Abstract
Human peripheral blood lymphocytes grown in vitro were stimulated with nonspecific mitogens and in mixed lymphocyte culture. The presence of IgM and thymus (T) surface markers on large and small lymphocytes was investigated by immunofluorescence and correlated with spontaneous rosette formation. All stimulated large lymphocytes formed spontaneous rosettes and all except pokeweed mitogen (PWM)-stimulated large lymphocytes had IgM and T markers. IgG, IgA, and light chain determinants were only detected on PWM-induced large lymphocytes. Thus, surface markers expressed on activated human lymphocytes may differ for different mitogens. IgM was always present on large cells which had the T markers, and it cannot be used to identify a lymphocyte as a bone marrow-derived (B) cell. Due to the overlap of surface markers, the classification into B and thymus-derived (T) cells ought to be restricted to functional phenomena of antibody-production or cell-mediated immunity.
Full text
PDF


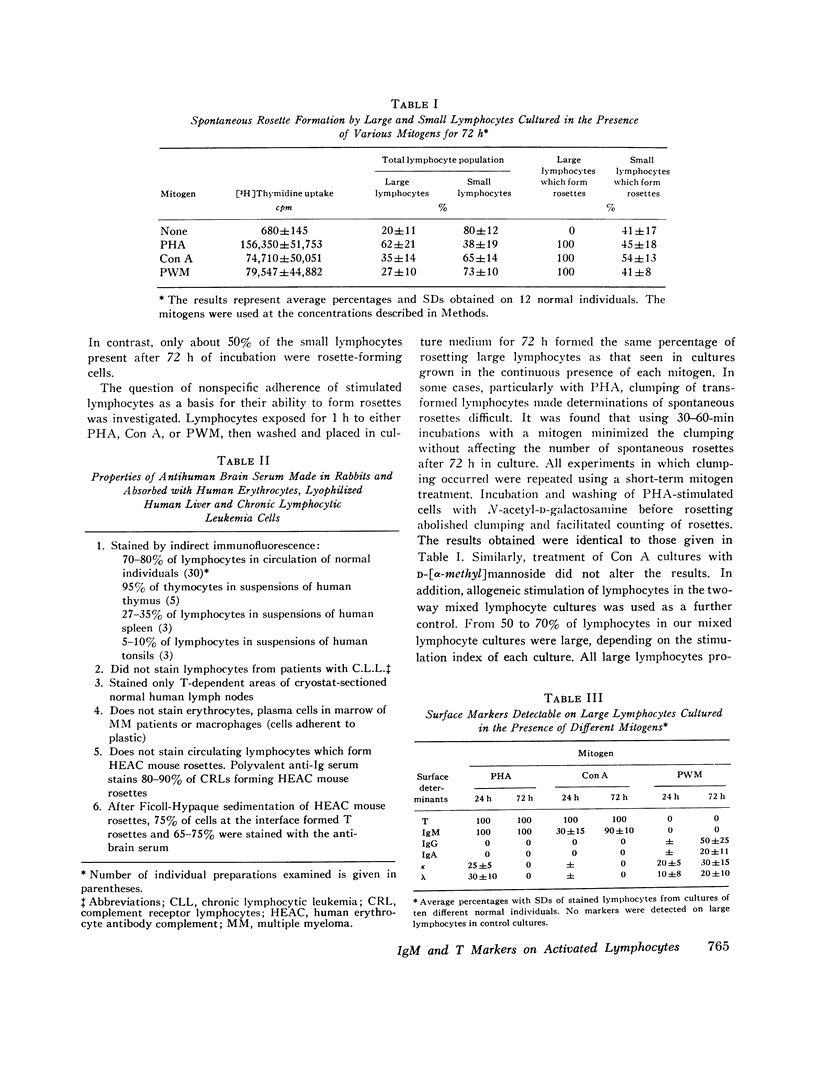
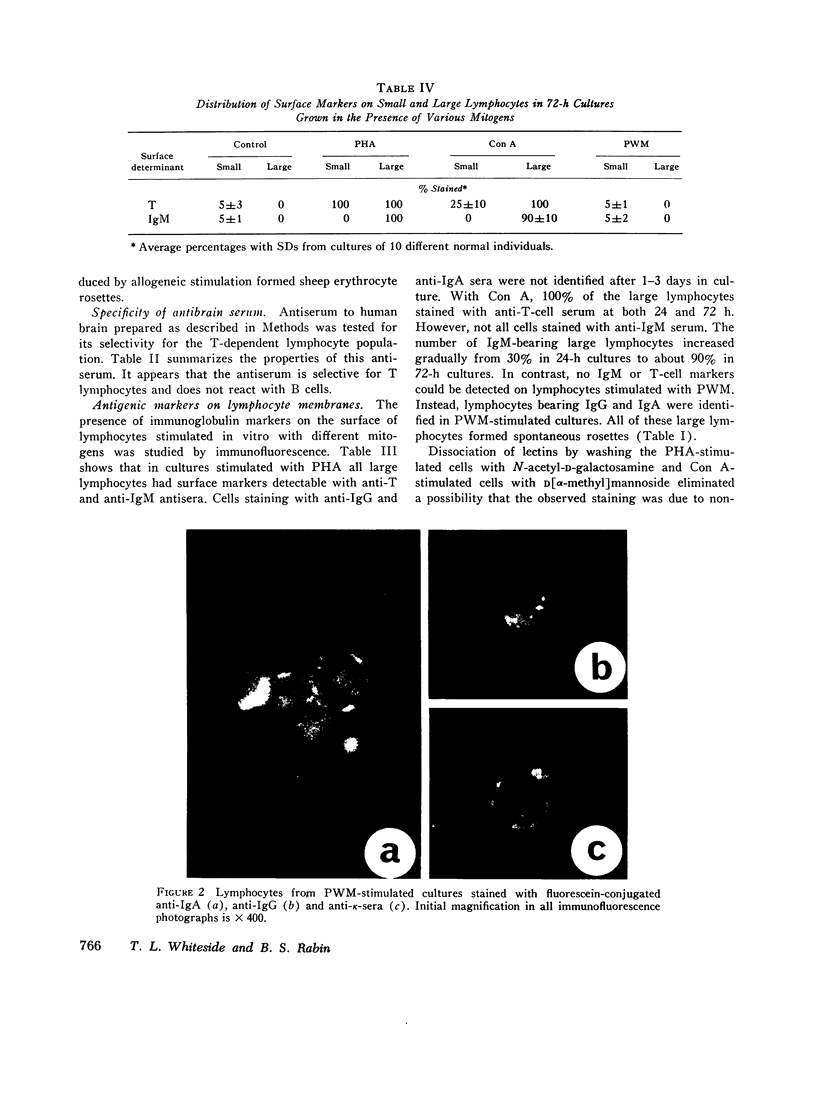
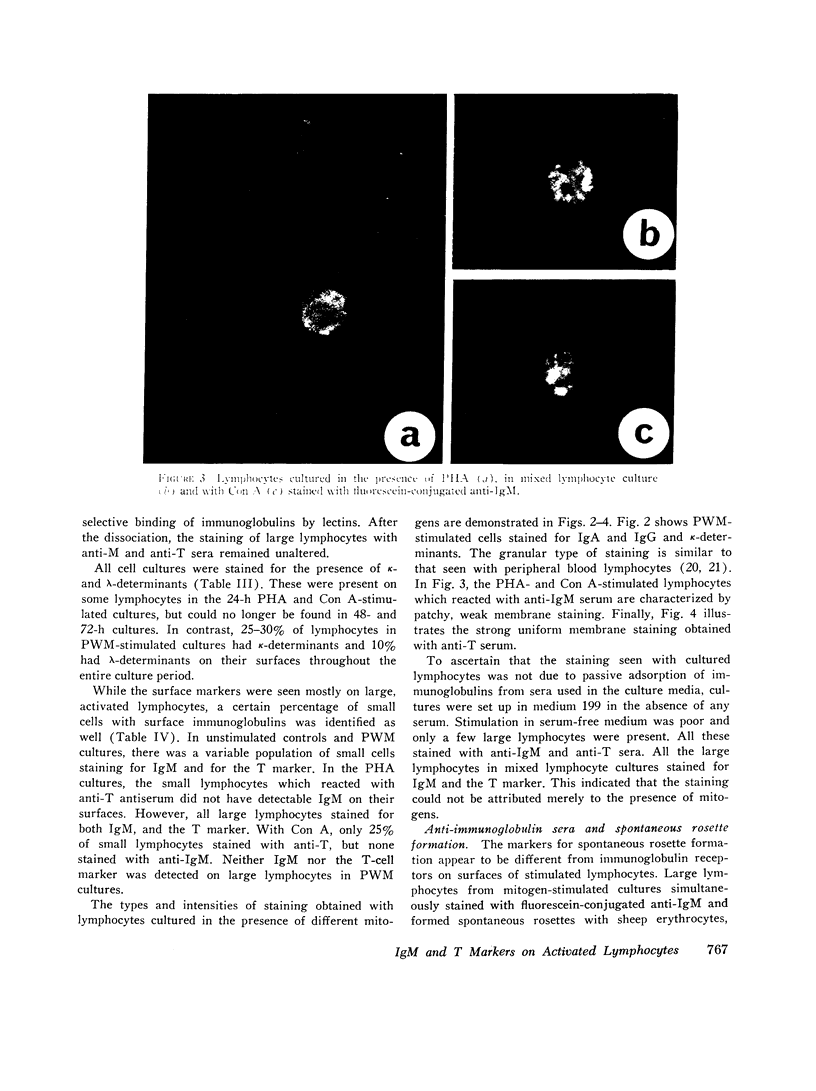




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentwich Z., Douglas S. D., Siegal F. P., Kunkel H. G. Human lymphocyte-sheep erythrocyte rosette formation: some characteristics of the interaction. Clin Immunol Immunopathol. 1973 Jul;1(4):511–522. doi: 10.1016/0090-1229(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Biberfeld P., Biberfeld G., Perlmann P. Surface immunoglobulin light chain determinants on normal and PHA-stimulated human blood lymphocytes studied by immunofluorescence and electronmicroscopy. Exp Cell Res. 1971 May;66(1):177–189. doi: 10.1016/s0014-4827(71)80027-7. [DOI] [PubMed] [Google Scholar]
- Binz H., Lindenmann J., Wigzell H. Cell-bound receptors for alloantigens on normal lymphocytes. I. Characterization of receptor-carrying cells by the use of antibodies to alloantibodies. J Exp Med. 1974 Apr 1;139(4):877–887. doi: 10.1084/jem.139.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chess L., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. I. Quantitative isolation of human T and B cells and response to mitogens. J Immunol. 1974 Oct;113(4):1113–1121. [PubMed] [Google Scholar]
- Chiao J. W., Pantic V. S., Good R. A. Human peripheral lymphocytes bearing both B-cell complement receptors and T-cell characteristics for sheep erythrocytes detected by a mixed rosette method. Clin Exp Immunol. 1974 Dec;18(4):483–490. [PMC free article] [PubMed] [Google Scholar]
- Cone R. E., Sprent J., Marchalonis J. J. Antigen-binding specificity of isolated cell-surface immunoglobulin from thymus cells activated to histocompatibility antigens. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2556–2560. doi: 10.1073/pnas.69.9.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickler H. B., Adkinson N. F., Jr, Terry W. D. Evidence for individual human peripheral blood lymphocytes bearing both B and T cell markers. Nature. 1974 Jan 25;247(5438):213–215. doi: 10.1038/247213a0. [DOI] [PubMed] [Google Scholar]
- Engers H. D., Unanue E. R. Antigen-binding thymic lymphocytes: specific binding of soluble antigen molecules and quantitation of surface receptor sites. J Immunol. 1974 Jan;112(1):293–304. [PubMed] [Google Scholar]
- Goldschneider I., Cogen R. B. Immunoglobulin molecules on the surface of activated T lymphocytes in the rat. J Exp Med. 1973 Jul 1;138(1):163–175. doi: 10.1084/jem.138.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F. Biological effects of anti-immunoglobulins: evidence for immunoglobulin receptors on 'T' and 'B' lymphocytes. Transplant Rev. 1970;5:45–75. doi: 10.1111/j.1600-065x.1970.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Torrigiani G., Roitt I. M. Blocking of the lymphocyte receptor site for cell mediated hypersensitivity and transplantation reactions by anti-light chain sera. Nature. 1969 May 31;222(5196):885–886. doi: 10.1038/222885a0. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Colón S., Campbell P., Rabellino E. Immunoglobulins on the surface of lymphocytes. V. Quantitative studies on the question of whether immunoglobulins are associated with T cells in the mouse. J Immunol. 1972 Oct;109(4):776–783. [PubMed] [Google Scholar]
- Grey H. M., Kubo R. T., Cerottini J. C. Thymus-derived (T) cell immunoglobulins. Presence of a receptor site for IgG and absence of large amounts of "buried" Ig determinants on T cells. J Exp Med. 1972 Nov 1;136(5):1323–1328. doi: 10.1084/jem.136.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström U., Zeromski J., Perlmann P. Immunoglobulin light chain determinants on unstimulated and stimulated human blood lymphocytes, assayed by indirect immunofluorescence. Immunology. 1971 Jun;20(6):1099–1111. [PMC free article] [PubMed] [Google Scholar]
- Hunt S. V., Williams A. F. The origin of cell surface immunoglobulin of marrow-derived and thymus-derived lymphocytes of the rat. J Exp Med. 1974 Mar 1;139(3):479–496. doi: 10.1084/jem.139.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling U., Rajewsky K. Evidence for surface-associated immunoglobulin on T and B lymphocytes. Eur J Immunol. 1971 Dec;1(6):447–452. doi: 10.1002/eji.1830010608. [DOI] [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Wigzell H., Aiuti F. Human lymphocyte subpopulations: classification according to surface markers and-or functional characteristics. Transplant Rev. 1973;16:163–195. doi: 10.1111/j.1600-065x.1973.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Belohradsky B., Yee K., Vogel J., Butcher D., Wybran J., Fudenberg H. H. Cellular interactions: scanning electron microscopy of human thymus-derived rosette-forming lymphocytes. Clin Immunol Immunopathol. 1974 Apr;2(3):301–309. doi: 10.1016/0090-1229(74)90048-8. [DOI] [PubMed] [Google Scholar]
- Ladoulis C. T., Gill T. J., 3rd, Chen S. H., Misra D. N. The structure and metabolism of lymphocyte membranes. Prog Allergy. 1975;18:205–288. [PubMed] [Google Scholar]
- Ladoulis C. T., Misra D. N., Estes L. W., Gill T. J., 3rd Lymphocyte plasma membranes. I. Thymic and splenic membranes from inbred rats. Biochim Biophys Acta. 1974 Jul 12;356(1):27–35. doi: 10.1016/0005-2736(74)90291-0. [DOI] [PubMed] [Google Scholar]
- Lamelin J. P., Lisowska-Bernstein B., Matter A., Ryser J. E., Vassalli P. Mouse thymus-independent and thymus-derived lymphoid cells. I. Immunofluorescent and functional studies. J Exp Med. 1972 Nov 1;136(5):984–1007. doi: 10.1084/jem.136.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J. F., Kettman J. R., Dutton R. W. Immunoglobulins on the surface of thymus-derived cells engaged in the initiation of a humoral immune response. J Exp Med. 1971 Sep 1;134(3 Pt 1):618–629. doi: 10.1084/jem.134.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. S., Cooper A. G., Wortis H. H. Scanning electron microscopy of human T-cell and B-cell rosettes. N Engl J Med. 1973 Sep 13;289(11):548–551. doi: 10.1056/NEJM197309132891102. [DOI] [PubMed] [Google Scholar]
- Lisowska-Bernstein B., Rinuy A., Vassalli P. Absence of detectable IgM in enzymatically or biosynthetically labeled thymus-derived lymphocytes. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2879–2883. doi: 10.1073/pnas.70.10.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowska-Bernstein B., Vassalli P. Mouse thymocyte immunoglobulin: can proteolysis explain difficulties in its detection? Biochem Biophys Res Commun. 1974 Nov 6;61(1):142–147. doi: 10.1016/0006-291x(74)90545-2. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Atwell J. L., Cone R. E. Isolation of surface immunoglobulin from lymphocytes from human and murine thymus. Nat New Biol. 1972 Feb 23;235(60):240–242. doi: 10.1038/newbio235240a0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Atwell J. L. Isolation and partial characterization of lymphocyte surface immunoglobulins. J Exp Med. 1972 Apr 1;135(4):956–971. doi: 10.1084/jem.135.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes N. F., Tolnai M. E., Silveira N. P., Gilbertsen R. B., Metzgar R. S. Technical aspects of the rosette tests used to detect human complement receptor (B) and sheep erythrocyte-binding (T) lymphocytes. J Immunol. 1973 Sep;111(3):860–867. [PubMed] [Google Scholar]
- Moroz C., Hahn Y. Cell-surface immunoglobulin human thymus cells and its biosynthesis in vitro. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3716–3720. doi: 10.1073/pnas.70.12.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Warner N. L., Lewis H., Sprent J. Quantitative features of a sandwich radioimmunolabeling technique for lymphocyte surface receptors. J Exp Med. 1972 Feb 1;135(2):405–428. doi: 10.1084/jem.135.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins W. D., Karnovsky M. J., Unanue E. R. An ultrastructural study of lymphocytes with surface-bound immunoglobulin. J Exp Med. 1972 Feb 1;135(2):267–276. doi: 10.1084/jem.135.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulin spots on the surface of rabbit lymphocytes. J Exp Med. 1970 Nov;132(5):1001–1018. doi: 10.1084/jem.132.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis B., Miller J. F., Forni L., Sprent J. Immunoglobulin on activated T cells detected by indirect immunofluorescence. Cell Immunol. 1974 Mar 15;10(3):476–482. doi: 10.1016/0008-8749(74)90139-7. [DOI] [PubMed] [Google Scholar]
- Rajapakse D. A., Mapamichail M., Holborow E. J. Immunoglobulin nature of PPD receptors on human T lymphocytes. Nat New Biol. 1973 Oct 3;245(144):155–157. doi: 10.1038/newbio245155a0. [DOI] [PubMed] [Google Scholar]
- Roelants G. E., Rydén A., Hägg L. B., Loor F. Active synthesis of immunoglobulin receptors for antigen by T lymphocytes. Nature. 1974 Jan 11;247(5436):106–108. doi: 10.1038/247106a0. [DOI] [PubMed] [Google Scholar]
- Roelants G., Forni L., Pernis B. Blocking and redistribution ("capping") of antigen receptors on T and B lymphocytes by anti-immunoglobulin antibody. J Exp Med. 1973 Apr 1;137(4):1060–1077. doi: 10.1084/jem.137.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelants G., Rydén A. Dose dependence of antigen binding to B and T lymphocytes. Nature. 1974 Jan 11;247(5436):104–106. doi: 10.1038/247104a0. [DOI] [PubMed] [Google Scholar]
- Santana V., Wedderburn N., Turk J. L. Demonstration of immunoglobulin on the surface of thymus lymphocytes. Immunology. 1974 Jul;27(1):65–73. [PMC free article] [PubMed] [Google Scholar]
- Smith R. W., Terry W. D., Buell D. N., Sell K. W. An antigenic marker for human thymic lymphocytes. J Immunol. 1973 Mar;110(3):884–887. [PubMed] [Google Scholar]
- Smith W. I., Ladoulis C. T., Misra D. N., Gill T. J., Bazin H. Lymphocyte plasma membranes. III. Composition of lymphocyte plasma membranes from normal and immunized rats. Biochim Biophys Acta. 1975 Apr 8;382(4):506–525. doi: 10.1016/0005-2736(75)90218-7. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Bianco C., Nussenzweig V., Uhr J. W. Cell surface immunoglobulin. IV. Distribution among thymocytes, bone mrrow cells, and their derived populations. J Exp Med. 1972 Jul 1;136(1):81–93. doi: 10.1084/jem.136.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S. R., Cooper M. D. T cells can bind antigen via cytophilic IgM antibody made by B cells. J Immunol. 1973 Jul;111(1):275–277. [PubMed] [Google Scholar]
- Yoshida T. O., Andersson B. Evidence for a receptor recognizing antigen complexed immunoglobulin on the surface of activated mouse thymus lymphocytes. Scand J Immunol. 1972;1(4):401–408. doi: 10.1111/j.1365-3083.1972.tb03306.x. [DOI] [PubMed] [Google Scholar]