Abstract
Background
Pig erythrocytes are potentially useful to solve the worldwide shortage of human blood for transfusion. Domestic pig erythrocytes, however, express antigens that are bound by human preformed antibodies. Advances in genetic engineering have made it possible to rapidly knock out the genes of multiple xenoantigens, namely galactose α1,3 galactose (aGal) and N-glycolylneuraminic acid (Neu5Gc). We have recently targeted the GGTA1 and CMAH genes with zinc finger endonucleases resulting in double knockout pigs that no longer express aGal or Neu5Gc and attract significantly fewer human antibodies. In this study, we characterized erythrocytes from domestic and genetically modified pigs, baboons, chimpanzees, and humans for binding of human and baboon natural antibody, and complement mediated lysis.
Methods
Distribution of anti Neu5Gc IgG and IgM in pooled human AB serum was analyzed by ELISA. Erythrocytes from domestic pigs (Dom), aGal knockout pigs (GGTA1 KO), aGal and Neu5Gc double knockout pigs (GGTA1/CMAH KO), baboons, chimpanzees, and humans were analyzed by flow cytometry for aGal and Neu5Gc expression. In vitro comparative analysis of erythrocytes was conducted with pooled human AB serum and baboon serum. Total antibody binding was accessed by hemagglutination; complement-dependent lysis was measured by hemolytic assay; IgG or IgM binding to erythrocytes was characterized by flow cytometry.
Results
The pooled human AB serum contained 0.38 μg/ml anti Neu5Gc IgG and 0.085 μg/ml anti Neu5Gc IgM. Both Gal and Neu5Gc were not detectable on GGTA1/CMAH KO erythrocytes. Hemagglutinaion of GGTA1/CMAH KO erythrocytes with human serum was 3.5-fold lower compared to GGTA1 KO erythrocytes, but 1.6-fold greater when agglutinated with baboon serum. Hemolysis of GGTA1/CMAH KO erythrocytes by human serum (25%) was reduced 9-fold compared to GGTA1 KO erythrocytes, but increased 1.64-fold by baboon serum. Human IgG binding was reduced 27-fold on GGTA1/CMAH KO erythrocytes compared to GGTA1 KO erythrocytes, but markedly increased 3-fold by baboon serum IgG. Human IgM binding was decreased 227-fold on GGTA1/CMAH KO erythrocytes compared to GGTA1 KO erythrocytes, but enhanced 5-fold by baboon serum IgM.
Conclusions
Removal of aGal and Neu5Gc antigens from pig erythrocytes significantly reduced human preformed antibody-mediated cytotoxicity but may have complicated future in vivo analysis by enhancing reactivity from baboons. The creation of the GGTA1/CMAH KO pig has provided the xenotransplantion researcher with organs and cells that attract fewer human antibodies than baboon and our closest primate relative, chimpanzee. These finding suggest that while GGTA1/CMAH KO erythrocytes may be useful for human transfusions, in vivo testing in the baboon may not provide a direct transplation to the clinic.
Keywords: Erythrocyte, xenoantigen, Neu5Gc, xenotransfusion
Introduction
The creation of GGTA1/CMAH gene double knock out (GGTA1/CMAH KO) pigs has made it possible to consider the clinical application of pig erythrocytes for transfusion (1-3). Historically, the problem with using domestic (Dom) pig erythrocytes in humans has been the same as organ transplantation, antigens on the surface of erythrocytes recognized by human antibody initiate rapid complement-dependent lysis (4). Developing α 1,3 galactosyltransferase knockout (GGTA1 KO) pigs overcame the immune response towards galactose α-1,3 galactose (aGal) epitopes reducing antibody binding by approximately 70% as compared to Dom pigs (5). Like other blood cells, erythrocytes express another well-known xenoantigen, N-glycolylneuraminic acid (Neu5Gc) (6-9). Neu5Gc is mediated by the enzyme cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH). A mutation in the CMAH gene, unique to humans, causes the absence of Neu5Gc in human, which is present in pigs and elevated the non-human primates, chimpanzees and baboons (10,11). Antibodies not directed towards aGal in most healthy human sera may be directed toward Neu5Gc epitopes on many pig cells including vascular endothelial cells, PBMC and erythrocytes (12). Anti-Neu5Gc antibodies in human serum are detectable in 85% of human population (6). Our recent progress in the creation of a GGTA1/CMAH KO pigs reduced antibody binding to peripheral blood cells by approximately 70% when compared to GGTA1 KO pigs (13). The GGTA1/CMAH KO pig has created a unique opportunity to revisit the use of pig erythrocytes clinically.
Previous in vitro studies of erythrocyte transfusion indicated that removing aGal epitopes by treatment with α-galactosidase or using erythrocytes from GGTA1 KO pigs reduced binding of human or baboon antibody (7, 8). When erythrocyte agglutination was compared to ABO matched or mismatched human serum the erythrocytes from GGTA1 KO pigs, but not Dom pigs, agglutinated at a rate comparable to ABO-mismatched human erythrocytes (9). In vivo studies in non-human primates showed that GGTA1 KO pig erythrocyte loss was delayed as compared to Dom pig erythrocytes (7, 8); further a combination of complement depletion from the non-human primate and treatment of the pig erythrocytes with α-galactosidase enabled their survival in circulation for 24 hours; if macrophages and complement were removed, the treated erythrocytes survived for 72 hours (7). Nevertheless GGTA1 KO erythrocytes were removed from circulation within a few minutes after intravenous infusion, which suggests that multiple mechanisms are involved in rejection of pig erythrocyte xenotransfusion (7, 8).
It is challenging to study GGTA1/CMAH KO cells in an animal model since all non-human primates express CMAH therefore lacking anti Neu5Gc antibody (14). The limitations of using chimpanzees or baboons as organ and cell donors or as in vivo models of xenotransplantation may have been due in part to differences in non-aGal carbohydrate expression. In this study, we evaluated the neuraminic acid and Neu5Gc expression on human, pig and non-human primate erythrocytes. We provide in vitro comparative analysis of human and baboon antibody-mediated hemagglutination, cytotoxicity and IgG/IgM binding to erythrocytes from genetically modified pigs important to xenotransplantation. While the baboon may not be an ideal model, our in vitro analysis supports further investigation into GGTA1/CMAH KO erythrocytes for xenotransfusion.
Materials and methods
Blood and serum
Pig blood was collected in heparinized vacuum tubes from Dom, GGTA1 KO, and GGTA1/CMAH KO pigs (13), which are predominantly landrace mixed breed pigs blood group O using Institutional Review Board and Institutional Animal Care and Use Committee approved protocols (IRB#0808 and IACUC#10345). Blood (baboon and chimpanzee) and serum (baboon only) were obtained from Southwest National Primate Research Center (Texas Biomedical Research Institute). Human blood was collected from healthy volunteers (type A and O) according to IRB protocol guidelines. Pooled human serum (blood type A and B) was purchased from Lonza Bioscience, Rockland .
Isolation of erythrocytes
Erythrocytes were isolated from whole blood using Ficoll-Paque Plus, and washed three times with phosphate-buffered saline (PBS). The erythrocytes were diluted 1:10 in PBS at room temperature.
ELISA
Total IgG/IgM and anti-Neu5Gc IgG/IgM in human serum was measured by ELISA as described previously (15, 16). Briefly, Total human IgG/IgM was captured on ELISA plates by mouse anti-human IgG or goat anti-human IgM antibodies (Sigma) and detected by peroxidase conjugated goat anti-human IgG and IgM (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). Anti-Neu5Gc antibody from human serum was measured by binding to Neu5Gc-PAA (250ng/well) (Glycotech) or Neu5Ac-PAA (250ng/well) (Glycotech) coated wells. Peroxidase conjugated goat anti-human IgG and IgM were used to detect bound antibody. As a control for non-specific human antibody binding to carbohydrate linked PAA, antibody bound to Neu5Ac-PAA was subtracted as background. The concentration of anti-Neu5Gc IgG/IgM was calculated by comparison to a purified IgG/IgM standard curve (Sigma) on each ELISA plate.
Flow cytometry
To access aGal and Neu5Gc expression, erythrocytes (1 × 106/sample) were prepared and stained at 4°C for 1h with fluorescein labeled Sambucus nigra lectin (SNA) (Vector Labs), Griffonia simplicfolia IB4 (IB4 lectin) Alexa Fluor 647 (Invitrogen, Grand Island), chicken anti-Neu5Gc antibody and isotope control (Sialix, Vista). Donkey anti chicken DyLight 649 antibody staining was followed at 4°C for 30 min (Jackson ImmunoReserch laboratories Inc.). To determine the relative concentration of human and baboon IgG/IgM binding, erythrocytes (1 × 106/sample) were incubated with a 4-fold serial dilution of heat-inactivated human or baboon serum at 4°C for 1h. Washed cells were stained at 4°C for 30 min with Donkey anti human IgG or IgM antibody (Jackson ImmunoResearch Laboratories Inc.). Controls consisted of erythrocytes unstained or stained with secondary antibody alone. Flow cytometry analysis was performed using an Accuri C6 flow cytometer and CFlow software (Accuri, Ann Arbor, MI, USA).
Hemagglutinaiton assay
The assay is carried out in 96-well round bottom plates as described previously (9). Briefly, erythrocytes were prepared and suspended at 2 × 107/well. After washing with HBSS, the erythrocytes were incubated with a 2-fold serial dilution of heat-inactivated human or baboon serum in HBSS at 4°C overnight. Control samples were treated with HBSS without serum. The images were scanned with an Epson Perfection 3200. Agglutination was based on the modified Marsh standard 5-point scale from 0= none through 4 =maximum.
Antibody-mediated complement dependent hemolytic assay
Erythrocytes were isolated as described above, suspended at 2 × 107/well in 96-well plates and treated at 4°C for 1h with 25% human or baboon serum. After washing twice, the cells were incubated at 37°C for 1h with rabbit complement with a 1:12 dilution. The control samples were treated with rabbit complement alone. The plates were centrifuged at 400g for 10min. The supernatant was collected and transferred into new plates. Released hemoglobin was measured in the supernatant at 541 nm using a SpectraMax M2e plate reader (Molecular Devices Corp. Sunnyvale, CA, USA) (7).
Statistics
GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA) was used to perform one-way ANOVA. Significant differences were considered at P ≤ 0.05.
Results
Distribution of total IgG/IgM and anti-Neu5Gc IgG/IgM in human serum AB pool
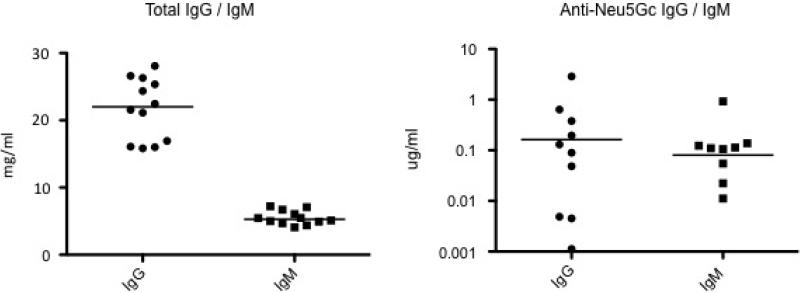
To directly access the characteristics of human serum AB pool, ELISA was performed to test the level of both total IgG/ IgM and anti-Neu5Gc IgG / IgM in the human AB pooled serum. Total IgG was 20.9 mg/ml in AB pool serum, and from15.8 to 28.1mg/ml in donor serum; total IgM was 5.5 mg/ml in AB pool, and from 4.1mg/ml to 7.2 mg/ml in donor serum (Figure 1, left panel). While anti Nue5Gc IgG was 0.38 μg/ml in human AB pooled serum, and individually from 0.001 to 24.1 μg/ml in donor serum; anti Nue5Gc IgM was 0.085 μg/ml in AB pool, and individually from 0 to 0.92 μg/ml in donor serum (Figure 1. right panel). Since the human serum AB pool had detectable levels of both anti Neu5Gc IgG and IgM, pooled human serum was used to characterize GGTA1/CMAH KO erythrocytes in comparison with baboon serum.
Figure 1.
Distribution of anti-Neu5Gc IgG/IgM in human serum by ELISA. The concentration of total IgG and IgM in pooled AB human serum and on-site donors was measured by ELISA (n=11) (left panel). The reactivity of human IgG and IgM to Neu5Gc-PAA in pooled AB human serum and donor serum (n=11) were measured in triplicate using anti-Nue5Ac-PAA for background subtraction (right panel).
Undetectable expression of both aGal and Neu5Gc on GGTA1/CMAH KO erythrocytes
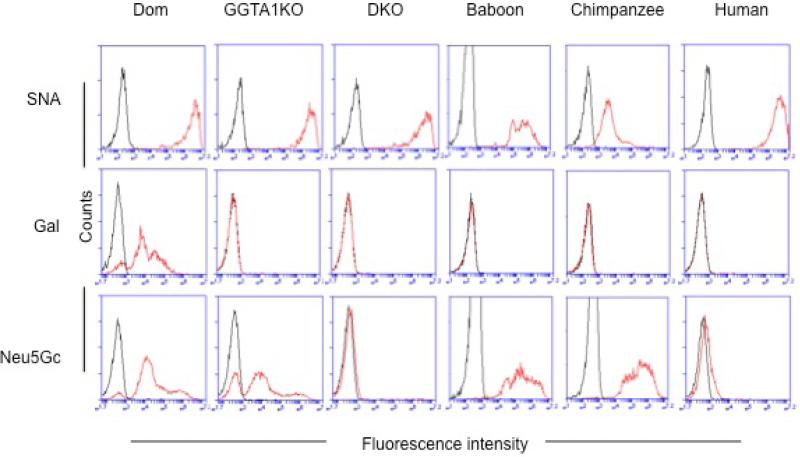
To access aGal and Neu5Gc expression on freshly isolated erythrocytes, flow cytometry analysis was performed (Figure 2). Neu5Ac was present on all erythrocytes from Dom, GGTA1 KO, GGTA1/CMAH KO pigs, baboons, chimpanzees, and humans (blood group A and O) (top panel); like humans and primates (baboon and chimpanzee), aGal was in the absence of both of GGTA1 KO and GGTA1/CMAH KO erythrocytes, except but Dom erythrocytes (middle pane). Similar to humans, Neu5Gc was absent on GGTA1/CMAH KO erythrocytes, but present on Dom, GGTA1 KO, baboons and chimpanzees erythrocytes (bottom panel).
Figure 2.
Expression of Gal and Neu5Gc on erythrocytes by flow cytometry. Erythrocytes from Dom, GGTA1 KO and GGTA1/CMAH KO pigs, baboons, chimpanzees, and humans were labeled with fluorescently conjugated Sambucus nigra (SNA) lectin (red line) to detect Neu5Ac linked α 1,6 galactose expression (top panel) were used as a positive control for the presence of erythrocytes. Fluorescently conjugated IB4-lectin was used to assess Gal expression (middle panel)(red line), and anti-Neu5Gc antibody was used to examine the expression of Neu5Gc epitopes (bottom panel)(red line). Unstained erythrocytes are shown as negative controls (black line) for SNA and IB4-lectins, and an isotype control for Neu5Gc. Histograms are representative of 3 individual experiments.
Hemagglutination by human or baboon serum
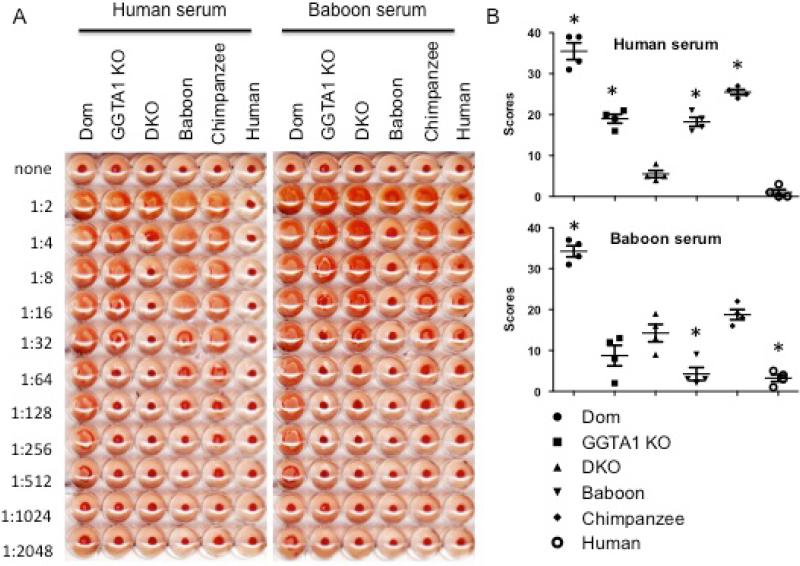
Hemagglutination was used to determine the relative amount of natural antibody in pooled human or baboon serum directed towards pig erythrocytes (Figure 3). Representative images of agglutination by a 2-fold serial dilution of heat-inactivated human or baboon serum were shown. When incubated with human serum GGTA1/CMAH KO erythrocytes agglutinated the least (1:8 dilution) followed by GGTA1 KO erythrocytes (1:64) and Dom erythrocytes (1:1024). Human serum agglutinated baboon and chimpanzee erythrocytes at higher dilutions compared with GGTA1/CMAH KO and GGTA1 KO erythrocytes. No agglutination was observed in human type O erythrocytes. Hemagglutination of different erythrocytes was measured by the modified Marsh scoring system (Figure 3 B). Values shown are the sum of scores for each cell type in the dilution series. For human serum-induced agglutination, the mean agglutination score (n=3) for Dom, GGTA1 KO and GGTA1/CMAH KO erythrocytes was 35.5, 19 and 5.5 respectively; the score of baboon and chimpanzee erythrocytes were 18.3 and 25.5 respectively; human erythrocytes was 1. For baboon serum-induced agglutination, the mean score (n=3) of Dom, GGTA1 KO and GGTA1/CMAH KO erythrocytes was 34.3, 8.8 and 14.3 respectively; the score of baboon, chimpanzee and human erythrocytes was 4.3, 18.8 and 3.3 respectively.
Figure 3.
Hemagglutination by human or baboon sera. Heat-inactivated human and baboon sera were serially diluted in HBSS and incubated with erythrocytes overnight at room temperature. Untreated erythrocytes were used as negative controls. The 96-well plates were scanned with Epson perfection 3200 and a representative image was shown (Panel A). The levels of agglutination in each well were measured by the modified Marsh scoring system scored from 0 to 4. The scores were expressed as the mean ± SEM, n=3 and *p<0.05 (Panel B).
RBC hemolysis by human or baboon serum
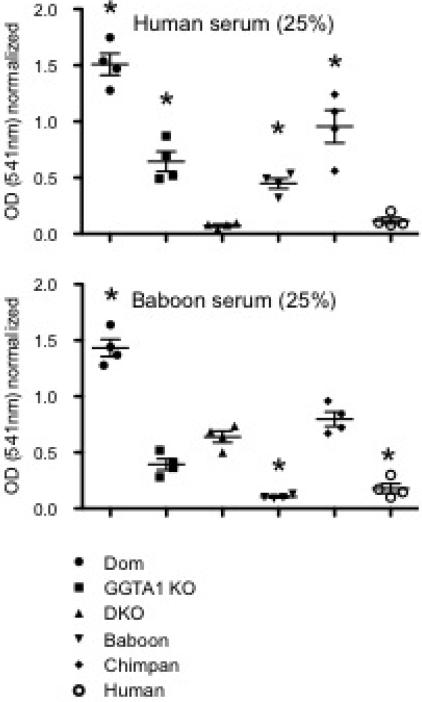
To evaluate antibody-mediated complement-dependent cytotoxicity by human or baboon serum we used a hemolytic assay measuring released hemoglobin at the optical density (OD) of 541 nm (Figure 4). For human serum-induced lysis, the mean OD values (n=3) of Dom, GGTA1 KO and GGTA1/CMAH KO erythrocytes were 1.5, 0.64 and 0.07; the mean OD values of baboons, chimpanzees and humans were 0.45, 0.96 and 0.11 respectively. For baboon serum-induced lysis, the mean OD values (n=3) of Dom, GGTA1 KO and GGTA1/CMAH KO erythrocytes were 1.4, 0.39 and 0.64 respectively; the mean OD values of baboons, chimpanzees and humans were 0.1, 0.8 and 0.18 respectively.
Figure 4.
Antibody-mediated complement-dependent hemolysis by human or baboon sera. Supernatants from erythrocytes incubated with human or baboon sera (25%) followed by rabbit complement were measured at 541nm with a spectrophotometer. Values were normalized to complement treatment in the absence of serum for each group. The results from individual experiments were expressed as the mean ± SEM, n=3 and *p<0.05.
Human or baboon IgG/IgM binding on erythrocytes
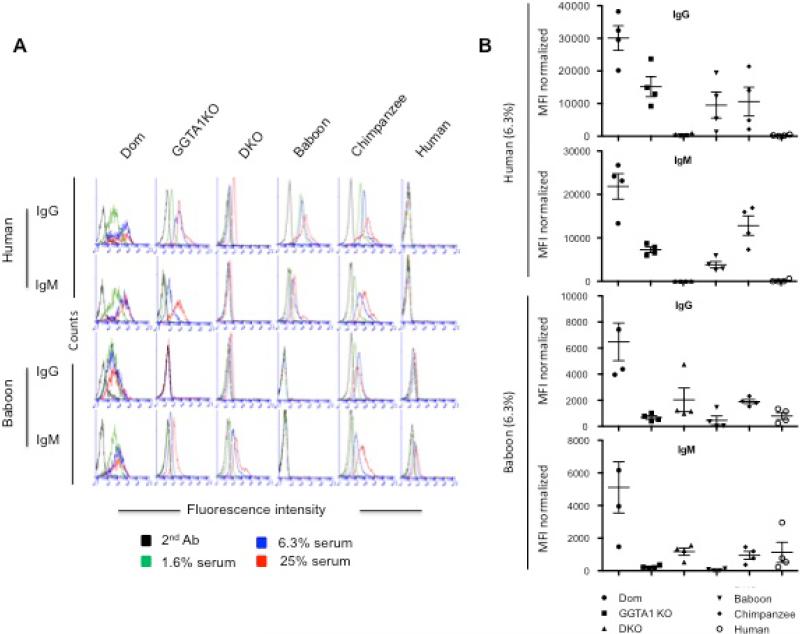
To assess the binding of human and baboon IgG/IgM towards erythrocytes from pigs, primates and humans, we used flow cytometry. Human and baboon serum was evaluated in a 4-fold serial dilution including 25%, 6.3% and 1.56% (Figure 5). In panel A, representative histograms showed that higher concentrations of human or baboon serum are correlated with higher IgG or IgM binding to erythrocytes from Dom, GGTA1 KO, GGTA1/CMAH KO, baboons, chimpanzees and humans. In panel B, bar graphs expressed a mean fluorescence intensity (MFI) of human or baboon IgG/M binding toward erythrocytes using 6.3% final sera (n=3) normalized by the subtraction of the MFI values for control cells incubated with the secondary antibody alone. Human IgG binding was reduced by 27-fold on GGTA1/CMAH KO erythrocytes compared to GGTA1 KO erythrocytes, (559 and 15187 respectively). IgG binding to GGTA1KO erythrocytes was 2-fold lower than Dom erythrocytes; the MFI of Dom, baboon and Chimpanzee Erythrocytes were 30079, 9532, 10613 respectively; human Erythrocytes as a control was 262; while human IgM binding (MFI) was decreased significantly by 227-fold on GGTA1/CMAH KO erythrocytes compared to GGTA1 KO, respectively 32 and 7278; and GGTA1 KO erythrocytes were also significantly Lower by 3-fold than Dom erythrocytes. The MFI of Dom, baboon and chimpanzee erythrocytes was 21858, 3803, and 12824 respectively. Human erythrocytes as a control was 161.
Figure 5.
Flow cytometry analysis of human or baboon IgG/IgM binding to erythrocytes. A 4-fold serial dilution of human or baboon sera was used to label erythrocytes from Dom, GGTA1 KO and GGTA1/CMAH KO pigs, as well as baboons, chimpanzees, and humans. Bound human and baboon antibody was detected with fluorescently conjugated anti-human IgG or IgM antibodies. Erythrocytes from each group were treated with secondary antibody only as a control. Histograms shown are representative of 3 experiments (Panel A). IgG/IgM binding was assessed after normalization with the MFI of erythrocytes incubated with only secondary antibody. The results from individual experiments using 6.3% final sera are expressed as mean ± SEM, n=3 and *p<0.05 (Panel B).
In contrast, baboon IgG binding (MFI) was markedly increased by 3-fold on GGTA1/CMAH KO erythrocytes compared to GGTA1 KO erythrocytes, (2034 and 681 respectively). Baboon IgG binding to Dom erythrocytes was significantly higher than to either GGTA1/CMAH KO or GGTA1 KO erythrocytes. The MFI of baboon IgG binding to Dom, chimpanzee, and human erythrocytes was 6477, 1898, and 799, respectively. Baboon erythrocytes were used as a control for baboon IgG binding and had an MFI of 460. Baboon IgM binding (MFI) was enhanced 5-fold to GGTA1/CMAH KO erythrocytes compared to GGTA1 KO erythrocytes, (1174 and 235 respectively). Baboon IgM binding to Dom erythrocytes was significantly higher than both GGTA1/CMAH KO and GGTA1 KO erythrocytes. The MFI of Dom, chimpanzee, and human erythrocytes was 5112, 950, and 1127 respectively. The MFI of baboon IgM binding to baboon erythrocytes was 52.
Discussion
Genetically engineering pigs without aGal expression (GGTA1 KO) delayed antibody-mediated rejection (AMR) of pig organs or cell transplantation in non-human primates (17). Similar to aGal, Neu5Gc is absent on human cells and has been defined as a non-Gal antigen target on human erythrocytes recognized by human preformed antibodies (6, 10). We have previously created pigs free of aGal and Neu5Gc that has the potential to be an organ and tissue donor to humans (13). The reactivity of human serum to peripheral blood monocytes (PBMC) from GGTA1/CMAH KO pigs was similar to the decrease in immunoreactivity seen in this study (13). These finding are not unique to our GGTA1/CMAH KO pigs. Thymocytes from mice with similar genetic modifications incubated with human serum also had a decrease in antibody binding (18). Our results have established that the erythrocytes from GGTA1/CMAH KO pigs are a significant advancement towards a plentiful alternative to human erythrocytes for transfusion.
The development of GGTA1/CMAH KO pigs confirmed that Neu5Gc on erythrocytes is an important binding target for non-Gal preformed antibody in human serum but not in baboon serum. The creation of the GGTA1/CMAH KO pigs has brought us closer than ever to clinical xenotransplantation, but our ability to test these pigs in established models is hampered by the expression of Neu5Gc in non-human primates. This study demonstrates the changes in immunoreactivity of human and baboon serum toward erythrocytes of GGTA1/CMAH KO, GGTA1 KO and Dom pigs and suggests that the baboon is a poor model of antigen binding after the loss of Neu5Gc in pigs. Surprisingly, baboon serum was much more reactive to the GGTA1/CMAH KO pig RBC when compared to human serum. Theoretically there could be an increase in existing antigen or creation of new antigen recognized by baboon IgG or IgM but more importantly the observed increase in hemolytic activity prevents further application of the baboon model.
Antibody binding, hemagglutination, and lysis defined the immediate effects of human antibody on the pig erythrocytes the way they may occur during transfusion. Cellular effects, however, may have an impact on the application of GGTA1/CMAH KO erythrocytes to xenotransfusion. The observation that baboon sera was more reactive to GGTA1/CMAH KO erythrocytes as compared to GGTA1 KO alone suggests that that there are significant changes to the antigenic surface of the erythrocytes. We have no evidence whether these changes are in the extracellular proteins or carbohydrates. But since the genetic modifications were to the glyco-synthetic pathway, we hypothesize that modification of the surface sialic acid profile has created a species-specific glycosylation pattern that made baboons more immunoreacitve to the GGTA1/CMAH KO pigs. Given our understanding of the role of terminal sialic acid in erythrocyte phagocytosis, this may present a barrier in both baboons and humans (19). Xenogeneic cells may be phagocytosed by human macrophages in the spleen and liver (7, 9, 20). The variation in carbohydrate may not be the only complication. A well described incompatibility between human and pig CD47-signal regulatory protein a (SIRPa) signaling protein may predispose any pig erythrocyte to an undetermined amount of phagocytosis (21, 22). Our description here of antibody bound to pig erythrocytes may predispose xenogenic erythrocyte to be cleared from circulation the way many bacteria or foreign particles would be phagocytosed (9). We have made an initial step in characterizing GGTA1/CMAH KO pig erythrocytes for xenotransfusion but there remain several important cellular issues to address. Additionally, it may be necessary to express the human CD47 or SIRPa protein in the GGTA1/CMAH KO pigs using an erythrocyte specific promoter (e.g., glycophorin A, hemoglobin) to provide sufficient protection from xenogeneic erythrocyte phagocytosis. In lieu of a non-human primate model the use of NOD/SCID/gc null mice with a reconstituted human immune system bred on a GGTA1/CMAH KO genetic background would provide a suitable in vivo model to further address macrophage-mediated rejection of GGTA1/CMAH KO erythrocytes (18, 23, 24). The humanized NOD/SCID/gc null mice are well documented and given our recent success with the creation of a GGTA1/CMAH KO pig we believe this approach is possible.
In conclusion, removal of aGal and Neu5Gc provided further protection from human antibody-meditated rejection of pig erythrocytes. Given the success of complement depletion in the non-human primate model of xenotransfusion, the expression of human complement regulatory or thromboregulatory proteins in the GGTA1/CMAH KO pigs may provide sufficient protection from antibody-mediated injury bringing us another step closer to clinical xenotransfusion.
Acknowledgements
This study was supported by IU Health Transplant Institute.
Abbreviations
- AMR
antibody-mediated rejection
- CMAH
cytidine monophosphate-N-acetylneuraminic acid hydroxylase
- Gal
Gala-1,3-Gal
- GGTA1 KO
a1,3-galactosyltransferase knockout
- GGTA1/CMAH KO
a1,3-lalactosyltransferase and cytidine monophosphate-N-acetylneuraminic acid hydroxylase double knockout
- Neu5Gc
N-glycolylneuraminic acid
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate-buffered saline
- RBC
red blood cell
Footnotes
Author contributions:
Zheng-Yu Wang: Concept/design, data collection/analysis/interpretation, drafting manuscript and statistics.
Christopher Burlak: Concept/design, drafting and revision of manuscript
Jose L. Estrada: Production of genetically modified pigs by somatic cell nuclear transfer, in vitro fertilization and critical review of manuscript.
Ping Li: Development of genetically modified pig cells.
Matthew F. Tector: Concept/design, critical review of manuscript
A. Joseph Tector: Critical review and approval of manuscript and secured funding.
References
- 1.COOPER DK. Porcine red blood cells as a source of blood transfusion in humans. Xenotransplantation. 2003;10:384–386. doi: 10.1034/j.1399-3089.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 2.ROUX FA, SAI P, DESCHAMPS JY. Xenotransfusions, past and present. Xenotransplantation. 2007;14:208–216. doi: 10.1111/j.1399-3089.2007.00404.x. [DOI] [PubMed] [Google Scholar]
- 3.COOPER DK, HARA H, YAZER M. Genetically engineered pigs as a source for clinical red blood cell transfusion. Clinics in laboratory medicine. 2010;30:365–380. doi: 10.1016/j.cll.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 4.GOOD AH, COOPER DK, MALCOLM AJ, IPPOLITO RM, KOREN E, NEETHLING FA, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplantation proceedings. 1992;24:559–562. [PubMed] [Google Scholar]
- 5.LI P, ESTRADA JL, BURLAK C, TECTOR AJ. Biallelic knockout of the alpha-1,3 galactosyltransferase gene in porcine liver-derived cells using zinc finger nucleases. The Journal of surgical research. 2013;181:e39–45. doi: 10.1016/j.jss.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 6.ZHU A, HURST R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 7.ECKERMANN JM, BUHLER LH, ZHU A, DOR FJ, AWWAD M, COOPER DK. Initial investigation of the potential of modified porcine erythrocytes for transfusion in primates. Xenotransplantation. 2004;11:18–26. doi: 10.1111/j.1399-3089.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- 8.ROUHANI FJ, DOR FJ, COOPER DK. Investigation of red blood cells from alpha1,3-galactosyltransferase-knockout pigs for human blood transfusion. Transfusion. 2004;44:1004–1012. doi: 10.1111/j.1537-2995.2004.04002.x. [DOI] [PubMed] [Google Scholar]
- 9.LONG C, HARA H, PAWLIKOWSKI Z, KOIKE N, D'ARVILLE T, YEH P, et al. Genetically engineered pig red blood cells for clinical transfusion: initial in vitro studies. Transfusion. 2009;49:2418–2429. doi: 10.1111/j.1537-2995.2009.02306.x. [DOI] [PubMed] [Google Scholar]
- 10.VARKI A. Loss of N-glycolylneuraminic acid in humans: Mechanisms, consequences, and implications for hominid evolution. American journal of physical anthropology. 2001;(Suppl 33):54–69. doi: 10.1002/ajpa.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MUCHMORE EA, Diaz S, Varki A. A structural difference between the cell surfaces of humans and the great apes. Amer. J. Physical Anthropology. 1998;107:87–198. doi: 10.1002/(SICI)1096-8644(199810)107:2<187::AID-AJPA5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.ZHU A, HURST R. Human natural antibodies that recognize nonalphaGal antigens on porcine red blood cells. Transplantation proceedings. 2000;32:872–873. doi: 10.1016/s0041-1345(00)01018-6. [DOI] [PubMed] [Google Scholar]
- 13.LUTZ AJ, LI P, ESTRADA JL, SIDNER RA, CHIHARA RK, DOWNEY SM, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and Galactose alpha-1,3-Galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]
- 14.VARKI A. N-glycolylneuraminic acid deficiency in humans. Biochimie. 2001;83:615–622. doi: 10.1016/s0300-9084(01)01309-8. [DOI] [PubMed] [Google Scholar]
- 15.BURLAK C, WANG ZY, CHIHARA RK, LUTZ AJ, WANG Y, ESTRADA JL, et al. Identification of human preformed antibody targets in GTKO pigs. Xenotransplantation. 2012;19:92–101. doi: 10.1111/j.1399-3089.2012.00695.x. [DOI] [PubMed] [Google Scholar]
- 16.TANGVORANUNTAKUL P, GAGNEUX P, DIAZ S, BARDOR M, VARKI N, VARKI A, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CHEN G, QIAN H, STARZL T, SUN H, GARCIA B, WANG X, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nature medicine. 2005;11:1295–1298. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.BASNET NB, IDE K, TAHARA H, TANAKA Y, OHDAN H. Deficiency of N-glycolylneuraminic acid and Galalpha1-3Galbeta1-4GlcNAc epitopes in xenogeneic cells attenuates cytotoxicity of human natural antibodies. Xenotransplantation. 2010;17:440–448. doi: 10.1111/j.1399-3089.2010.00610.x. [DOI] [PubMed] [Google Scholar]
- 19.BROCK LG, DELPUTTE PL, WALDMAN JP, NAUWYNCK HJ, REES MA. Porcine sialoadhesin: a newly identified xenogeneic innate immune receptor. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12:3272–3282. doi: 10.1111/j.1600-6143.2012.04247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.REES MA, BUTLER AJ, BRONS IG, NEGUS MC, SKEPPER JN, FRIEND PJ. Evidence of macrophage receptors capable of direct recognition of xenogeneic epitopes without opsonization. Xenotransplantation. 2005;12:13–19. doi: 10.1111/j.1399-3089.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 21.HU Z, VAN ROOIJEN N, YANG YG. Macrophages prevent human red blood cell reconstitution in immunodeficient mice. Blood. 2011;118:5938–5946. doi: 10.1182/blood-2010-11-321414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.IDE K, OHDAN H, KOBAYASHI T, HARA H, ISHIYAMA K, ASAHARA T. Antibody- and complement-independent phagocytotic and cytolytic activities of human macrophages toward porcine cells. Xenotransplantation. 2005;12:181–188. doi: 10.1111/j.1399-3089.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 23.KING M, PEARSON T, SHULTZ LD, LEIF J, BOTTINO R, TRUCCO M, et al. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clinical immunology. 2008;126:303–314. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 24.ITO M, HIRAMATSU H, KOBAYASHI K, SUZUE K, KAWAHATA M, HIOKI K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]