Abstract
Background. The human B-cell response to natural influenza virus infection has not been extensively investigated at the polyclonal level.
Methods. The overall B-cell response of patients acutely infected with the 2009 pandemic influenza A(H1N1)pdm09 virus (A[H1N1]pdm09) was analyzed by determining the reactivity of plasmablast-derived polyclonal antibodies (PPAbs) to influenza proteins. Recipients of inactivated influenza vaccine containing the same A(H1N1)pdm09 strain were studied for comparison.
Results. During acute infection, robust plasmablast responses to the infecting virus were detected, characterized by a greater PPAb reactivity to the conserved influenza virus nuclear protein and to heterovariant and heterosubtypic hemagglutinins, in comparison to responses to the inactivated A(H1N1)pdm09 vaccine. In A(H1N1)pdm09 vaccinees, the presence of baseline serum neutralizing antibodies against A(H1N1)pdm09, suggesting previous exposure to natural A(H1N1)pdm09 infection, did not affect the plasmablast response to vaccination, whereas repeated immunization with inactivated A(H1N1)pdm09 vaccine resulted in significantly reduced vaccine-specific and cross-reactive PPAb responses.
Conclusions. Natural A(H1N1)pdm09 infection and inactivated A(H1N1)pdm09 vaccination result in very distinct patterns of B-cell activation and priming. These differences are likely to be associated with differences in protective immunity, especially cross-protection against heterovariant and heterosubtypic influenza virus strains.
Keywords: influenza virus infection, influenza vaccine, B-cell response, antibody, plasmablast
The 2009 pandemic due to influenza A(H1N1) virus (A[H1N1]pdm09) and recent human cases of infection due to highly pathogenic avian influenza A(H5N1) and A(H7N9) viruses emphasize the urgent need to adequately prepare for influenza pandemics. Vaccination is considered the most effective means to protect against influenza. Two types of seasonal influenza vaccines are currently available in the United States: inactivated influenza vaccine (IIV) and live, attenuated influenza vaccine (LAIV). In children 6 months to 18 years of age, LAIV has been consistently more efficacious than IIV against both antigenically matched and drifted strains [1–3]. LAIV was recently recommended as the preferred vaccine for healthy children 2–8 years of age by the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices [4]. In adults <50 years of age, LAIV is either equally effective or, in some reports, somewhat less effective than IIV [1, 5, 6].
Both wild-type infection and IIV immunization, but not LAIV immunization, efficiently induce serum antibody responses against influenza virus antigens. Influenza hemagglutinin (HA)–specific serum antibody levels after wild-type influenza virus infection or IIV immunization are good predictors of substantial protection against infection with antigenically matched strains, but not antigenically drifted variant strains [7–12]. With the exception of studies involving the generation of recombinant monoclonal antibodies with broad neutralizing activity from patients with acute influenza [13, 14], the B-cell responses to natural influenza virus infection have not been well characterized.
Since the 2009 influenza pandemic, A(H1N1)pdm09 has been the annual H1N1 component of seasonal influenza vaccines, and most vaccine recipients have received IIV. The influenza vaccine coverage rate in the United States was 60%, and vaccine effectiveness was estimated as 62% for the 2013–2014 influenza season [15]. Despite the consistent inclusion of A(H1N1)pdm09 in the vaccine formulations since 2010, A(H1N1)pdm09 became the dominant strain isolated from patients with influenza in the 2013–2014 influenza season for the first time since 2010. Moreover, changes in the antigenicity of the circulating A(H1N1)pdm09 were not detected in 2013–2014 [15], suggesting a lack of immunological selective pressure on the circulating virus. In the current study, we collected blood samples from patients with polymerase chain reaction (PCR)–confirmed acute A(H1N1)pdm09 infection during the 2013–2014 influenza season and generated plasmablast-derived polyclonal antibodies (PPAbs) [16]. PPAbs are representative of the overall antibody repertoire of infection- or vaccination-activated B cells; analysis of these antibodies avoids interference by preexisting cross-reactive serum antibodies, a pitfall difficult to avoid when using serum-based assays. Reactivities of PPAbs from these infected patients and from recipients of IIV to proteins from the infecting virus and from heterovariant and heterosubtypic influenza virus strains were examined. In addition, priming of the immune system by natural infection versus IIV immunization was evaluated by examining the PPAb response to a subsequent immunization with IIV. To our knowledge, this is the first comparison of the characteristics of B-cell responses in natural influenza virus infection versus responses to IIV immunization at the polyclonal level.
MATERIALS AND METHODS
Participants, Vaccines, and Blood Sample Collection
Patients with acute influenza-like illness (ILI) were enrolled at Stanford Hospital and Clinics during the 2013–2014 influenza season. A nasopharyngeal swab was tested by the Stanford Hospital Virology Laboratory with the eSensor Respiratory Viral Panel (GenMark) to identify infecting virus. A blood sample was collected at the time of enrollment.
For comparison, healthy volunteers aged 18–30 years who participated in 2 influenza vaccine studies at Stanford University during the 2010–2011 (n = 14) or 2011–2012 (n = 39) influenza seasons were included in this study. The 2010 volunteers received 1 dose of the 2010 IIV (Fluzone, Sanofi Pasteur) by intramuscular injection. None of these subjects received monovalent A(H1N1)pdm09 vaccine in 2009. The 2011 volunteers were randomized to receive intramuscular IIV or intradermal IIV (Fluzone Intradermal, Sanofi Pasteur). None of these subjects received seasonal influenza vaccine in 2010. A subset of the 2010 and 2011 participants who were available to participate in the study again (n = 23) in 2012 were re-enrolled and received 1 dose of seasonal IIV by the same route as their prior immunization in 2010 or 2011. The 2010 and 2011 IIV formulations contained the same 3 influenza virus strains: A/California/7/2009(H1N1)-like virus (A[H1N1]pdm09), A/Perth/16/2009(H3N2)-like virus, and B/Brisbane/60/2008-like virus. The 2012 IIV contained A/California/7/2009(H1N1)-like virus, A/Victoria/361/2011(H3N2)-like virus, and B/Wisconsin/1/2010-like virus. Serum was collected on days 0 and 28 ± 4 after each vaccination to measure levels of neutralizing antibodies against A(H1N1)pdm09 by influenza virus neutralization assay as previously described [17]. A blood sample was collected at day 6–8 after vaccination for PPAb studies.
All studies were approved by the Stanford Institutional Review Board, and written informed consent was obtained from all participants.
Analysis of Influenza Virus–Specific PPAbs
B cells were isolated from blood samples by using the RosetteSep Human B-cell Enrichment Cocktail (Stemcell Technologies) and cultured to collect PPAbs [16]. Enzyme-linked immunosorbent assays (ELISAs) were performed as described previously [17]. In brief, 96-well plates (Greiner) were coated with purified, cold-adapted influenza A(H1N1)pdm09 (kindly provided by Dr H. Jin of MedImmune Vaccines) at 106 fluorescent focus forming units per well or with recombinant HA from 293 cells (Immune Technology), matrix protein 1 (M1) from Escherichia coli (Immune Technology), or nucleoprotein (NP) from insect cells (Imgenex) at 5 µg/mL. Plates were blocked and then incubated with 10-fold serially diluted PPAbs starting at 100-fold dilution. Wells incubated with complete medium without human immunoglobulin were used to determine background. Plates were washed and incubated with peroxidase-conjugated goat anti–immunoglobulin G (IgG) γ antibody or goat anti–immunoglobulin A (IgA) α antibody (KPL) and developed with TMB substrate (KPL). The OD450nm was measured. Background was subtracted, and the area under curve (AUC) of each serially diluted sample was calculated as described elsewhere [17].
Statistical Analysis
Hypothesis testing used 2-sample and paired-sample t tests, as indicated. For comparison of immune priming by influenza virus infection versus IIV immunization, because the 2 data sets to be compared had some participants in common, comparisons of means used perturbation resampling [18] with bias correction [19] to estimate P values. Sequential Bonferroni adjustment [20] was used to adjust for multiple comparisons across all hypothesis tests in each figure. Analyses were performed in SAS v. 9.4 (SAS Institute). Code is available upon request. Hypothesis tests were declared statistically significant for P < .05.
RESULTS
Natural A(H1N1)pdm09 Infection Induces B-Cell Responses to Influenza Virus Proteins
During the 2013–2014 influenza season, we enrolled 12 patients with acute ILI (Table 1), including 9 patients infected with A(H1N1)pdm09, 1 infected with influenza B virus, 1 infected with metapneumovirus, and 1 infected with coronavirus. Blood samples were collected on the enrollment day, from 2 to 8 days after the onset of illness. PPAbs were derived from samples [16] and tested by ELISA for binding reactivity to A(H1N1)pdm09 (Figure 1A and 1B). For control, we used a pool of PPAbs derived from blood samples collected on days 6–8 after vaccination from a group of recipients of the 2011 IIV, which contained A(H1N1)pdm09. A(H1N1)pdm09-specific binding was detected in the PPAb pool from the IIV recipients and from individual PPAb samples from 6 of the 7 A(H1N1)pdm09-infected patients who presented at day 4 or later after symptom onset (Figure 1B). Binding was not detected in the PPAb samples collected from A(H1N1)pdm09-infected patients on days 2 or 3 or from patients infected with influenza B virus, metapneumovirus, or coronavirus. In A(H1N1)pdm09-reactive PPAb samples from A(H1N1)pdm09-infected patients, the binding activity of IgG was significantly higher than that of IgA (P = .01, by the paired t test). These results suggest that, in patients infected with A(H1N1)pdm09, substantial virus-specific plasmablast responses are detectable in the blood 4 days after symptom onset and that the IgG response is dominant.
Table 1.
Clinical Information of Patients With Acute Influenza-Like Illnessa
| Patient | Sex | Age, y | Time Sample Collection, db | Infecting Virusc |
|---|---|---|---|---|
| 1 | Male | 78 | 8 | A(H1N1)pdm09 |
| 2 | Female | 52 | 7 | A(H1N1)pdm09 |
| 3 | Male | 24 | 3 | A(H1N1)pdm09 |
| 4 | Male | 56 | 6 | A(H1N1)pdm09 |
| 5 | Female | 31 | 7 | Metapneumovirus |
| 6 | Female | 44 | 6 | Coronavirus OC43 |
| 7 | Female | 59 | 4 | A(H1N1)pdm09 |
| 8 | Male | 22 | 4 | A(H1N1)pdm09 |
| 9 | Female | 32 | 8 | A(H1N1)pdm09 |
| 10 | Male | 53 | 7 | A(H1N1)pdm09 |
| 11 | Female | 43 | 6 | Influenza B virus |
| 12 | Female | 33 | 2 | A(H1N1)pdm09 |
Abbreviation: A(H1N1)pdm09, 2009 pandemic influenza A(H1N1).
a Diagnosis of influenza-like illness was based on fever (temperature of ≥100°F) and a cough and/or sore throat in the absence of known cause other than influenza.
b Data are days after symptom onset.
c Determined by diagnostic polymerase chain reaction assays, using the eSensor Respiratory Viral Panel.
Figure 1.
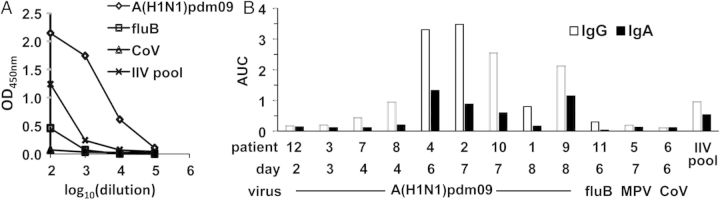
2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09) binding activity of plasmablast-derived polyclonal antibodies (PPAbs). PPAbs from patients with acute influenza-like illness (ILI) were prepared from blood samples collected 2–8 days after disease onset. A pool of PPAbs from a subset (n = 23) of 2010/2011 inactivated influenza vaccine (IIV) recipients (IIV pool) was also tested. This PPAb pool was assembled by combining equal amounts of immunoglobulin G (IgG) from each PPAb sample prepared with blood samples collected 6–8 days after IIV receipt. A, IgG enzyme-linked immunosorbent assay (ELISA) titration curves of 4 serially diluted PPAb samples: representative ILI patients 4 (A[H1N1]pdm09), 11 (influenza B virus [fluB]), and 6 (coronavirus [CoV]) and the PPAb pool from IIV recipients (IIV pool). B, IgG and immunoglobulin A (IgA) binding activity, shown as area under curve (AUC) of ELISA titration curves of individual ILI PPAb samples and the IIV PPAb pool. Patient number, days between disease onset and blood collection, and infecting agent are shown below the relevant bars in the graph.
Next, we examined the IgG binding of PPAb to 3 individual influenza virus proteins: HA of A(H1N1)pdm09 and NP and M1 of influenza A(H1N1). Substantial binding to HA was detected in 5 of 6 PPAb samples from A(H1N1)pdm09-infected patients (Figure 2A). NP-specific binding was detected in all 6 patient samples, whereas M1-specific binding was detected in only 3. In contrast, the IIV PPAb pool only bound to the HA antigen but not to NP or M1. The PPAb from the influenza B virus–infected patient did not bind any of the influenza A virus proteins (Figure 2A).
Figure 2.
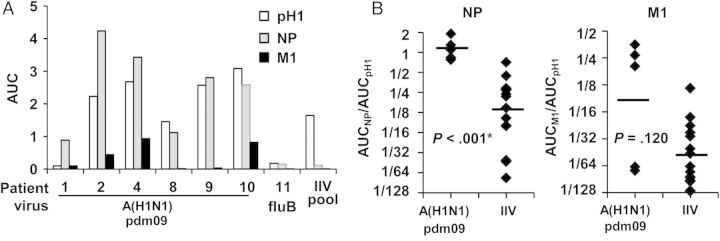
Immunoglobulin G (IgG) plasmablast-derived polyclonal antibody (PPAb) binding to individual influenza virus proteins. A, Six 2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09)–infected patient PPAb samples with detectable binding to A(H1N1)pdm09 virus, PPAb sample from a patient infected with influenza B virus (fluB), and PPAb pool from inactivated influenza vaccine (IIV) recipients (IIV pool) were tested for IgG binding activity to the hemagglutinin (HA) protein of A(H1N1)pdm09 (pH1) and to the nucleoprotein (NP) and matrix 1 protein (M1) of influenza A(H1N1). B, IgG binding activity for NP and M1 were normalized to that for HA by calculating the area under the curve (AUC) ratios (ie, AUCNP/AUCHA and AUCM1/AUCHA) for each PPAb sample. Normalized data from infected patients and the IIV recipients are compared. The A(H1N1)pdm09-infected patient PPAbs with detectable binding to HA (n = 5) and PPAbs from a group of 15 randomly selected 2010/2011 IIV recipients were used for this analysis. The selection of this group was not based on any biological parameters. Horizontal bars indicate the geometric mean of the AUC ratio. The P values were determined by unpaired t tests and adjusted by sequential Bonferroni adjustment for multiple comparisons. The asterisk indicates a statistically significant difference after the adjustment.
We then compared PPAb reactivity to the 3 influenza virus proteins between the A(H1N1)pdm09-infected patients and a group of 15 IIV recipients. Since the precise kinetics of the peripheral plasmablast response in influenza virus infection are not known, the observed reactivity of PPAb samples collected on different days after disease onset might not represent the peak plasmablast response. Therefore, instead of comparing the reactivity to each influenza virus protein directly, we normalized the NP and M1 binding activity to HA reactivity, since HA is the primary antigenic target of IIV. These normalized reactivities provide information about the relative pattern of PPAb responses to different influenza virus proteins. As shown in Figure 2B, the normalized NP reactivity was significantly higher in the A(H1N1)pdm09-infected patients than in the IIV recipients. The mean of normalized binding activity for M1 was also higher in the infected patients than in the IIV recipients, but the difference was not statistically significant, perhaps because of the small sample size and large variability among the infected patients. Taken together, these results indicate that, in addition to a B-cell response to the variable HA, patients infected with A(H1N1)pdm09 developed B-cell responses to the conserved NP that were significantly higher than those mounted by IIV recipients despite the fact that IIV preparations contain large amounts of NP [21, 22].
Natural Influenza Virus Infection Induces Cross-reactive B-Cell Responses to Heterovariant and Heterosubtypic HA
We tested the PPAb samples from A(H1N1)pdm09-infected patients and IIV recipients for their ability to bind full-length HA proteins of 3 influenza A virus strains: the homotypic infecting A(H1N1)pdm09 strain (pH1), the heterovariant A/Brisbane/59/2007(H1N1) strain (sH1), and the heterosubtypic avian A/Vietnam/1203/2004(H5N1) strain (H5) and to the HA2 peptide of H5. Each A(H1N1)pdm09-infected PPAb sample with detectable pH1 binding also bound to sH1 and H5 (Figure 3A). In addition, these PPAbs bound to the HA2 portion of H5, which contains the conserved major antigenic site of the stalk domain. In agreement with our previous reports [17, 23], the IIV PPAb pool also bound the heterovariant sH1. These results indicate that a cross-reactive PPAb response to heterovariant and heterosubtypic HAs was induced in patients with acute A(H1N1)pdm09 infection and that cross-reactivity was in part mediated by the binding to the conserved HA stalk domain.
Figure 3.
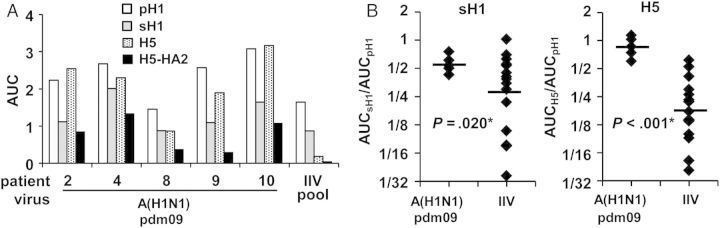
Cross-reactive plasmablast-derived polyclonal antibody (PPAb) binding activity to influenza virus hemagglutinin (HA). A, The 2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09)–infected patient PPAb samples with detectable binding to the homologous HA of A(H1N1)pdm09 (pH1; n = 5) and the inactivated influenza vaccine (IIV) PPAb pool were tested for immunoglobulin G (IgG) binding to the HA of the heterovariant H1N1 strain A/Brisbane/59/2007 (sH1), the heterosubtypic avian H5N1 strain A/Vietnam/1203/2004 (H5), and the HA2 domain of H5 (H5-HA2). B, IgG binding activity for sH1 and H5 were normalized to that for pH1. The A(H1N1)pdm09-infected patient PPAbs with detectable binding to pH1 (n = 5) and PPAbs from the 15 randomly selected individual IIV recipients were included for this analysis. Horizontal bars indicate geometric mean of the area under the curve (AUC) ratio. The P values were determined by unpaired t tests and adjusted by sequential Bonferroni adjustment for multiple comparisons. The asterisks indicate a statistically significant difference after the adjustment.
Next we normalized the sH1 and H5 reactivity to the homotypic pH1 reactivity and compared these normalized cross-reactivities of PPAbs from infected and IIV immunized groups. As shown in Figure 3B, the normalized sH1 and H5 reactivities were both significantly higher in the infected patients than in the IIV recipients, indicating that plasmablast responses to influenza virus infection had greater relative cross-reactivity against heterovariant and heterosubtypic HAs than those induced by IIV.
Immune Priming by Natural Influenza Virus Infection and IIV Immunization Differs
For this analysis, we identified a subset of 43 individuals aged 18–30 years who did not receive a monovalent A(H1N1)pdm09 vaccine in 2009 (by self-report) in our cohort of healthy IIV recipients. Before the A(H1N1)pdm09 pandemic, individuals younger than 30 years had little A(H1N1)pdm09-reactive serum antibodies [24]. At the time of enrollment, 26 of 43 individuals had detectable serum neutralizing antibodies against A(H1N1)pdm09 with a geometric mean titer of 84 (range 16–1995), suggesting that they had been previously infected with wild-type A(H1N1)pdm09. Seventeen subjects were seronegative (titer lower than 10) for A(H1N1)pdm09. All 43 individuals were immunized for the first time with A(H1N1)pdm09-containing trivalent IIV in 2010/2011 or 2011/2012 by either intramuscular or intradermal injection. Of note, no detectable differences in the frequency of vaccine-specific antibody secreting cells or in vaccine-specific PPAb reactivity were observed in samples from patients vaccinated intramuscularly versus those vaccinated intradermally (data not shown). The IIV from both years had identical strain composition and contained A(H1N1)pdm09 as their H1N1 component.
We compared the B-cell responses after IIV immunization in baseline A(H1N1)pdm09-seronegative and A(H1N1)pdm09-seropositive individuals by measuring the PPAb reactivity to the homotypic pH1, heterovariant sH1, and heterosubtypic H5 proteins. Significant differences were not detected in the binding to pH1, sH1, or H5 between the seronegative and seropositive individuals (Figure 4A), suggesting that previous natural infection with A(H1N1)pdm09 did not result in detectable differences in the plasmablast response to homotypic or heterovariant HAs after IIV immunization. Of note, the mean PPAb binding to the homotypic pH1 was higher in the seropositive individuals than the seronegative individuals, although the difference was no longer significant after adjustment for multiple comparisons.
Figure 4.
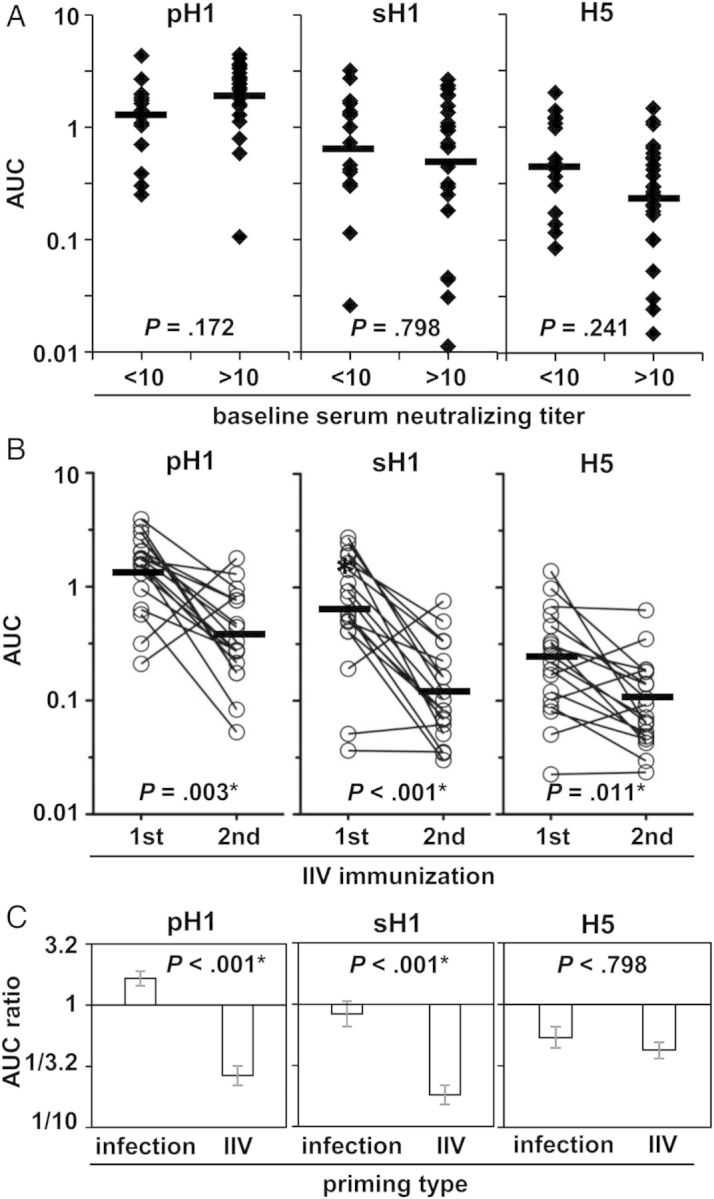
Priming effect of natural 2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09) infection versus inactivated influenza vaccine (IIV) immunization. Forty-three healthy individuals who did not receive a monovalent A(H1N1)pdm09 vaccine in 2009 and were immunized with an A(H1N1)pdm09-containing IIV for the first time were included for this analysis. A, Plasmablast-derived polyclonal antibodies (PPAbs) were collected after the first immunization with the A(H1N1)pdm09-containing IIV in 2010 or 2011. The PPAb reactivities were analyzed by enzyme-linked immunosorbent assay (ELISA) for binding to the hemagglutinin (HA) protein of homologous A(H1N1)pdm09 (pH1), heterovariant A/Brisbane/59/2007(H1N1) (sH1), and heterosubtypic A/Vietnam/1203/2004(H5N1) (H5). The baseline seronegative (A[H1N1]pdm09 neutralizing titer <10, n = 17) and baseline seropositive (A[H1N1]pdm09 neutralizing titer >10, n = 26) vaccinees were compared. B, A subset (n = 18) of the 2010/2011 IIV vaccinees were immunized with the A[H1N1]pdm09-containing IIV in 2012. The PPAbs collected after each immunization were analyzed by ELISA for binding to the HAs pH1, sH1, and H5. C, Comparison of the priming effect of natural A[H1N1]pdm09 infection versus that of IIV immunization. The priming effect of A(H1N1)pdm09 infection was defined as the ratio of the mean area under the curve (AUC) for baseline seropositive individuals to the mean AUC for baseline seronegative individuals. The priming effect of IIV was defined as the ratio of the mean AUC of the second immunization to the mean AUC of the first immunization. Horizontal bars in panels A and B indicate geometric mean AUCs. Hypotheses were tested with unpaired (A) or paired (B) t tests. Because the 2 data sets (in panels A and B) had some subjects in common, analysis used perturbation resampling [18], which is essentially a smoothed bootstrap. The P values were adjusted by sequential Bonferroni adjustment for multiple comparisons across all 9 tests in the figure. The asterisk indicates a statistically significant difference after the adjustment.
In the subsequent 2012–2013 influenza season, 18 of 43 2010 or 2011 IIV recipients received the 2012 IIV, which contained the same A(H1N1)pdm09 component. Comparison of responses to the first versus the second IIV immunization as assessed by PPAb reactivity to the 3 HA proteins reflects the priming effect of inactivated A(H1N1)pdm09 vaccine (the first immunization) on the B-cell response to subsequent vaccination with the same vaccine (the second immunization). PPAb reactivities to pH1, sH1, and H5 were all significantly lower after the second IIV immunization than after the first immunization (Figure 4B). In agreement with these results, the levels of A(H1N1)pdm09-specific serum neutralizing antibodies increased significantly after the first and second IIV immunization, but the fold-increase of titers after the second immunization was significantly lower than that after the first (Supplementary 1). Therefore, priming with inactivated A(H1N1)pdm09 vaccine reduced the plasmablast response to a subsequent immunization with the same vaccine.
Finally, we compared the fold difference in PPAb reactivity between the seronegative and seropositive subjects (Figure 4A) and the fold difference between the first and second IIV immunizations (Figure 4B). These differences represent the priming effects of A(H1N1)pdm09 infection versus inactivated A(H1N1)pdm09 immunization, respectively. As shown in Figure 4C, the priming effects were significantly different for PPAb reactivity to pH1 and sH1 but not to H5. Taken together, these results show that priming with inactivated A(H1N1)pdm09, but not with A(H1N1)pdm09 infection, results in significantly reduced plasmablast responses to a subsequent immunization with the same inactivated A(H1N1)pdm09 vaccine.
DISCUSSION
We observed a vigorous B-cell response in patients acutely infected with A(H1N1)pdm09, as demonstrated by broad PPAb reactivity against select A(H1N1)pdm09 structural proteins and against HAs derived from a heterovariant H1N1 strain and a heterosubtypic avian H5N1 strain. Compared with the B-cell response in IIV recipients, influenza virus infection elicited greater B-cell responses to the conserved NP, as well as to the heterovariant and heterosubtypic HAs. We also identified distinct priming effects after infection versus IIV immunization: wild-type A(H1N1)pdm09 infection did not affect the antigen recall response to a subsequent immunization with an IIV containing the same strain-specific HA, whereas IIV immunization resulted in significantly reduced homotypic and cross-reactive PPAb responses after a subsequent immunization with the same IIV strain.
On day 7 after vaccination with either IIV [25–27] or LAIV [28, 29] (and unpublished data), specific plasmablasts reach their peak concentration in the circulation. In a recent human challenge study with wild-type influenza virus, virus-specific plasmablasts were detected at day 7 after infection [30]. This study did not, however, examine the kinetics of the infection-induced plasmablast response or the antibody specificities of these plasmablasts. The kinetics of the B-cell response in naturally infected patients is likely to be more variable than the kinetics in volunteers who receive standardized vaccines or who are experimentally challenged with wild-type influenza virus preparations at a prespecified time and dose. This supposition is supported by the report that plasmablast-derived influenza virus–specific monoclonal antibodies were isolated from influenza virus–infected patients 9–31 days after the onset of symptoms [13]. In our current study, we detected substantial PPAb responses in infected patients for several days after the onset of symptoms, although multiple sampling was not performed to identify the peak day. Other limitations of the current study are the lack of randomization in the comparisons of infected versus vaccinated individuals and the seronegative versus seropositive individuals, and the small number and wide age range and underlying diseases of the infected patients examined. The only elderly patient (patient 1) had substantial PPAb reactivity to NP but not HA; this differed from the response of all the younger patients. We previously reported a weaker plasmablast response in elderly subjects than in younger individuals after immunization with IIV, and HA is the primary antigenic component in this vaccine [31]. It is not clear whether the lack of a detectable HA-specific PPAb reactivity in this elderly patient was due to off-peak sample collection or to a generally reduced HA-specific response. Thus, we suggest that the kinetics of the plasmablast response, including its quantitative and qualitative characteristics over the disease course, should be addressed in future studies of defined subject populations experimentally challenged with wild-type virus.
In addition to plasmablasts specific for the HA antigen of the infecting virus, natural A(H1N1)pdm09 infection elicited a greater plasmablast response to the conserved NP and to HAs from a heterovariant H1N1 strain and an avian H5N1 strain than IIV immunization. Recently, we reported that LAIV also induces proportionally greater plasmablast responses to NP and variant HA proteins than IIV [17]. Our findings suggest that LAIV immunization recapitulates some distinct qualitative characteristics of wild-type influenza virus infection in terms of B-cell response, but at a much lower magnitude. Thus, wild-type influenza virus and LAIV may induce protective immunity through similar mechanisms that are distinct from those of IIV. Of note, it has been proposed that antibodies targeting conserved virus components, including the stalk domain of HA from the group 1 influenza A viruses [32, 33] and the NP [22], have potential as the basis of universal influenza vaccines that would offer protection against a broad range of seasonal and pandemic influenza viruses. This is consistent with the data showing that LAIV is superior to IIV in inducing cross-protection [3]. The basis for differences in B-cell responses to influenza virus infection and IIV immunization is currently unknown and should be addressed in future studies that include analyses of immunoglobulin gene sequences and antibody functions of the activated B-cell repertoire at the clonal level [34] and also, of course, T-cell responses. Elucidating different aspects of immune responses to natural influenza virus infection and identifying the immune correlates with protective immunity will be important for development of next-generation influenza vaccines.
A recent influenza cohort study revealed high rates of asymptomatic natural influenza virus infection in the community; infection was only detected by strain-specific seroconversion [35]. It will be interesting to determine whether such asymptomatic influenza virus infection differs from symptomatic cases or from immunization with LAIV in terms of protective immune responses and to determine the factors that affect the outcome (symptomatic vs asymptomatic) of natural infection. This information could lead to a new approach for influenza vaccine development that is based on reducing influenza pathogenicity while preserving immunogenicity.
Because of the repeated exposure to influenza virus infection and vaccination, the B-cell response to influenza vaccination is an antigen recall response in almost all individuals [25], except for very young children who are naive to influenza virus infection and vaccination. Activation of antigen-specific B cells occurs in germinal centers of lymphoid tissues with the help of antigen-specific follicular helper T-cells and results in generation of plasmablasts and switched memory B cells that express high-affinity antibodies [36, 37]. Exposure to a newly emerged strain, such as A(H1N1)pdm09, primes strain-specific B cells and T-cells to generate memory cells that in turn alter the B-cell response to a subsequent vaccination or infection. In the current study, we showed that priming with IIV resulted in diminished strain-specific recall responses to a subsequent IIV immunization, whereas such suppression was not observed in individuals with prior natural A(H1N1)pdm09 infection. LAIV immunization also does not result in detectable differences in the serum antibody and plasmablast responses to IIV or LAIV immunization in the subsequent year [29]. A recent study found that immunization with a live, attenuated avian H5N1 influenza vaccine, which stimulated virtually no serum antibodies, effectively primed the immune system, resulting in a robust serum antibody response to a subsequent immunization with an inactivated H5N1 vaccine including broader cross-reactivity, compared with repeated immunization with the inactivated vaccine alone [38]. In mice primed with a T-cell–dependent antigen, a second immunization with an epitope-matched T-cell independent antigen results in induction of tolerant memory B cells rather than a recall response [39]. A substantial CD4+ T-cell response was detected in volunteers experimentally infected with wild-type influenza viruses [40]. In another human study a specific subset of ICOS+CXCR3+CXCR5+CD4+ follicular helper T-cells correlated with the induction of antibodies to IIV in previously primed individuals but not in naive subjects [41]. Thus, deficiencies in the magnitude or functional properties of the CD4+ T-cell repertoire specific for IIV are likely a contributing factor to the reduced B-cell response in repeated IIV immunization. The efficacy of repeated IIV immunization has been an issue of debate for some time [42–46]. However, this controversy has not previously focused specifically on the generation of humoral immunity in recipients of repeated IIV immunization in comparison to those previously primed by natural infection. Since annual immunization with IIV is common practice, the implication of our findings to the long-term effectiveness of influenza vaccination needs to be carefully evaluated, especially in the context of maximizing protective immunity in vulnerable populations, such as elderly individuals.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank our study subjects, for their participation; C. Zhang, for technical assistance; B. Pinsky, for performing the diagnostic PCR assay; H. Jin, for providing purified A(H1N1)pdm09; S. Mackey, for coordinating the clinical study; S. Swope, N. Mastman, T. Trela, and M. Ugur, for enrolling subjects, administering vaccine, and collecting samples and clinical data; and A. Goel, T. Quan, R. Fleischman, S. Batra, and I. Chang, for screening and scheduling subjects and providing regulatory and clinical data management support.
Financial support. This work was supported by the National Institutes of Health (NIH; grants AI090019, AI057229, AI089987, P01AI097092, and U19AI089987); the National Center for Research Resources, NIH (Clinical and Translational Science Award UL1RR025744); and the Center for Research on Influenza Pathogenesis (a National Institute of Allergy and Infectious Diseases–funded Center of Excellence for Influenza Research and Surveillance; contract HHSN272201400008C).
Potential conflicts of interest. H. B. G. is on the scientific advisory board of Novartis Vaccines, a major producer of influenza vaccines, and a consultant for Vaxart and PaxVax, both of which are developers of novel influenza vaccines. The Icahn School of Medicine owns intellectual property in the area of influenza vaccines for which A. G.-S. is an inventor. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ambrose CS, Levin MJ, Belshe RB. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respi Viruses. 2011;5:67–75. doi: 10.1111/j.1750-2659.2010.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrose CS, Wu X, Belshe RB. The efficacy of live attenuated and inactivated influenza vaccines in children as a function of time postvaccination. Pediatr Infect Dis J. 2010;29:806–11. doi: 10.1097/INF.0b013e3181e2872f. [DOI] [PubMed] [Google Scholar]
- 3.Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356:685–96. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 4.Grohskopf LA, Olsen SJ, Sokolow LZ, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP) - United States, 2014–15 Influenza Season. MMWR Morb Mortal Wkly Rep. 2014;63:691–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Monto AS, Ohmit SE, Petrie JG, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009;361:1260–7. doi: 10.1056/NEJMoa0808652. [DOI] [PubMed] [Google Scholar]
- 6.Ohmit SE, Victor JC, Rotthoff JR, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006;355:2513–22. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burlington DB, Clements ML, Meiklejohn G, Phelan M, Murphy BR. Hemagglutinin-specific antibody responses in immunoglobulin G, A, and M isotypes as measured by enzyme-linked immunosorbent assay after primary or secondary infection of humans with influenza A virus. Infect Immun. 1983;41:540–5. doi: 10.1128/iai.41.2.540-545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70:767–77. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clements ML BR, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24:157–60. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treanor J, Wright PF. Immune correlates of protection against influenza in the human challenge model. Dev Biol (Basel) 2003;115:97–104. [PubMed] [Google Scholar]
- 11.Beyer WE, Palache AM, de Jong JC, Osterhaus AD. Cold-adapted live influenza vaccine versus inactivated vaccine: systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine. 2002;20:1340–53. doi: 10.1016/s0264-410x(01)00471-6. [DOI] [PubMed] [Google Scholar]
- 12.Couch RB, Atmar RL, Keitel WA, et al. Randomized comparative study of the serum antihemagglutinin and antineuraminidase antibody responses to six licensed trivalent influenza vaccines. Vaccine. 2012;31:190–5. doi: 10.1016/j.vaccine.2012.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wrammert J, Koutsonanos D, Li GM, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–93. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomson CA, Wang Y, Jackson LM, et al. Pandemic H1N1 influenza infection and vaccination in humans induces cross-protective antibodies that target the hemagglutinin stem. Front Immun. 2012;3 doi: 10.3389/fimmu.2012.00087. 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flannery B, Thaker SN, Clippard J, et al. Interim estimates of 2013–14 seasonal influenza vaccine effectiveness - United States, february 2014. MMWR Morb Mortal Wkly Rep. 2014;63:137–42. [PMC free article] [PubMed] [Google Scholar]
- 16.He XS, Sasaki S, Narvaez CF, et al. Plasmablast-derived polyclonal antibody response after influenza vaccination. J Immunol Methods. 2011;365:67–75. doi: 10.1016/j.jim.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki S, Holmes TH, Albrecht RA, et al. Distinct cross-reactive B-cell responses to live attenuated and inactivated influenza vaccines. J Infect Dis. 2014;210:865–74. doi: 10.1093/infdis/jiu190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin Z, Ying Z, Wei LJ. A simple resampling method by perturbing the minimand. Biometrika. 2001;88:381–90. [Google Scholar]
- 19.Manly BFJ. Randomization, bootstrap and Monte Carlo methods in biology. 2nd ed. London: Chapman & Hall; 1997. [Google Scholar]
- 20.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;43:223–5. [Google Scholar]
- 21.Garcia-Canas V, Lorbetskie B, Bertrand D, Cyr TD, Girard M. Selective and quantitative detection of influenza virus proteins in commercial vaccines using two-dimensional high-performance liquid chromatography and fluorescence detection. Anal Chem. 2007;79:3164–72. doi: 10.1021/ac0621120. [DOI] [PubMed] [Google Scholar]
- 22.Lamere MW, Moquin A, Lee FE, et al. Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance. J Virol. 2011;85:5027–35. doi: 10.1128/JVI.00150-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He XS, Sasaki S, Baer J, et al. Heterovariant cross-reactive B-cell responses induced by the 2009 pandemic influenza virus A subtype H1N1 vaccine. J Infect Dis. 2013;207:288–96. doi: 10.1093/infdis/jis664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 25.Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.el-Madhun AS, Cox RJ, Soreide A, Olofsson J, Haaheim LR. Systemic and mucosal immune responses in young children and adults after parenteral influenza vaccination. J Infect Dis. 1998;178:933–9. doi: 10.1086/515656. [DOI] [PubMed] [Google Scholar]
- 27.Halliley JL, Kyu S, Kobie JJ, et al. Peak frequencies of circulating human influenza-specific antibody secreting cells correlate with serum antibody response after immunization. Vaccine. 2010;28:3582–7. doi: 10.1016/j.vaccine.2010.02.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki S, Jaimes MC, Holmes TH, et al. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol. 2007;81:215–28. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki S, He XS, Holmes TH, et al. Influence of prior influenza vaccination on antibody and B-cell responses. PLoS One. 2008;3:e2975. doi: 10.1371/journal.pone.0002975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang KY, Li CK, Clutterbuck E, et al. Virus-specific antibody secreting cell, memory B-cell, and sero-antibody responses in the human influenza challenge model. J Infect Dis. 2014;209:1354–61. doi: 10.1093/infdis/jit650. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki S, Sullivan M, Narvaez CF, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121:3109–19. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sui J, Hwang WC, Perez S, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16:265–73. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang TT, Tan GS, Hai R, et al. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A. 2010;107:18979–84. doi: 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang N, He J, Weinstein JA, et al. Lineage structure of the human antibody repertoire in response to influenza vaccination. Sci Transl Med. 2013;5:171ra119. doi: 10.1126/scitranslmed.3004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayward AC, Fragaszy EB, Bermingham A, et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med. 2014;2:445–54. doi: 10.1016/S2213-2600(14)70034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–35. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shulman Z, Gitlin AD, Targ S, et al. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–7. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talaat KR, Luke CJ, Khurana S, et al. A live attenuated influenza A(H5N1) vaccine induces long-term immunity in the absence of a primary antibody response. J Infect Dis. 2014;209:1860–9. doi: 10.1093/infdis/jiu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haniuda K, Nojima T, Ohyama K, Kitamura D. Tolerance induction of IgG+ memory B cells by T cell-independent type II antigens. J Immunol. 2011;186:5620–8. doi: 10.4049/jimmunol.1100213. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson TM, Li CK, Chui CS, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18:274–80. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 41.Bentebibel SE, Lopez S, Obermoser G, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5:176ra132. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoskins TW, Davies JR, Smith AJ, Miller CL, Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet. 1979;1:33–5. doi: 10.1016/s0140-6736(79)90468-9. [DOI] [PubMed] [Google Scholar]
- 43.Keitel WA, Cate TR, Couch RB, Huggins LL, Hess KR. Efficacy of repeated annual immunization with inactivated influenza virus vaccines over a five year period. Vaccine. 1997;15:1114–22. doi: 10.1016/s0264-410x(97)00003-0. [DOI] [PubMed] [Google Scholar]
- 44.Beyer WE, de Bruijn IA, Palache AM, Westendorp RG, Osterhaus AD. The plea against annual influenza vaccination? ‘The Hoskins' Paradox’ revisited. Vaccine. 1998;16:1929–32. doi: 10.1016/s0264-410x(98)00123-6. [DOI] [PubMed] [Google Scholar]
- 45.Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A. 1999;96:14001–06. doi: 10.1073/pnas.96.24.14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLean HQ, Thompson MG, Sundaram ME, et al. Impact of repeated vaccination on vaccine effectiveness against influenza A(H3N2) and B during 8 seasons. Clin Inf Dis. 2014;59:1375–85. doi: 10.1093/cid/ciu680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


