Abstract
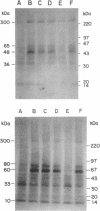
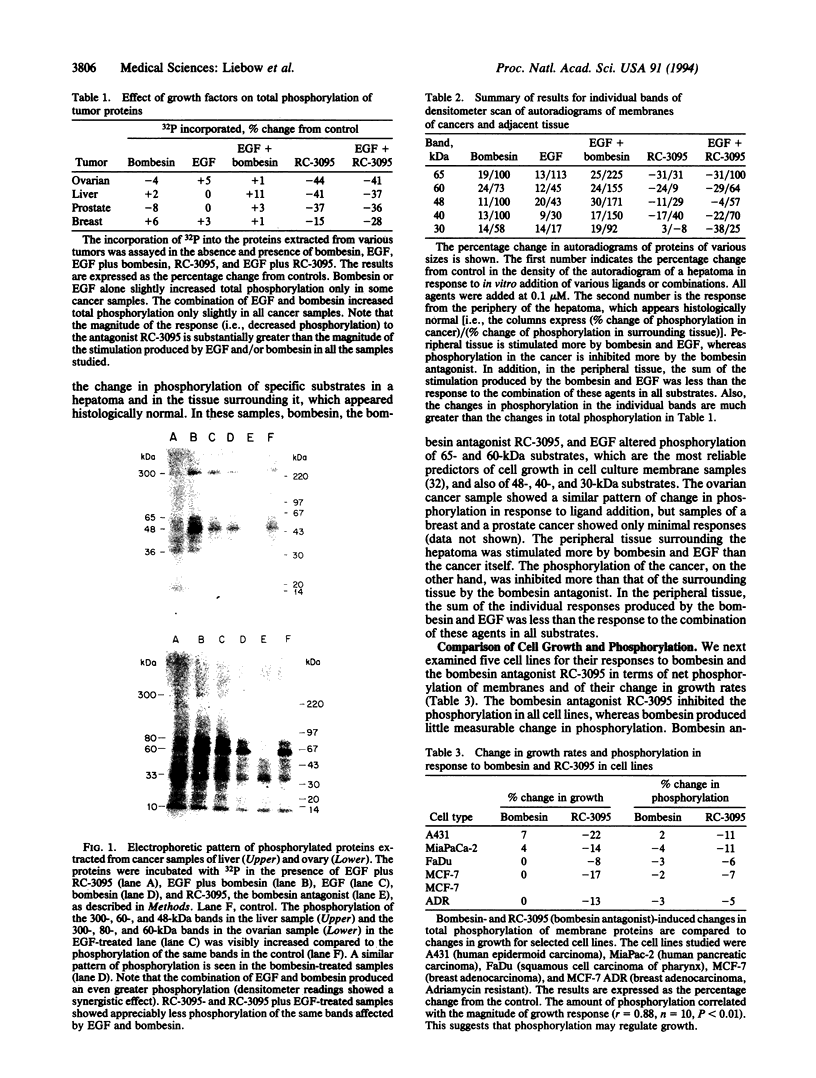
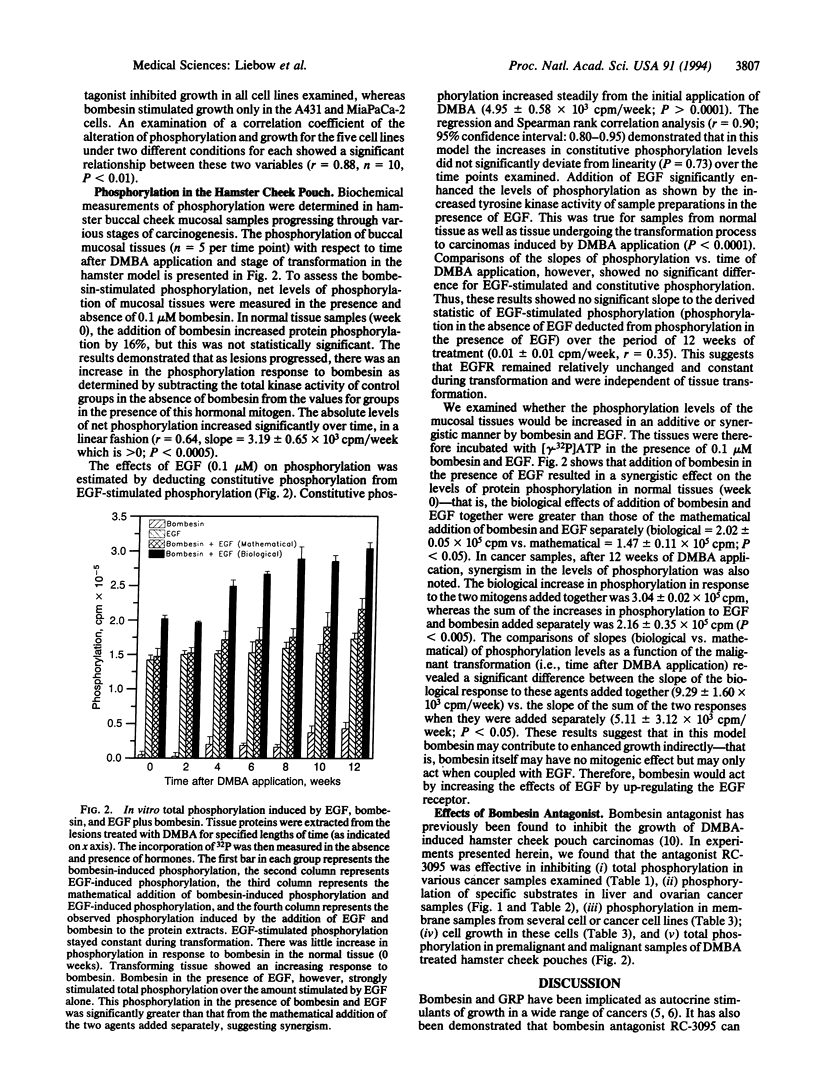
Bombesin and gastrin-releasing peptide act as autocrine mitogens in various cancers. Bombesin antagonist RC-3095 inhibited growth in some cancers and slowed the progression of premalignant lesions, possibly by down-regulating epidermal growth factor (EGF) receptors. Since the EGF receptor mitogen response involves tyrosine kinase stimulation, we tested the hypotheses that bombesin stimulates, and RC-3095 inhibits, phosphorylation; EGF and bombesin promote the phosphorylation of the same substrates; and EGF and bombesin act synergistically on phosphorylation. Therefore, in vitro assays for phosphorylation were performed in the presence or absence of EGF, bombesin, RC-3095, and combinations in samples derived from tumor, tissue surrounding tumor, cell lines, and normal and transforming tissue derived from the 9,10-dimethyl-1,2-benzanthracene-induced squamous cell lesions of the hamster cheek pouch. Bombesin increased, and RC-3095 decreased, phosphorylation in these samples. In the human hepatoma sample and surrounding tissue, these ligands altered the phosphorylation of the same substrates affected by EGF. EGF and bombesin stimulated phosphorylation synergistically in the hamster samples and the hepatoma. Bombesin-induced phosphorylation was greater in tissue surrounding the hepatoma, whereas RC-3095 was more effective in inhibiting phosphorylation in the hepatoma itself. This cancer, therefore, could be endogenously stimulated by gastrin-releasing peptide. These observations support the hypothesis that bombesin stimulates growth of tissues and tumors by amplifying the phosphorylation response to EGF. The growth inhibitory response to RC-3095, or other bombesin analogues, of individual tumors may be prognosed by in vitro phosphorylation assays using the samples from the patient's tumor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowen S., Stanley K., Selva E., Davis R. J. Constitutive phosphorylation of the epidermal growth factor receptor blocks mitogenic signal transduction. J Biol Chem. 1991 Jan 15;266(2):1162–1169. [PubMed] [Google Scholar]
- Brown M. R., Carver K., Fisher L. A. Bombesin: central nervous system actions to affect the autonomic nervous system. Ann N Y Acad Sci. 1988;547:174–182. doi: 10.1111/j.1749-6632.1988.tb23885.x. [DOI] [PubMed] [Google Scholar]
- Carney D. N., Cuttitta F., Moody T. W., Minna J. D. Selective stimulation of small cell lung cancer clonal growth by bombesin and gastrin-releasing peptide. Cancer Res. 1987 Feb 1;47(3):821–825. [PubMed] [Google Scholar]
- Cohen S., Ushiro H., Stoscheck C., Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982 Feb 10;257(3):1523–1531. [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Denner L. A., Schrader W. T., O'Malley B. W., Weigel N. L. Hormonal regulation and identification of chicken progesterone receptor phosphorylation sites. J Biol Chem. 1990 Sep 25;265(27):16548–16555. [PubMed] [Google Scholar]
- Denner L. A., Weigel N. L., Maxwell B. L., Schrader W. T., O'Malley B. W. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990 Dec 21;250(4988):1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- Driscoll W. J., Lee Y. C., Strott C. A. Regulation of adrenocortical pregnenolone-binding protein activity by phosphorylation/dephosphorylation. Phosphatase-mediated inactivation is reversed by cytosolic kinase. J Biol Chem. 1990 Jul 25;265(21):12306–12311. [PubMed] [Google Scholar]
- Gill G. N. Regulation of EGF receptor expression and function. Mol Reprod Dev. 1990 Sep;27(1):46–53. doi: 10.1002/mrd.1080270110. [DOI] [PubMed] [Google Scholar]
- Harvey S., Baidwan J. S., Attardo D. Homologous and heterologous regulation of somatostatin-binding sites on chicken adenohypophysial membranes. J Endocrinol. 1990 Dec;127(3):417–425. doi: 10.1677/joe.0.1270417. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lammers R., Van Obberghen E., Ballotti R., Schlessinger J., Ullrich A. Transphosphorylation as a possible mechanism for insulin and epidermal growth factor receptor activation. J Biol Chem. 1990 Oct 5;265(28):16886–16890. [PubMed] [Google Scholar]
- Lee M. T., Liebow C., Kamer A. R., Schally A. V. Effects of epidermal growth factor and analogues of luteinizing hormone-releasing hormone and somatostatin on phosphorylation and dephosphorylation of tyrosine residues of specific protein substrates in various tumors. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1656–1660. doi: 10.1073/pnas.88.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebow C., Crean D. H., Schally A. V., Mang T. S. Peptide analogues alter the progression of premalignant lesions, as measured by Photofrin fluorescence. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1897–1901. doi: 10.1073/pnas.90.5.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebow C., Hierowski M., duSapin K. Hormonal control of pancreatic cancer growth. Pancreas. 1986;1(1):44–48. doi: 10.1097/00006676-198601000-00009. [DOI] [PubMed] [Google Scholar]
- Liebow C., Lee M. T., Kamer A. R., Schally A. V. Regulation of luteinizing hormone-releasing hormone receptor binding by heterologous and autologous receptor-stimulated tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2244–2248. doi: 10.1073/pnas.88.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. R., Chen W. S., Lazar C. S., Carpenter C. D., Gill G. N., Evans R. M., Rosenfeld M. G. Protein kinase C phosphorylation at Thr 654 of the unoccupied EGF receptor and EGF binding regulate functional receptor loss by independent mechanisms. Cell. 1986 Mar 28;44(6):839–848. doi: 10.1016/0092-8674(86)90006-1. [DOI] [PubMed] [Google Scholar]
- Mahmoud S., Palaszynski E., Fiskum G., Coy D. H., Moody T. W. Small cell lung cancer bombesin receptors are antagonized by reduced peptide bond analogues. Life Sci. 1989;44(5):367–373. doi: 10.1016/0024-3205(89)90231-2. [DOI] [PubMed] [Google Scholar]
- Milovanovic S. R., Radulovic S., Groot K., Schally A. V. Inhibition of growth of PC-82 human prostate cancer line xenografts in nude mice by bombesin antagonist RC-3095 or combination of agonist [D-Trp6]-luteinizing hormone-releasing hormone and somatostatin analog RC-160. Prostate. 1992;20(4):269–280. doi: 10.1002/pros.2990200403. [DOI] [PubMed] [Google Scholar]
- Moss J. A., Francis G. L., Ross M., Wallace J. C., Ballard F. J. Insulin-like growth factor (IGF)-I and IGF-II binding to an IGF binding protein. An investigation using chemical modification of tyrosine residues as a structural probe for the sites of interaction. J Biol Chem. 1991 Jan 15;266(2):909–914. [PubMed] [Google Scholar]
- Radulovic S., Cai R. Z., Serfozo P., Groot K., Redding T. W., Pinski J., Schally A. V. Biological effects and receptor binding affinities of new pseudononapeptide bombesin/GRP receptor antagonists with N-terminal D-Trp or D-Tpi. Int J Pept Protein Res. 1991 Dec;38(6):593–600. doi: 10.1111/j.1399-3011.1991.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Radulovic S., Miller G., Schally A. V. Inhibition of growth of HT-29 human colon cancer xenografts in nude mice by treatment with bombesin/gastrin releasing peptide antagonist (RC-3095). Cancer Res. 1991 Nov 1;51(21):6006–6009. [PubMed] [Google Scholar]
- Rosen H., Dalkin A., Haisenleder D., Friberg R. D., Ortolano G., Barkan A. Dexamethasone alters responses of pituitary gonadotropin-releasing hormone (GnRH) receptors, gonadotropin subunit messenger ribonucleic acids, and gonadotropins to pulsatile GnRH in male rats. Endocrinology. 1991 Feb;128(2):654–660. doi: 10.1210/endo-128-2-654. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALLEY J. J. Experimental carcinogenesis in the cheek pouch of the Syrian hamster. J Dent Res. 1954 Apr;33(2):253–262. doi: 10.1177/00220345540330021201. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Allosteric regulation of the epidermal growth factor receptor kinase. J Cell Biol. 1986 Dec;103(6 Pt 1):2067–2072. doi: 10.1083/jcb.103.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szepeshazi K., Schally A. V., Cai R. Z., Radulovic S., Milovanovic S., Szoke B. Inhibitory effect of bombesin/gastrin-releasing peptide antagonist RC-3095 and high dose of somatostatin analogue RC-160 on nitrosamine-induced pancreatic cancers in hamsters. Cancer Res. 1991 Nov 1;51(21):5980–5986. [PubMed] [Google Scholar]
- Szepeshazi K., Schally A. V., Groot K., Halmos G. Effect of bombesin, gastrin-releasing peptide (GRP)(14-27) and bombesin/GRP receptor antagonist RC-3095 on growth of nitrosamine-induced pancreatic cancers in hamsters. Int J Cancer. 1993 May 8;54(2):282–289. doi: 10.1002/ijc.2910540220. [DOI] [PubMed] [Google Scholar]
- Szepeshazi K., Schally A. V., Halmos G., Groot K., Radulovic S. Growth inhibition of estrogen-dependent and estrogen-independent MXT mammary cancers in mice by the bombesin and gastrin-releasing peptide antagonist RC-3095. J Natl Cancer Inst. 1992 Dec 16;84(24):1915–1922. doi: 10.1093/jnci/84.24.1915. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey J. C., Lechner J. F., Harris C. C. Bombesin and the C-terminal tetradecapeptide of gastrin-releasing peptide are growth factors for normal human bronchial epithelial cells. Exp Cell Res. 1984 Jul;153(1):245–248. doi: 10.1016/0014-4827(84)90466-x. [DOI] [PubMed] [Google Scholar]
- Yano T., Pinski J., Groot K., Schally A. V. Stimulation by bombesin and inhibition by bombesin/gastrin-releasing peptide antagonist RC-3095 of growth of human breast cancer cell lines. Cancer Res. 1992 Aug 15;52(16):4545–4547. [PubMed] [Google Scholar]
- Yano T., Pinski J., Szepeshazi K., Halmos G., Radulovic S., Groot K., Schally A. V. Inhibitory effect of bombesin/gastrin-releasing peptide antagonist RC-3095 and luteinizing hormone-releasing hormone antagonist SB-75 on the growth of MCF-7 MIII human breast cancer xenografts in athymic nude mice. Cancer. 1994 Feb 15;73(4):1229–1238. doi: 10.1002/1097-0142(19940215)73:4<1229::aid-cncr2820730417>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]