Abstract
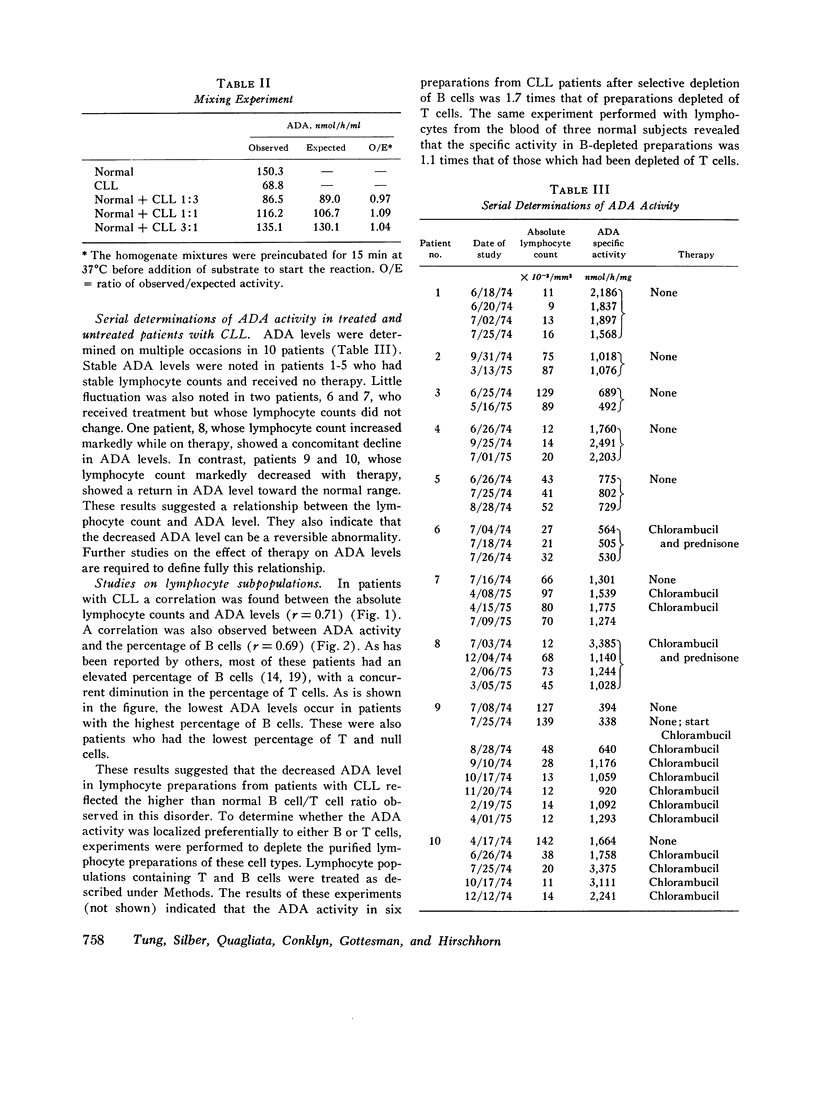
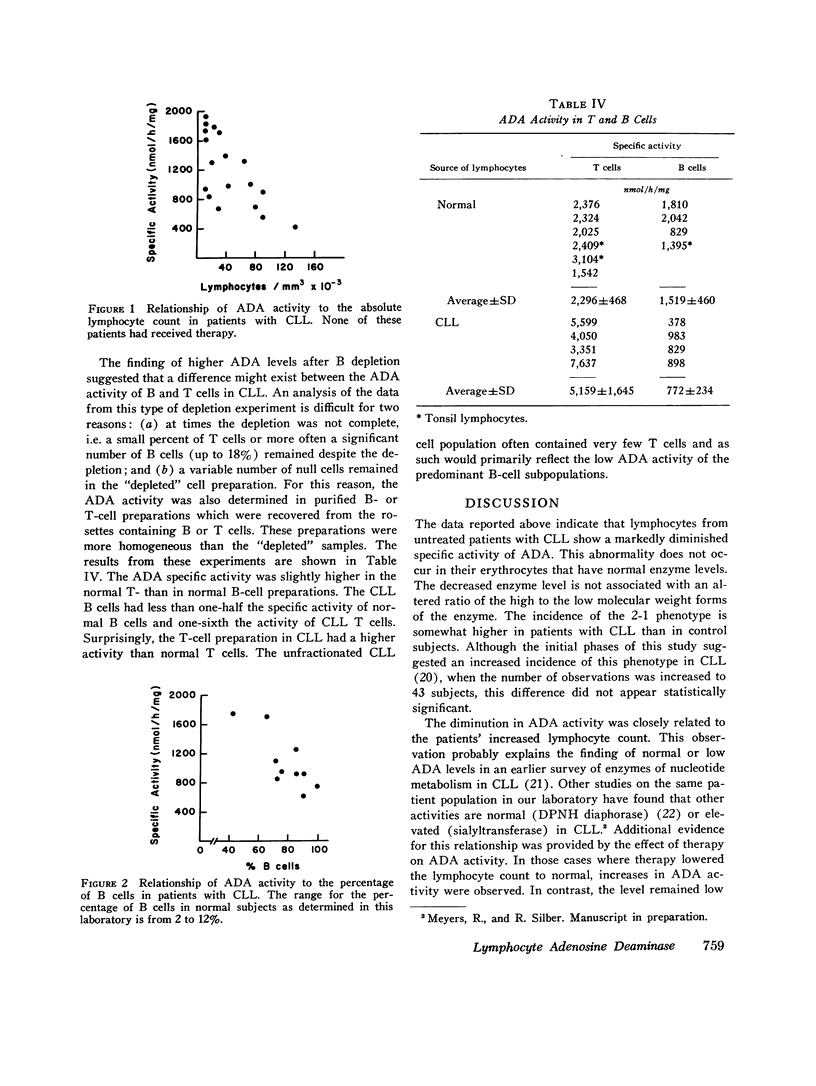
The level, phenotypes, and isozyme distribution of adenosine deaminase (ADA) were determined in lymphocytes from patients with chronic lymphocytic leukemia (CLL). The ADA level in lymphocytes from patients with untreated CLL was consistently lower than in lymphocytes from normal subjects. No significant differences were found in the phenotype or isozyme distribution. In untreated patients, the ADA level was inversely correlated with the lymphocyte count and the percentage of bursa-equivalent (B) cells. After therapy, a diminution in the lymphocyte count was associated with an increase of ADA activity towards normal levels. The ADA levels were slightly higher in the thymus-derived (T) than in the B lymphocytes from normal subjects. The B cells had lower activity than T cells in patients with CLL. They also had a lower activity than the B cells from normal subjects. The ADA level was 2.3-fold higher in T cells from patients with CLL than in normal T cells. The decrease in ADA levels is an anomaly that is reversible and appears to be a reflection of the proliferation of abnormal B cells in this disorder.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battistuzzi G., Scozzari R., Santolamazza P., Terrenato L., Modiano G. Comparative activity of red cell adenosine deaminase allelic forms. Nature. 1974 Oct 25;251(5477):711–713. doi: 10.1038/251711a0. [DOI] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome J. D., Zucker-Franklin D., Weiner M. S., Bianco C., Nussenzweig V. Leukemic cells with membrane properties of thymus-derived (T) lymphocytes in a case of Sézary's syndrome: morphologic and immunologic studies. Clin Immunol Immunopathol. 1973 Apr;1(3):319–329. doi: 10.1016/0090-1229(73)90049-4. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Edwards Y. H., Hopkinson D. A., Harris H. Adenosine deaminase isozymes in human tissues. Ann Hum Genet. 1971 Oct;35(2):207–219. doi: 10.1111/j.1469-1809.1956.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Levytska V. Alterations in isozymes of adenosine deaminase during stimulation of human peripheral blood lymphocytes. Cell Immunol. 1974 Jun;12(3):387–395. doi: 10.1016/0008-8749(74)90095-1. [DOI] [PubMed] [Google Scholar]
- Hopkinson D. A., Cook P. J., Harris H. Further data on the adenosine deaminase (ADA) polymprphism and a report of a new phenotype. Ann Hum Genet. 1969 May;32(4):361–367. doi: 10.1111/j.1469-1809.1969.tb00087.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lopes J., Nachbar M., Zucker-Franklin D., Silber R. Lymphocyte plasma membranes: analysis of proteins and glycoproteins by SDS-gel electrophoresis. Blood. 1973 Jan;41(1):131–140. [PubMed] [Google Scholar]
- Lopes J., Zucker-Franklin D., Silber R. Heterogeneity of 5'-nucleotidase activity in lymphocytes in chronic lymphocytic leukemia. J Clin Invest. 1973 May;52(5):1297–1300. doi: 10.1172/JCI107298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkman R., Gelfand E. W., Rosen F. S., Sanderson A., Hirschhorn R. Severe combined immunodeficiency and adenosine deaminase deficiency. N Engl J Med. 1975 Apr 3;292(14):714–719. doi: 10.1056/NEJM197504032921402. [DOI] [PubMed] [Google Scholar]
- Pincus S., Bianco C., Nussenzweig V. Increased proportion of complement-receptor lymphocytes in the peripheral blood of patients with chronic lymphocytic leukemia. Blood. 1972 Sep;40(3):303–310. [PubMed] [Google Scholar]
- Polmar S. H., Wetzler E. M., Stern R. C., Hirschhorn R. Restoration of in-vitro lymphocyte responses with exogenous adenosine deaminase in a patient with severe combined immunodeficiency. Lancet. 1975 Oct 18;2(7938):743–746. doi: 10.1016/s0140-6736(75)90726-6. [DOI] [PubMed] [Google Scholar]
- Quagliata F., Faig D., Conklyn M., Silber R. Studies on the lymphocyte 5'-nucleotidase in chronic lymphocytic leukemia, infectious mononucleosis, normal subpopulations, and phytohemagglutinin-stimulated cells. Cancer Res. 1974 Dec;34(12):3197–3202. [PubMed] [Google Scholar]
- RUNDLES R. W., COONRAD E. V., ARENDS T. Serum proteins in leukemia. Am J Med. 1954 Jun;16(6):842–853. doi: 10.1016/0002-9343(54)90449-0. [DOI] [PubMed] [Google Scholar]
- Richards F., 2nd, Spurr C. L., Pajak T. F., Blake D. D., Raben M. Thymic irradiation. An approach to chronic lymphocytic leukemia. Am J Med. 1974 Dec;57(6):862–869. doi: 10.1016/0002-9343(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Rubin A. D., Havemann K., Dameshek W. Studies in chronic lymphocytic leukemia: further studies of the proliferative abnormality of the blood lymphocyte. Blood. 1969 Feb;33(2):313–328. [PubMed] [Google Scholar]
- Scholar E. M., Calabresi P. Identification of the enzymatic pathways of nucleotide metabolism in human lymphocytes and leukemia cells. Cancer Res. 1973 Jan;33(1):94–103. [PubMed] [Google Scholar]
- Shevach E. M., Herberman R., Frank M. M., Green I. Receptors for complement and immunoglobulin on human leukemic cells and human lymphoblastoid cell lines. J Clin Invest. 1972 Aug;51(8):1933–1938. doi: 10.1172/JCI106999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth J. F., Harrap K. R. Adenosine deaminase activity in leukaemia. Br J Cancer. 1975 May;31(5):544–549. doi: 10.1038/bjc.1975.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]
- Zimmer J., Khalifa A. S., Lightbody J. J. Decreased lymphocyte adenosine deaminase activity in acute lymphocytic leukemia children and their parents. Cancer Res. 1975 Jan;35(1):68–70. [PubMed] [Google Scholar]


