Abstract
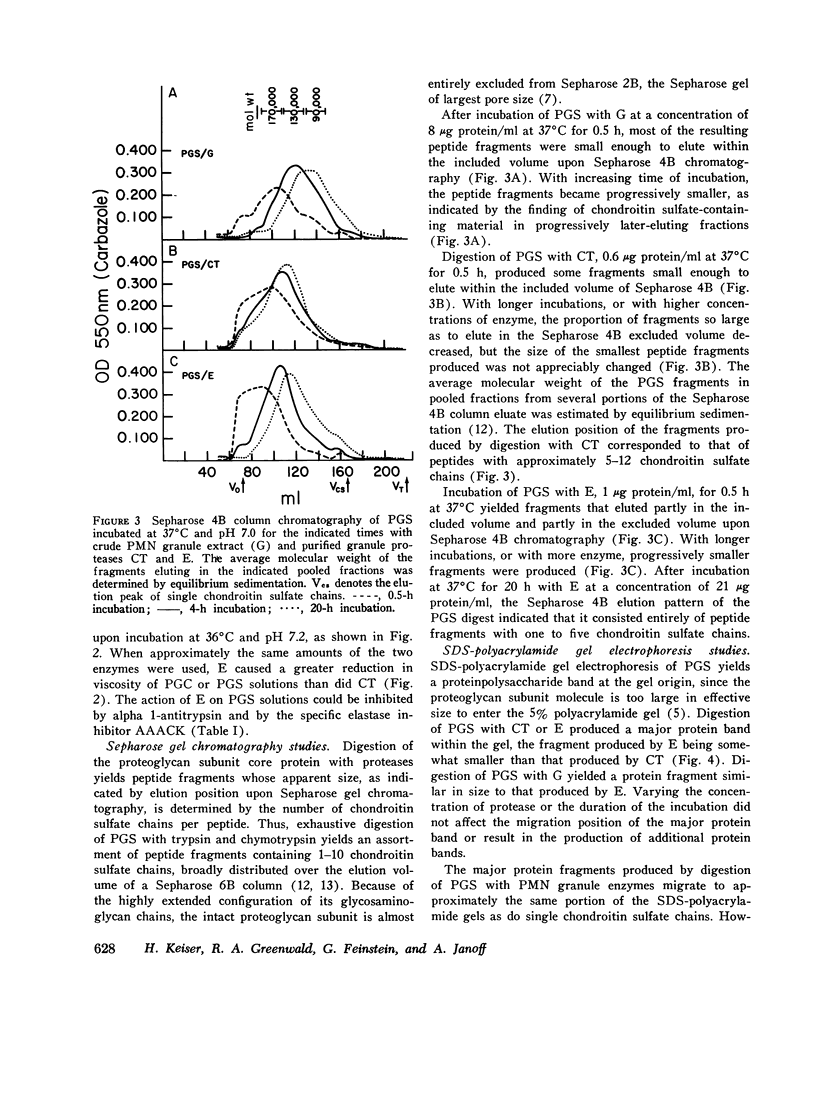
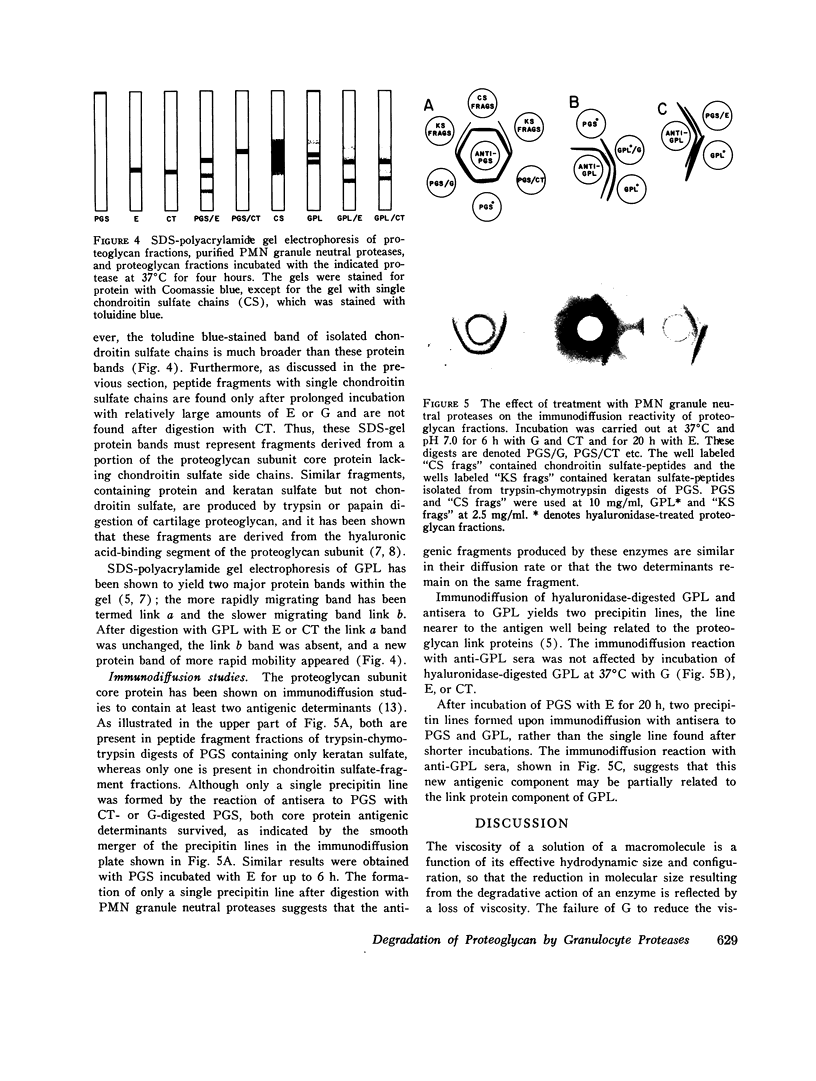
Extracts of human peripheral blood polymorphonuclear leukocyte granules, and two purified proteases derived from such extracts, an elastase and a chymotrypsin-like enzyme, degrade isolated bovine nasal cartilage proteoglycan at neutral pH. Viscosity studies indicate that the leukocyte granule extracts lack hyaluronidase activity and that their degradative effect on proteoglycan at physiological pH is due entirely to proteolytic action. Sepharose 4B gel chromatography and SDS-polyacrylamide gel electrophoresis of proteoglycan fractions treated with leukocyte granule enzymes at pH 7.0 indicate that they degrade one of the proteoglycan link proteins, release a fragment from the hyaluronic acid-binding portion of the proteoglycan subunit core protein, and break down the remainder of the proteoglycan subunit molecule into peptide fragments with varying numbers of chondroitin sulfate chains. Immunodiffusion studies indicate that the antigenic determinants of the proteoglycan subunit core protein and the link proteins survive treatment with granule proteases. Similar degradation of human articular cartilage proteoglycan by granule neutral proteases can be presumed to occur, in view of the similarity of structure of human articular and bovine nasal cartilage proteoglycans. The release of granule enzymes in the course of neutrophil-mediated inflammation can thus result in the degradation of cartilage matrix proteoglycan, leading to cartilage destruction and joint injury.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Davidson E. A. Lysosomal hyaluronidase from rat liver. II. Properties. J Biol Chem. 1967 Feb 10;242(3):441–444. [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- BOLLET A. J., BONNER W. M., Jr, NANCE J. L. THE PRESENCE OF HYALURONIDASE IN VARIOUS MAMMALIAN TISSUES. J Biol Chem. 1963 Nov;238:3522–3527. [PubMed] [Google Scholar]
- Feinstein G., Janoff A. A rapid method for purification of human granulocyte cationic neutral proteases: purification and characterization of human granulocyte chymotrypsin-like enzyme. Biochim Biophys Acta. 1975 Oct 22;403(2):477–492. doi: 10.1016/0005-2744(75)90076-5. [DOI] [PubMed] [Google Scholar]
- Feinstein G., Janoff A. A rapid method of purification of human granulocyte cationic neutral proteases: purification and further characterization of human granulocyte elastase. Biochim Biophys Acta. 1975 Oct 22;403(2):493–505. doi: 10.1016/0005-2744(75)90077-7. [DOI] [PubMed] [Google Scholar]
- Gregory J. D. Multiple aggregation factors in cartilage proteoglycan. Biochem J. 1973 Jun;133(2):383–386. doi: 10.1042/bj1330383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Characterization of chondroitin sulfate isolated from trypsin-chymotrypsin digests of cartilage proteoglycans. Arch Biochem Biophys. 1974 Nov;165(1):427–441. doi: 10.1016/0003-9861(74)90182-9. [DOI] [PubMed] [Google Scholar]
- Herman J. H., Dennis M. V. Immunopathologic studies in relapsing polychondritis. J Clin Invest. 1973 Mar;52(3):549–558. doi: 10.1172/JCI107215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J. H., Wiltse D. W., Dennis M. V. Immunopathologic significance of cartilage antigenic components in rheumatoid arthritis. Arthritis Rheum. 1973 May-Jun;16(3):287–297. doi: 10.1002/art.1780160302. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Robinson H. C. The structure and composition of cartilage keratan sulphate. Biochem J. 1974 Aug;141(2):517–526. doi: 10.1042/bj1410517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Feinstein G., Malemud C. J., Elias J. M. Degradation of cartilage proteoglycan by human leukocyte granule neutral proteases--a model of joint injury. I. Penetration of enzyme into rabbit articular cartilage and release of 35SO4-labeled material from the tissue. J Clin Invest. 1976 Mar;57(3):615–624. doi: 10.1172/JCI108317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Scherer J. Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968 Nov 1;128(5):1137–1155. doi: 10.1084/jem.128.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser H., DeVito J. Immunochemical studies of fragments of bovine nasal cartilage proteoglycan subunit. Connect Tissue Res. 1974;2(4):273–282. doi: 10.3109/03008207409152256. [DOI] [PubMed] [Google Scholar]
- Keiser H. Immunological studies of bovine nasal cartilage proteoglycan "link proteins". Biochemistry. 1975 Dec 2;14(24):5304–5307. doi: 10.1021/bi00695a012. [DOI] [PubMed] [Google Scholar]
- Keiser H. Immunological studies of the fragments of bovine cartilage proteoglycan produced by chondroitinase-trypsin digestion. Arch Biochem Biophys. 1975 Jun;168(2):622–629. doi: 10.1016/0003-9861(75)90294-5. [DOI] [PubMed] [Google Scholar]
- Keiser H., Sandson J. I. Immunodiffusion and gel-electrophoretic studies of human articular cartilage proteoglycan. Arthritis Rheum. 1974 May-Jun;17(3):219–228. doi: 10.1002/art.1780170304. [DOI] [PubMed] [Google Scholar]
- Keiser H., Shulman H. J., Sandson J. I. Immunochemistry of cartilage proteoglycan. Immunodiffusion and gel-electrophoretic studies. Biochem J. 1972 Jan;126(1):163–169. doi: 10.1042/bj1260163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus G. S., Daniels J. R., Lian J., Burleigh M. C. Role of granulocyte collagenase in collagen degradation. Am J Pathol. 1972 Sep;68(3):565–578. [PMC free article] [PubMed] [Google Scholar]
- Lotke P. A., Granda J. L. Alterations in the permeability of articular cartilage by proteolytic enzymes. Arthritis Rheum. 1972 May-Jun;15(3):302–308. doi: 10.1002/art.1780150312. [DOI] [PubMed] [Google Scholar]
- Mankin H. J. Biochemical and metabolic abnormalities in osteoarthritic human cartilage. Fed Proc. 1973 Apr;32(4):1478–1480. [PubMed] [Google Scholar]
- Neil G. L., Niemann C., Hein G. E. Structural specificity of alpha-chymotrypsin: polypeptide substrates. Nature. 1966 May 28;210(5039):903–907. doi: 10.1038/210903a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Electron microscopic studies of proteoglycan aggregates from bovine articular cartilage. J Biol Chem. 1975 Mar 10;250(5):1877–1883. [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Macromolecular models of proteinpolysaccharides from bovine nasal cartilage based on electron microscopic studies. J Biol Chem. 1970 Aug 25;245(16):4123–4130. [PubMed] [Google Scholar]
- SCHUBERT M. INTERCELLULAR MACROMOLECULES CONTAINING POLYSACCHARIDES. Biophys J. 1964 Jan;4:SUPPL119–SUPPL138. doi: 10.1016/s0006-3495(64)86933-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Treuhaft P. S., MCCarty D. J. Synovial fluid pH, lactate, oxygen and carbon dioxide partial pressure in various joint diseases. Arthritis Rheum. 1971 Jul-Aug;14(4):475–484. doi: 10.1002/art.1780140407. [DOI] [PubMed] [Google Scholar]