendix Table 3).
Figure 1.
Selection process for study inclusion in the meta-analysis. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; RCT, randomized controlled trial; SS, sub-Saharan.
Fifty-six studies reported CD4 count at presentation (N = 295 455) and 71 studies reported CD4 count at ART initiation (N = 549 702), with 6 studies reporting data on both (Table 1). The majority of articles (82 [68%]) were from the period 2006–2009, with only 17 (14%) reporting data from 2010 or later. Relatively few studies focused on PMTCT (6%) or opportunistic infections (4%). Among studies reporting CD4 count at presentation, 9 (15%) studies employed active recruitment methods such as community-based HIV testing. The included studies spanned 27 countries in sub-Saharan Africa. South Africa (N = 21 [18%]) and Uganda (N = 16 [13%]) yielded the most estimates. Approximately half of the articles (63 [52%]) included data spanning ≤3 years, whereas 43 (36%) articles spanned 3–5 years, and 12 (10%) articles spanned ≥6 years.
Table 1.
Summary of Articles and Population Included in Meta-analysis to Estimate Trends in CD4 Count in Sub-Saharan Africa During 2002–2012
| Characteristic | Presentation |
Initiation |
||||
|---|---|---|---|---|---|---|
| Articles, No. (%) | Sample Size, No. (%) | CD4 Count, Cells/µL, Mean | Article Count, No. (%) | Sample Size, No. (%) | CD4 Count, Cells/µL, Mean | |
| Total sample | 56 (100) | 5276 (100) | 286 | 71 (100) | 7742 (100) | 147 |
| Country of origin | ||||||
| Ethiopia | 4 (7.1) | 1719 (0.6) | 208 | 7 (9.9) | 12 041 (2.2) | 120 |
| Kenya | 5 (8.9) | 15 706 (5.3) | 300 | 4 (5.6) | 906 (0.2) | 217 |
| Nigeria | 6 (10.7) | 5405 (1.8) | 275 | 5 (7.0) | 7070 (1.3) | 154 |
| South Africa | 13 (23.2) | 12 509 (4.2) | 257 | 10 (14.1) | 96 626 (17.6) | 123 |
| Tanzania | 1 (1.8) | 2408 (0.8) | 136 | 4 (5.6) | 82 027 (14.9) | 127 |
| Uganda | 5 (8.9) | 2276 (0.8) | 370 | 11 (15.5) | 40 182 (7.3) | 119 |
| Other/multiplea | 22 (39.3) | 255 432 (86.5) | 257 | 30 (42.3) | 310 850 (56.5) | 164 |
| Imputed year | ||||||
| 2002–2003 | 1 (1.8) | 81 (0.0) | 452 | 5 (7.0) | 2329 (2.1) | 156 |
| 2004 | 4 (7.1) | 378 (0.5) | 296 | 6 (8.5) | 1479 (1.6) | 145 |
| 2005 | 2 (3.6) | 473 (0.3) | 278 | 4 (5.6) | 11 356 (8.3) | 186 |
| 2006 | 8 (14.3) | 1621 (4.4) | 196 | 18 (25.4) | 11 275 (36.9) | 147 |
| 2007 | 10 (17.9) | 4632 (15.7) | 281 | 19 (26.8) | 6477 (22.4) | 139 |
| 2008 | 10 (17.9) | 3181 (10.8) | 322 | 6 (8.5) | 5278 (5.8) | 127 |
| 2009 | 9 (16.1) | 20 792 (63.3) | 247 | 8 (11.3) | 15 420 (22.4) | 151 |
| 2010 | 5 (8.9) | 2489 (4.2) | 319 | 4 (5.6) | 617 (0.4) | 144 |
| 2011–present | 7 (12.5) | 323 (0.8) | 344 | 1 (1.4) | 236 (0.0) | 186 |
| Years spanned by data collection | ||||||
| 0–3 | 36 (64.3) | 426 (5.2) | 295 | 30 (42.3) | 1258 (6.9) | 139 |
| 3–5 | 16 (28.6) | 14 576 (78.9) | 281 | 30 (42.3) | 13 054 (71.2) | 144 |
| ≥6 | 3 (5.4) | 15 414 (15.7) | 198 | 10 (14.1) | 12 024 (21.9) | 176 |
| Unknown | 1 (1.8) | 669 (0.2) | 311 | 1 (1.4) | 79 (0.0) | 180 |
| PMTCT-focus studies | ||||||
| Not PMTCT focus | 52 (92.9) | 5655 (99.5) | 278 | 68 (95.8) | 7919 (98.0) | 139 |
| PMTCT focus | 4 (7.1) | 351 (0.5) | 395 | 3 (4.2) | 3736 (2.0) | 313 |
| Opportunistic infections studies | ||||||
| Not OI focus | 52 (92.9) | 5647 (99.4) | 295 | 69 (97.2) | 7801 (97.9) | 148 |
| OI focus | 4 (7.1) | 450 (0.6) | 168 | 2 (2.8) | 5730 (2.1) | 112 |
| HIV screening | ||||||
| Passive | 47 (83.9) | 6108 (97.2) | 269 | 68 (95.8) | 8045 (99.5) | 143 |
| Active | 7 (12.5) | 1122 (2.7) | 405 | 2 (2.8) | 1198 (0.4) | 268 |
| Both | 2 (3.6) | 263 (0.2) | 263 | 1 (1.4) | 242 (0.0) | 170 |
Abbreviations: HIV, human immunodeficiency virus; OI, opportunistic infection; PMTCT, prevention of mother-to-child transmission.
a Other countries (number of studies from countries not listed above or from studies without data disaggregated by country): Benin (1), Botswana (3), Burkina Faso (1), Burundi (2), Cameroon (5), Congo (2), Cote d'Ivoire (5), Democratic Republic of the Congo (1), Gambia (2), Guinea (1), Guinea-Bissau (1), Kenya (2), Malawi (7), Mozambique (6), Namibia (1), Rwanda (5), Senegal (1), South Africa (1), Swaziland (1), Tanzania (1), Zambia (4).
The estimated mean CD4 count at presentation to care was 250 cells/µL (95% CI, 147–354 cells/µL) in 2002 and 309 cells/µL (95% CI, 237–381 cells/µL) in 2012. The mean CD4 count at ART initiation was 152 cells/µL (95% CI, 115–189 cells/µL) in 2002 and 140 cells/µL (95% CI, 101–181 cells/µL) in 2012. In the primary meta-regression models with a single covariate representing time in years, we found no significant trend in CD4 count at presentation to care (β = 5.8 cells per year; 95% CI, −10.7 to 22.4 cells/year; P = .48; Table 2, Figure 2A) or at ART initiation (β = −1.1 cells per year; 95% CI, −8.4 to 6.2 cells/year; P = .76; Table 2, Figure 2B). When we modeled time with a linear spline term, we did not find evidence that there was a change in the trend before vs after the 2009 WHO guidelines (for CD4 at presentation, P = .11; for CD4 at initiation, P = .65). We did not find evidence of a curvilinear trend in CD4 count at presentation (P = .07 for quadratic term) or CD4 count at ART initiation (P = .85 for quadratic term).
Table 2.
Random-Effects Meta-regression Models Estimating Trends in CD4 Count at Time of Presentation to HIV Care and Antiretroviral Therapy Initiation—Sub-Saharan Africa, 2002–2013
| Model | Presentation to Care |
Initiation of Antiretroviral Therapy |
||||||
|---|---|---|---|---|---|---|---|---|
| Studies | β-Coefficient | 95% CI | P Value | Studies | β-Coefficient | 95% CI | P Value | |
| Primary analyses | 56 | 5.84 | −10.7 to 22.4 | .48 | 71 | −1.13 | −8.4 to 6.2 | .76 |
| Subanalyses | ||||||||
| Opportunistic infections studies excluded | 52 | 5.39 | −11.3 to 22.1 | .52 | 69 | −0.55 | −8.0 to 6.9 | .88 |
| PMTCT studies excluded | 52 | 3.81 | −14.1 to 21.7 | .67 | 68 | 0.45 | −4.8 to 5.7 | .87 |
| South Africa only | 13 | 39.9 | 9.2 to 70.7 | .016 | 10 | 2.36 | −22.5 to 27.2 | .83 |
| Uganda only | … | … | … | … | 11 | 4.20 | −4.0 to 12.4 | .28 |
| Sensitivity analyses | ||||||||
| Excluded studies >3 years | 43 | 1.38 | −18.3 to 21.1 | .89 | 41 | 5.50 | −1.6 to 12.6 | .13 |
| Disaggregated by yeara | 56 | 6.52 | −7.3 to 20.4 | .35 | 71 | 3.91 | −0.7 to 8.5 | .10 |
Abbreviations: CI, confidence interval; PMTCT, prevention of mother-to-child transmission.
a Model disaggregated by year, estimated using bootstrapping to account for clustering of data within studies.
Figure 2.
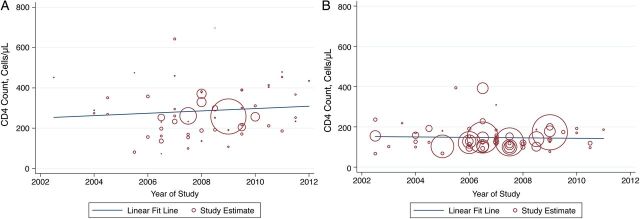
Temporal trends in CD4 count at presentation to care (A) and initiation of antiretroviral therapy (B) in sub-Saharan Africa during 2002–2013.
Sensitivity analyses did not result in substantive changes to our findings. The estimated trends were similar after excluding studies with a focus on opportunistic infections or PMTCT (Table 2). We found similar results in an analysis restricted to studies reporting ≤3 years of data, suggesting that aggregation did not bias us toward null findings. When we disaggregated data by year for the 14 articles that reported disaggregated data, the estimates remained similar. Restricting estimation to the subsample of South African studies, we found a statistically significant trend in CD4 count at presentation (β = 39.9; 95% CI, 9.2–70.7; P = .02; Table 2), but no evidence of a trend in CD4 count at ART initiation (β = 2.4; 95% CI, −22.5 to 27.2; P = .83; Table 2). Restricting estimation to the 11 Ugandan studies reporting CD4 count at ART initiation, we did not find evidence of an upward trend (β = 4.2; 95% CI, −4.0 to 12.4; P = .28).
DISCUSSION
Our findings suggest that neither CD4 count at presentation to HIV care nor CD4 count at the time of ART initiation have appreciably changed in sub-Saharan Africa during the past decade. Our sample of >500 000 HIV-infected persons in 24 countries included in the ART initiation analyses accounts for approximately 1 of every 20 people who initiated ART in the region during the observation period [1]. The CIs exclude the possibility of large upward trends in CD4 count, and our estimates were robust to numerous sensitivity analyses. These results reinforce 2 critical challenges to achieving the goal of an AIDS-free generation: (1) Despite remarkable increases in ART availability in the region, there remains a substantial gap between CD4 count at presentation to care and CD4 count at ART initiation; and (2) even the most well-designed and well-supported ART programs will have limited capacity to maximize the health benefits of ART and prevent new infections if people continue to present to care during late stages of disease.
Prior estimates of trends in HIV care delivery in the region have shown largely similar findings. Whereas multisite cohorts in sub-Saharan Africa have shown modest increases in CD4 count at presentation (10 cells/year) [20, 21], meaningful trends were most evident in women and younger persons [22]. Nonetheless, the beneficial effects of ART in terms of improvements in HIV-related health and life expectancy in the region have been well documented [2, 3, 7]. Taken together, these studies suggest that, while many countries have made progress in combating the HIV epidemic, there is considerable more progress to be made. Our collective ability to reduce HIV incidence in sub-Saharan Africa—from nearly 1 000 000 new infections annually to zero—will largely depend on identification and treatment of the remaining 40% of HIV-infected persons who are eligible for but are not receiving ART.
Recent WHO recommendations to initiate ART for all persons with CD4 counts ≤500 cells/µL [11] have spurred debate about the benefits of adopting this threshold as well as debate about the extent to which existing health systems can accommodate the expansion of treatment availability [23–25]. Our estimates indicate that, if present trends continue, it will be some time before such debates actually relate to real-world policy or programmatic concerns, because most HIV-infected persons present to care or initiate ART at CD4 counts well below even the current policy threshold. We therefore argue that greater resources should be focused on both earlier diagnosis of HIV and expedited linkage to ART initiation [9].
Promotion of early diagnosis and referral to HIV care will arguably have the greatest impacts on HIV-related health status and secondary transmission. As of 2010, <40% of HIV-infected persons in sub-Saharan Africa were aware of their status [26]. While much attention has been paid to the treatment cascade, relatively little has been paid to the earliest stage, HIV diagnosis [27], possibly because of the physical separation between many HIV diagnostic and treatment facilities in the region [28]. HIV testing and referral may be undermined by a wide array of social forces such as generalized poverty, the immense stigma attached to HIV, and disinterest in enrolling in HIV care or initiating ART while feeling healthy [12, 14, 29, 30]. Multifaceted, scalable interventions are urgently needed to address these barriers [31–33].
Improving the timeliness of ART initiation will also require significant additional investment. Notwithstanding the laudable implementation of ART services for >10 million HIV-infected persons during the past decade, the estimated 169-cell difference in CD4 cells/µL at presentation vs ART initiation in 2012 suggests that a sizeable gap between ART eligibility and initiation has persisted. This phenomenon was particularly evident in South Africa, in which we showed a widening gap between CD4 count at presentation and CD4 count at ART initiation. Moreover, we did not observe any evidence for increasing CD4 counts at ART initiation after 2009, when new WHO guidelines recommended increasing eligibility to 350 cells/µL, although it might be premature to detect such a trend because national guidelines often lag beyond international recommendations. Health systems–related causes of delays between diagnosis and ART initiation are likely multifactorial, and may include delays in laboratory testing and results reporting, requirements for multiple pretherapy visits for CD4 count testing and adherence counseling, and drug stock-outs [13, 34, 35]. While effective test-and-treat strategies might partially mitigate these delays, other considerations that have shown promise include decentralization of care, point-of-care CD4 count testing, and removal of pre-ART “readiness assessments” [35–37]. Ongoing international commitments by the US President's Emergency Plan for AIDS Relief (PEPFAR), national ministries of health, and other nongovernmental partners will certainly be a sine qua non to enabling ART access for the remaining approximately 7 million HIV-infected persons who are eligible for but not receiving ART [3, 38]. Unfortunately, annual PEPFAR funding has remained approximately unchanged since 2009 [39]. These data reinforce the value of support provided by PEPFAR and other donors, but also that additional resources and strategies must be explored, to promote earlier diagnosis of HIV and initiation of ART on the scale required to stem HIV transmission at the population level [40].
Our findings should be interpreted with 4 limitations in mind. First, the HIV-infected persons in our sample may differ from HIV-infected persons in the general population. For example, published articles may be more likely to be based on data from centers partnering with academic or research institutions, which often provide additional financial resources and/or health infrastructure for study sites. Such an association could bias our CD4 count estimates upward due to the impact of these resources on systems-level effects (but would not bias our estimates of trends in CD4 counts unless these resources had differential systems-level impacts over time). The fact that our ART initiation sample represents >500 000 persons in 26 countries—approximately 1 in 20 of all HIV-infected persons who initiated ART during the observation period [1]—should further mitigate concerns about the extent to which our data reflect actual population-level trends. A second potential limitation is that we may have missed unpublished studies or those exclusively indexed in databases other than PubMed. However, for publication bias to undermine our primary findings, there would need to be a disproportionate number of unpublished studies either (1) conducted early in the decade and showing extremely low CD4 counts, (2) conducted late in the decade and showing extremely high CD4 counts, or (3) conducted at any time and showing an upward trend. Although any of these scenarios are plausible, we find them to be unlikely, given that publication bias would be expected to mask unpublished studies showing lack of progress. Third, it should be noted that a minority of our estimates were derived from data collected after 2010. As more data accumulate and are disseminated, more recent trends are likely to be revealed with greater clarity. Last, relatively few studies (7 of 120) included estimates of both CD4 count at presentation and ART initiation, limiting our ability to make definitive conclusions about gaps in linkage to ART care.
In summary, despite progress in making ART available to millions of HIV-infected persons in sub-Saharan Africa, CD4 counts at presentation to care and at ART initiation have not appreciably changed in the past decade. To reduce HIV-related morbidity and mortality at the population level, and to decrease secondary transmission, intensified efforts to increase demand for ART through active testing and facilitated referral should be a priority. Meanwhile, continued financial investments by multinational partners and the implementation of creative interventions to mitigate delays between presentation to care and ART initiation are needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank Dr Elvin Geng for generously providing data for use in this analysis.
Author contributions. M. J. S. developed the study concept design, contributed to data acquisition and analysis, wrote the first draft of the manuscript, and performed critical revision of the manuscript. C. K. N. contributed to the study concept design, contributed to data acquisition, and performed critical revision of the manuscript. I. T. K., I. V. B., and D. R. B. contributed to the study concept design and performed critical revision of the manuscript. A. C. T. developed the study concept design, led the data analysis, and performed critical revision of the manuscript. All authors have read and approved of the submitted draft of the manuscript and agree to be held accountable for all aspects of the work.
Financial support. The authors received no specific funding for this study. The authors acknowledge salary support from the US National Institutes of Health (grant numbers K23MH099916, K23MH096620, K23MH097667, and R01MH090326).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.United Nations Joint Programme on HIV/AIDS. UNAIDS report on the global AIDS epidemic. 2013. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. Accessed 17 March 2014.
- 2.Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339:961–5. doi: 10.1126/science.1230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendavid E, Holmes CB, Bhattacharya J, Miller G. HIV development assistance and adult mortality in Africa. JAMA. 2012;307:2060–7. doi: 10.1001/jama.2012.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–71. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siedner MJ, Musinguzi N, Tsai AC, et al. Treatment as long-term prevention: sustained reduction in HIV sexual transmission risk with use of antiretroviral therapy in rural Uganda. AIDS. 2014;28:267–71. doi: 10.1097/QAD.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills EJ, Bakanda C, Birungi J, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med. 2011;155:209–16. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- 8.Fauci AS, Folkers GK. Toward an AIDS-free generation. JAMA. 2012;308:343–4. doi: 10.1001/jama.2012.8142. [DOI] [PubMed] [Google Scholar]
- 9.McNairy ML, El-Sadr WM. Antiretroviral therapy for the prevention of HIV transmission: what will it take? Clin Infect Dis. 2014;58:1003–11. doi: 10.1093/cid/ciu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odhiambo JO, Kellogg TA, Kim AA, et al. Antiretroviral treatment scale-up among persons living with HIV in Kenya: results from a nationally representative survey. J Acquir Immune Defic Syndr. 2014;66(suppl 1):S116–22. doi: 10.1097/QAI.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Geneva, Switzerland:: WHO; 2013. Consolidated guidelines on the use of antiretroviral drugs for treatment and preventing HIV infection. Recommendations for a public health approach. [Google Scholar]
- 12.Castro A, Farmer P. Understanding and addressing AIDS-related stigma: from anthropological theory to clinical practice in Haiti. Am J Public Health. 2005;95:53–9. doi: 10.2105/AJPH.2003.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26:2059–67. doi: 10.1097/QAD.0b013e3283578b9b. [DOI] [PubMed] [Google Scholar]
- 14.Lankowski AJ, Siedner MJ, Bangsberg DR, Tsai AC. Impact of geographic and transportation-related barriers on HIV outcomes in sub-Saharan Africa: a systematic review. AIDS Behav. 2014;18:1199–223. doi: 10.1007/s10461-014-0729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16(3 suppl 2):18640. doi: 10.7448/IAS.16.3.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Lesko CR, Cole SR, Zinski A, Poole C, Mugavero MJ. A systematic review and meta-regression of temporal trends in adult CD4(+) cell count at presentation to HIV care, 1992–2011. Clin Infect Dis. 2013;57:1027–37. doi: 10.1093/cid/cit421. [DOI] [PubMed] [Google Scholar]
- 18.Abaasa AM, Todd J, Ekoru K, et al. Good adherence to HAART and improved survival in a community HIV/AIDS treatment and care programme: the experience of The AIDS Support Organization (TASO), Kampala, Uganda. BMC Health Serv Res. 2008;8:241. doi: 10.1186/1472-6963-8-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Geneva, Switzerland: WHO; 2009. Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. Available at: http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf . Accessed 21 April 2014. [Google Scholar]
- 20.Lahuerta M, Wu Y, Hoffman S, et al. Advanced HIV disease at entry into HIV care and initiation of antiretroviral therapy during 2006–2011: findings from four sub-Saharan African countries. Clin Infect Dis. 2014;58:432–41. doi: 10.1093/cid/cit724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avila D, Althoff KN, Mugglin C, et al. IeDEA and ART Cohort Collaborations; Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr. 2014;65:e8–16. doi: 10.1097/QAI.0b013e3182a39979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman S, Wu Y, Laheurta M, et al. Advanced disease at enrollment in HIV care in four sub-Saharan African countries: change from 2006 to 2011 and multilevel predictors in 2011. AIDS. 2014;28:2429–38. doi: 10.1097/QAD.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maman D, Pujades-Rodriguez M, Nicholas S, et al. Response to antiretroviral therapy: improved survival associated with CD4 above 500 cells/µl. AIDS. 2012;26:1393–8. doi: 10.1097/QAD.0b013e328352d054. [DOI] [PubMed] [Google Scholar]
- 24.De Cock KM, El-Sadr WM. When to start ART in Africa—an urgent research priority. N Engl J Med. 2013;368:886–9. doi: 10.1056/NEJMp1300458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallant JE, Mehta SH, Sugarman J. Universal antiretroviral therapy for HIV infection: should US treatment guidelines be applied to resource-limited settings? Clin Infect Dis. 2013;57:884–7. doi: 10.1093/cid/cit382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization, United Nations Children's Fund, United Nations Joint Programme on HIV/AIDS. Geneva, Switzerland: WHO,; 2010. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report 2010. [Google Scholar]
- 27.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNairy ML, El-Sadr WM. The HIV care continuum: no partial credit given. AIDS. 2012;26:1735–8. doi: 10.1097/QAD.0b013e328355d67b. [DOI] [PubMed] [Google Scholar]
- 29.Katz IT, Essien T, Marinda ET, et al. Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS. 2011;25:2177–81. doi: 10.1097/QAD.0b013e32834b6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musheke M, Ntalasha H, Gari S, et al. A systematic review of qualitative findings on factors enabling and deterring uptake of HIV testing in sub-Saharan Africa. BMC Public Health. 2013;13:220. doi: 10.1186/1471-2458-13-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweat M, Morin S, Celentano D, et al. Community-based intervention to increase HIV testing and case detection in people aged 16–32 years in Tanzania, Zimbabwe, and Thailand (NIMH Project Accept, HPTN 043): a randomised study. Lancet Infect Dis. 2011;11:525–32. doi: 10.1016/S1473-3099(11)70060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. US Department of State. http://www.state.gov/r/pa/prs/ps/2011/09/172389.htm . Accessed 20 April 2014.
- 33. National Institutes of Health. http://www.nih.gov/news/health/sep2013/niaid-30.htm. Accessed 20 April 2014.
- 34.Pasquet A, Messou E, Gabillard D, et al. Impact of drug stock-outs on death and retention to care among HIV-infected patients on combination antiretroviral therapy in Abidjan, Cote d'Ivoire. PLoS One. 2010;5:e13414. doi: 10.1371/journal.pone.0013414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siedner MJ, Lankowski A, Haberer JE, et al. Rethinking the “pre” in pre-therapy counseling: no benefit of additional visits prior to therapy on adherence or viremia in Ugandans initiating ARVs. PLoS One. 2012;7:e39894. doi: 10.1371/journal.pone.0039894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bedelu M, Ford N, Hilderbrand K, Reuter H. Implementing antiretroviral therapy in rural communities: the Lusikisiki model of decentralized HIV/AIDS care. J Infect Dis. 2007;196(suppl 3):S464–8. doi: 10.1086/521114. [DOI] [PubMed] [Google Scholar]
- 37.Jani IV, Sitoe NE, Alfai ER, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378:1572–9. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 38.Katz IT, Bassett IV, Wright AA. PEPFAR in transition—implications for HIV care in South Africa. N Engl J Med. 2013;369:1385–7. doi: 10.1056/NEJMp1310982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.US President's Emergency Plan for AIDS Relief. Shared responsibility—strengthening results from an AIDS-free generation: latest PEPFAR funding. 2014. Available at: http://www.pepfar.gov/documents/organization/189671.pdf. Accessed 3 May 2014.
- 40.Hontelez JA, Lurie MN, Barnighausen T, et al. Elimination of HIV in South Africa through expanded access to antiretroviral therapy: a model comparison study. PLoS Med. 2013;10:e1001534. doi: 10.1371/journal.pmed.1001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.