Abstract
Osteoclast-associated receptor (OSCAR) is widely expressed on human myeloid cells. Collagen types (Col)I, II, and III have been described as OSCAR ligands, and ColII peptides can induce costimulatory signaling in receptor activator for NF-κB–dependent osteoclastogenesis. In this study, we isolated collagen as an OSCAR-interacting protein from the membranes of murine osteoblasts. We have investigated a functional outcome of the OSCAR–collagen interaction in human monocyte-derived dendritic cells (DCs). OSCAR engagement by ColI/II-induced activation/maturation of DCs is characterized by upregulation of cell surface markers and secretion of cytokines. These collagen-matured DCs (Col-DCs) were efficient drivers of allogeneic and autologous naive T cell proliferation. The T cells expanded by Col-DCs secreted cytokines with no clear T cell polarization pattern. Global RNA profiling revealed that multiple proinflammatory mediators, including cytokines and cytokine receptors, components of the stable immune synapse (namely CD40, CD86, CD80, and ICAM-1), as well as components of TNF and TLR signaling, are transcriptional targets of OSCAR in DCs. Our findings indicate the existence of a novel pathway by which extracellular matrix proteins locally drive maturation of DCs during inflammatory conditions, for example, within synovial tissue of rheumatoid arthritis patients, where collagens become exposed during tissue remodeling and are thus accessible for interaction with infiltrating precursors of DCs.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease that primarily targets the peripheral diarthrodial joints. It is characterized by the presence of autoantibodies, synovial inflammation, pannus formation, cartilage damage, and bone erosion. The molecular mechanisms involved in RA pathogenesis remain obscure. A multistep mechanism of RA development has been proposed (1, 2). First, unknown environmental factors cause alterations in posttranslational protein modifications, leading to recognition of autoantigens and the loss of immune tolerance. Next, a tissue-specific inflammatory response is elicited in the joints. Activated immune cells accumulate in the synovial tissue and secrete cytokines, chemokines, and matrix-degrading enzymes, causing tissue damage and remodeling. By way of a positive feedback loop, the local inflammation is sustained and finally progresses into a systemic disorder.
The cellular infiltrates in the synovial tissue are mainly composed of blood-derived cells, including T and B cells, as well as macrophages and dendritic cells (DCs) differentiated from monocytes in the RA synovial microenvironment. Cells of the myeloid lineage are regarded as pivotal regulators of RA. Both immature and mature DC subsets are present in large numbers within the RA joint, and strong evidence supports the importance of DCs in synovial inflammation (3, 4). DCs are professional APCs that are highly capable of inducing T cell responses. In RA, the DCs are essential for initiation and perpetuation by Ag presentation as well as for cytokine and chemokine secretion (5, 6). Furthermore, DCs are capable of transdifferentiating into osteoclasts, contributing directly to bone erosion (7, 8). The maturation state of the DCs is crucial for determining the balance between tolerance and immunity. Under homeostatic conditions, DCs are largely immature and are thought to induce tolerance. Changes in the microenvironment stimulate maturation of DCs, which can promote immunogenicity.
A human ortholog of murine OSCAR was identified by Merck et al. (9). OSCAR is expressed on cells of myeloid origin, and it signals through the ITAM-harboring adaptor protein FcRγ (9, 10). In DCs, cross-linking of OSCAR with specific mAbs enhanced Ag presentation and promoted a semimature phenotype, causing chemokine but not proinflammatory cytokine secretion. In synergy with TLR signaling, but not alone, OSCAR cross-linking enhanced the ability of DCs to induce naive CD4+ T cell proliferation (9, 11, 12). It was concluded that OSCAR can induce a semimatured phenotype in DCs, which is characterized by expression of costimulatory molecules but an inability to secrete proinflammatory cytokines. Such semimatured DCs are implicated in the induction of tolerance.
Recent studies have assessed the role of OSCAR in RA. OSCAR is expressed on mononuclear cells surrounding synovial microvessels and on multinucleated giant cells at the bone resorption areas. OSCAR was also found to be upregulated in peripheral blood monocytes from RA patients compared with healthy subjects, and its expression correlated to disease activity (13).
In the present study we aimed to examine OSCAR function in DCs with relevance to RA pathogenesis. We identified collagen types (Col)I/II as OSCAR ligands and investigated the functional outcomes of OSCAR–collagen interaction in DCs. During the preparation of this manuscript, Trowsdale and colleagues (14) identified ColI, -II and -III to be ligands of OSCAR (14). The authors showed that within joints collagens are exposed to OSCAR-expressing osteoclasts and osteoclast precursors. In vitro, OSCAR-binding collagen peptides promoted receptor activator for NF-κB ligand–induced osteoclast differentiation from blood-derived monocytes, indicating that OSCAR is an activating receptor on osteoclast precursor cells. In the present study, we show that OSCAR–collagen interaction provides an activating signal also in monocyte-derived DCs. This indicates an important, yet poorly recognized, role of the extracellular matrix (ECM) in the arthritic joint, where ColI/II become exposed due to ongoing tissue remodeling, thus providing activating signals to myeloid cells to drive a sustained inflammation. Blocking the OSCAR signaling, for example, with a mAb, may be considered as a novel therapeutic approach for RA.
Materials and Methods
Proteins and Abs for functional assays
The extracellular domain (ECD) of human OSCAR and OSCAR–Fc fusion protein were produced by recombinant technique as follows. The fragment encoding ECD (amino acid residues 1–227; sequence ID NP_573399) was amplified by RT-PCR using human universal cDNA (Clontech) as template, cloned into the pJSV002 expression vector, and sequenced. The recombinant protein was expressed in HEK293-6E cells. The purified recombinant protein is an N-glycosylated monomer of ∼25 kDa. For expression of OSCAR–Fc protein, the ECD-encoding cDNA was cloned in-frame with a sequence encoding the Fc fragment of human IgG4. The sequence was verified and the protein was expressed in HEK293-6E cells. The purified protein is an N-glycosylated dimer of ∼100 kDa. For cell binding studies, OSCAR-ECD was labeled using an Alexa Fluor 488 protein staining kit (Life Technologies) according to the manufacturer’s protocol.
A panel of murine mAbs against OSCAR-ECD and isotype controls were generated using hybridoma technology and then humanized using recombinant technology. The CDR of the generated mAb was grafted on a germline-encoded, human-mutated IgG1 Fc part, Fc-hIgG1.1 (15, 16). Five point mutations introduced in the Fc-hIgG1.1 hinder its binding to high-affinity Fc receptors. As isotype controls, an anti-OSCAR nonblocking high-affinity mAb as well as anti-TNP hIgG1.1 were used. Most blocking experiments were performed with the use of several different high-affinity anti-OSCAR–blocking mAbs and the two above-mentioned isotype controls with identical results. For simplicity, data are shown only for 1 μg/ml mAb unless specified otherwise. For functional assays, the following commercially available mAbs were used: anti-CD3 mAb (clone OKT3; eBioscience), anti-CD3/CD28 expander beads (Dynal), and anti–integrin α2β1 mAb (clone BHA2.1; Millipore). ColI purified from human skin was purchased from Sigma-Aldrich. ColII purified from human cartilage was purchased from Millipore. ColIII purified from human placenta was purchased from Millipore. ColIV from human placenta was purchased from Sigma-Aldrich. Endotoxin level in the protein solutions was measured using the turbidimetric kinetic Limulus amebocyte lysate assay (reagents supplied by Charles River Laboratories). All proteins used for functional assays in this study were essentially endotoxin-free.
Identification of OSCAR ligand
OSCAR-binding proteins were purified from membranes of MC3T3-E1 cells by affinity chromatography on an OSCAR–Fc matrix followed by identification of hits using a liquid chromatography–mass spectrometry (LC-MS) approach. In brief, for extraction of membrane proteins, the cells were washed in PBS, scraped with a plastic policeman, and homogenized mechanically using a glass pastel homogenizer in the buffer containing 10 mM imidazole and 1 mM EDTA (pH 7.4) on ice. The homogenate was spun at 20,000 × g for 10 min, and the pellet was resuspended in the homogenization buffer. This washing cycle was repeated three times with one last time in a buffer containing 2 mM imidazole/1 mM EDTA (pH 7.4). The pellet was then washed twice in 50 mM Tris-HCl/150 mM NaCl (pH 8.2), centrifuged, and resuspended in a RIPA buffer (150 mM NaCl, 50 mM Tris-HCl [pH 8.2], 1 mM EDTA, 1% Nonidet P-40 [NP-40], 1 mg/ml BSA, protease inhibitor [cOmplete, Roche] and phosphatase inhibitor mixture [Sigma-Aldrich]) for 30 min on ice. The protein extracts were clarified by centrifugation at 20,000 × g. Protein concentration was measured using a bicinchoninic acid protein assay (Pierce).
Preparation of an affinity resin was performed as follows. Human OSCAR–Fc IgG4 or Fc IgG4 (control) recombinant proteins were bound to a protein G–Sepharose 4B Fast Flow affinity matrix (Sigma-Aldrich) according to the manufacturer’s protocol and then chemically cross-linked as described in Schneider et al. (16). This technique allows optimal orientation of OSCAR-Fc on the beads and thus efficient binding of interacting proteins with minimal/no leakage of the bait during the elution process. After cross-linking, the beads were thoroughly washed in 0.1 M borate buffer, equilibrated in 5 ml 0.5 M Tris-HCl (pH 8.2), and sedimented by centrifugation. Cell membrane extracts in RIPA buffer were added to the affinity beads. NaCl concentration was adjusted to final 0.5 M and the beads were incubated for 3 h at 4°C with gentle agitation. The beads were then washed sequentially with three buffers containing 1) 0.5 M NaCl, 50 mM Tris-HCl, 1 mM EDTA, and 0.5% NP-40 (pH 8.2); 2) 150 mM NaCl, 50 mM Tris-HCl, 0.5% NP-40, and 0.1% SDS (pH 8.2); and 3) 150 mM NaCl and 0.5% CHAPS. The latter wash was repeated twice. The proteins bound to the affinity matrix were eluted in 50 mM diethylamine/0.5% CHAPS (pH 11.5) and pH was immediately adjusted to neutral using PBS and borate buffer. The samples were subjected to a 4–12% SDS-PAGE. The gels were stained using a ProteoSilver plus silver stain kit (Sigma-Aldrich). The bands differentially bound to the OSCAR–Fc matrix were subjected to LC-MS analysis as follows. Bands were excised from the SDS-PAGE gel, destained with the SilverQuest silver staining kit (Invitrogen), reduced with DTT, alkylated with iodoacetamide, and digested with trypsin with a slightly modified version of the method described in Shevchenko et al. (17).
LC-MS analysis
Peptide mixtures were analyzed by nanoscale liquid chromatography–electrospray ionization–tandem MS on an Easy-nLC (Proxeon Biosystems) connected to an LTQ Orbitrap mass spectrometer (Thermo Fisher Scientific). Reversed phase chromatography was performed with 0.1% formic acid (buffer A) and acetonitrile/0.1% formic acid (buffer B). Peptides were trapped on a precolumn (Proxeon Biosystems) and separated on a 10-cm fused-silica column (internal diameter 75 μm) packed with 3-μm ReproSil-Pur C18-AQ (Proxeon Biosystems). MS analysis was performed on the LTQ Orbitrap in data-dependent mode, where one MS survey scan was followed by MS fragmentation spectra of the four most intense ions.
Analysis of the MS data were performed with Proteome Discoverer 1.4 (Thermo Fisher Scientific). Peak lists of the MS fragmentation spectra were generated and searched in MASCOT against the mammalian part of the SwissProt database with oxidation (P), oxidation (M), and deamidated (NQ) as dynamic modifications and carbamidomethyl (C) as fixed modification. Results were filtered for a false discovery rate (FDR) of 0.01 and a minimum MASCOT peptide score of 25.
Binding of OSCAR to collagen
Binding of the soluble extracellular domain of OSCAR to human collagens was tested by surface plasmon resonance (SPR) on a ProteOn instrument (Bio-Rad). Human collagens were immobilized on a GLC sensor chip using the amine coupling kit (Bio-Rad). The experiment was performed at 15°C using HBS-EP+ (GE Healthcare) as running buffer for the immobilization and HBS-EP+/0.1% BSA for binding analysis. The chip was activated with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride/normal human serum (diluted 2:1 with H2O) according to instructions. Collagens were diluted in 10 mM NaAc buffer (pH 4.5) to 25 mg/ml and injected for 200 s followed by quenching with ethanolamine. OSCAR-ECD was injected at 2 μM, 1 μM, 500 nM, 250 nM, and 125 nM for 200 s at a flow of 60 μl/min followed by regeneration with 10 mM glycine (pH 2.0) for 30 s. Binding curves were double referenced by subtraction of the signals from reference cells and a buffer injection using the ProteOn Manager software.
Competition of OSCAR binding to collagen
Competition of anti-OSCAR mAb with OSCAR binding to collagen was measured by SPR on a Biacore T100 instrument using the same buffers as described above.
Human ColI was diluted with 10 mM NaAc (pH 4.5) to a final concentration of 2.5 μg/ml and immobilized on a CM5 sensor chip (GE Healthcare) using the amine coupling kit (GE Healthcare). Immobilization was carried out at 4°C with HBS-EP+ as buffer using 200 resonance units as a target level for the immobilized ligand. OSCAR was diluted to 25 nM in HBS-EP+ buffer containing 0.1% serum albumin and increasing concentrations of the anti-OSCAR Abs from 0 to 20 μg/ml. The OSCAR/mAb solutions were injected for 240 s followed by 200 s dissociation at a flow rate of 50 μl/min at 4°C followed by regeneration with 10 mM glycine (pH 2.0). Binding curves were double referenced by subtraction of the signals from reference cells and a buffer injection and further analyzed using the Biacore T100 evaluation software.
Cells
Buffy coats from healthy individuals were obtained anonymously from the Blood Bank, Rigshospitalet, Copenhagen, Denmark. All donors gave informed consent according to the protocol approved by the Ethics Committee for Copenhagen, Denmark, for research use (approval no. H-D-2008-113). PBMCs were freshly purified by Ficoll-Plaque density centrifugation. Monocytes were purified from PBMCs using the CD14 MicroBeads positive selection kit (Miltenyi Biotec). To induce DC differentiation, monocytes were cultured in RPMI 1640/10% FCS in the presence of 50 ng/ml recombinant human GM-CSF and 50 ng/ml recombinant human IL-4 (Novo Nordisk) for 6 d. These cells are referred to as immature DCs (iDCs). Naive CD4+RA+ cells were purified from PBMCs using a human naive CD4+ T cell enrichment kit (StemCell Technologies). For freezing, T cells were resuspended at a density of 7 × 106/ml in RPMI 1640/50% FBS/10% DMSO, and cryovials were placed in a Mr. Frosty container (Thermo Fisher Scientific) at −80°C overnight and then transferred into a −140°C freezer. Viability of the thawed cells was ∼95% as measured using a NucleoCounter (ChemoMetec).
DC culture on collagen
Untreated virgin polystyrene 24-well plates were coated with ColI or ColII in PBS at ∼3 μg/cm2 overnight at 4°C. iDCs (1 × 106) were added per well after being preincubated for 20 min with indicated mAb. The cells were cultured for 20 h unless otherwise specified. ColI and ColII had similar effects in all experiments. ColII was always slightly more potent than ColI. Functional data in the manuscript are shown for ColII only unless otherwise specified.
Allogeneic and autologous MLR
For allogeneic MLRs freshly purified cells were used. For autologous reactions, T cells were kept frozen in RPMI 1640/50% FBS/10% DMSO until the autologous DCs became matured. T cells (1 × 105) were cocultured in triplicate wells with 1 × 103 DCs in RPMI 1640/10% FCS growth medium. No live DCs were detected in the coculture at the time of termination of the experiment. To assess T cell proliferation pulse labeling of cells with [3H]thymidine was used to monitor DNA synthesis. Briefly, at day 4 of coculture the cells were pulsed with 0.5 μCi [3H]thymidine (PerkinElmer) for 18 h. [3H]thymidine incorporation was measured by liquid scintillation counting using a TopCount NXT (PerkinElmer).
Cytokine secretion
Multiplex analysis of cytokines was performed using the BioPlex 200 cytokine assay platform (Bio-Rad). IL-23 (p19/p40) was detected using the Platinum ELISA kit (eBioscience).
Expression of cell surface markers
For analysis of cell surface marker expression, the following Abs were used: BD multicolor PE-conjugated mouse monoclonal anti-human CD86, PerCP-Cy5.5–conjugated mouse anti-human CD209, allophycocyanin-conjugated mouse anti-human CD83 (BD Biosciences), FITC-conjugated mouse anti-human HLA-DR (BD Pharmingen), allophycocyanin-conjugated mouse anti-human CD14 (BD Pharmingen), PE-conjugated mouse anti-human OSCAR (clone 11.1CN5; Beckman Coulter), and Pacific Blue–conjugated anti-human CD3 (BD Pharmingen). Cells were washed twice in PBS with 2% FCS and then stained for 30 min at 37°C in PBS with 2% FCS in the presence of 1% human serum albumin to block unspecific binding. Cells were rinsed three times in PBS with 2% FCS prior to flow cytometry analysis. Data acquisition was done using a FACSFortessa (BD Biosciences). Only live cells, that is, negative for allophycocyanin-H7 dead cell stain (Invitrogen/Life Technologies) were analyzed. For compensation single stain was used with one drop of negative control beads and anti-mouse IgG beads (BD Biosciences). For the dead cell marker compensation, amine reactive compensation beads were used (Invitrogen/Life Technologies). Data analysis was performed using Kaluza software version 1.2 (Beckman Coulter).
Microarray analysis
Total RNA was obtained 4 and 20 h after stimulating DCs with ColI/II in the absence or presence of anti-OSCAR mAb or isotype control mAb. RNA was extracted using TRIzol (Invitrogen/Life Technologies) followed by purification with an RNeasy MinElute cleanup kit (Qiagen). The RNA integrity was evaluated on an Agilent 2100 bioanalyzer using Agilent RNA 6000 Nano Kit chips (Agilent Technologies), with RNA integrity number scores of ≥9.9. Microarray experiments were performed according to the protocols supplied by Affymetrix and run on the HT HG-U133+ PM chip from Affymetrix using an Affymetrix GeneTitan machine in a 96-well layout. Array data were normalized using the robust multiarray average algorithm with a custom chip definition file obtained from BrainArray (University of Michigan) based on Ensembl gene annotations (ENSG). Microarray data were analyzed for significant differences using Qlucore Omics Explorer (v2.2) software. A paired two-group comparison was performed with Benjamini–Hochberg correction for multiple testing applying a FDR of 5% and a biological threshold of >2-fold regulation. Differentially expressed genes were subjected to gene ontology analysis for biological processes using Ingenuity Pathway Analysis. The dataset and technical information compliant with minimum information about a microarray experiment can be found at the EMBL-EBI ArrayExpress depository, accession no. E-MTAB-2904 (http://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-2904/).
Statistical analysis
Statistical analyses were performed in GraphPad Prism 5 using Student two-tailed t tests. A p value <0.05 was considered statistically significant. Unless otherwise indicated, means and SD are shown.
Results
Identification of OSCAR ligand
It was previously shown that a putative ligand for murine OSCAR is expressed on the cell surface of osteoblasts (18). In the present study, we screened a panel of cell lines for expression of a putative ligand for human OSCAR by monitoring binding of fluorescently labeled OSCAR-ECD to the surface of live cells. A murine MC3T3-E1 preosteoblast cell line was selected for purification of the OSCAR ligand. Using affinity chromatography followed by liquid chromatography–tandem MS analysis of eluted proteins, we have identified α1 ColI as OSCAR-interacting protein. Direct interaction of OSCAR with native human ColI an ColII was confirmed by SPR, whereas no binding was detected to ColIII or ColIV (Supplemental Fig. 1). Thus, ColI and ColII serve as naturally occurring OSCAR ligands.
Anti-OSCAR mAb capable of blocking OSCAR–collagen interaction as well as nonblocking mAb were selected for further functional assays using SPR (Supplemental Fig. 2).
OSCAR–collagen signaling promotes DC survival
It has previously been found that cross-linking of OSCAR with an mAb promoted survival of monocyte-derived DCs (11). In the present study, we tested whether the naturally occurring OSCAR ligands, namely ColI/II, could promote DC survival. We differentiated iDCs from peripheral blood monocytes in the presence of GM-CSF and IL-4. This method allows generation of cells phenotypically resembling “inflammatory” DCs, which differentiate in vivo from blood-derived precursors during inflammation (19). The iDCs were plated onto either uncoated plates with or without GM-CSF, or onto collagen-coated plates without GM-CSF in the absence or presence of anti-OSCAR–blocking mAb or an isotype control mAb. We assessed cell viability by monitoring the intracellular reducing environment (an indicator of cellular well-being) with alamarBlue and by detecting cell death using annexin V/propidium iodine staining. After 7 d of incubation, the reducing potential was significantly decreased in DCs cultured on plastic without GM-CSF and in collagen-coated plates in the presence of anti-OSCAR mAb. In contrast, the cells maintained the reducing environment upon culturing with GM-CSF or on collagen (Fig. 1). Already after 3 d, a large percentage of annexin V+ apoptotic cells was detected in cultures lacking GM-CSF, whereas most cells cultured on collagen were alive (Table I). The latter effect was completely abolished by treatment of cells with anti-OSCAR mAb. As control, treatment with Enbrel (etanercept, the p75TNFR-Fc fusion protein), a drug currently used for treatment of RA, was used, as it has been shown that TNF-α signaling supports DC survival (20). We show that Enbrel treatment partially inhibits collagen-induced survival of DCs. Our data indicate that OSCAR–collagen signaling can promote DC survival under the conditions of growth factor withdrawal.
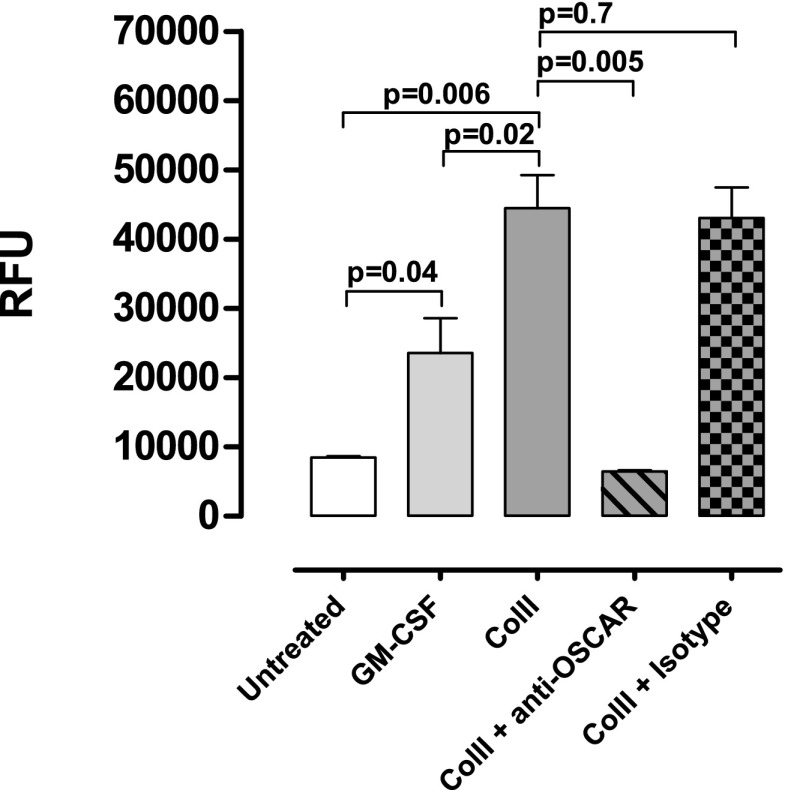
FIGURE 1.
OSCAR–collagen signaling promotes DC survival. Human monocytes were purified and differentiated into iDCs by GM-CSF and IL-4 (50 ng/ml each) for 6 d. Then, 1 × 106 iDCs/ml were cultured on either uncoated or ColII-coated plates. To the ColII-coated wells, OSCAR-blocking Ab (1 μg/ml) or control Ab (1 μg/ml) was added. GM-CSF (50 ng/ml) was added as a positive control of survival. As a negative control, iDCs were left in growth factor–free medium. After 7 d, cell viability was assessed using alamarBlue. Data are expressed as mean of biological triplicates ± SD. Data shown are from one representative donor out of five analyzed.
Table I. DC viability analyzed by flow cytometry.
| Alive (PI−/Annexin V−) | Early Apoptotic (PI−/Annexin V+) | Necrotic (PI+/Annexin V−) | Late Apoptotic (PI+/Annexin V+) | |
|---|---|---|---|---|
| Untreated | 18.1 ± 1.3 | 30.6 ± 0.5 | 11.8 ± 1.2 | 39.7 ± 1.9 |
| GM-CSF | 65.0 ± 5.6** | 17.4 ± 1.7** | 2.7 ± 1.3* | 14.9 ± 3.0 *** |
| ColII | 83.9 ± 1.4*** | 9.1 ± 0.3*** | 0.4 ± 0.1** | 6.6 ± 1.1** |
| ColII + anti-OSCAR | 13.4 ± 1.1* | 31.0 ± 0.3 | 14.2 ± 3.5 | 41.5 ± 4.1 |
| ColII + isotype | 80.6 ± 3.8** | 10.4 ± 1.6** | 0.3 ± 0.1** | 8.7 ± 2.1** |
| ColII + Enbrel | 59.3 ± 4.4** | 20.7 ± 2.0* | 0.8 ± 0.1** | 19.1 ± 2.6* |
iDCs were cultured on ColII-coated plates. Cells were untreated or treated with either 50 ng/ml GM-CSF, 1 μg/ml anti-OSCAR, 1 μg/ml isotype control, or 10 μg/ml Enbrel. After 3 d of culture cells were stained for annexin V and propidium iodide (PI) and analyzed via flow cytometry. For flow cytometry analysis, single cells were gated on with side scatter (SSC) height versus SSC width, followed by SSC versus forward scatter where debris was gated out. The percentages of cell populations were analyzed based on a dot plot (annexin V versus PI). Data are expressed as means ± SD of triplicate wells treated independently. Data are shown for one representative donor out of four analyzed.
p < 0.05, **p < 0.01, ***p < 0.001 versus untreated.
Collagen induces secretion of cytokines and chemokines by monocyte-derived DCs
Cross-linking of OSCAR in iDCs has been shown to induce secretion of IL-8, IL-12p40, M-CSF, MCP-1/CCL2, and MDC/CCL22, whereas no secretion of IL-1β, IL-6, IL-10, GM-CSF, IL-12p70, IP-10/CXCL10, and TNF-α was detected (9, 11). We tested whether collagen can induce secretion of cytokines via OSCAR signaling. Culturing of iDCs on ColII-coated plates caused a profound induction of multiple cytokines secretion, including TNF-α, IL-6, IL-8, IL-10, IL-13, IL-23, and RANTES (CCL5). This activation of DCs was completely abolished by anti-OSCAR–blocking mAb (Table II). Meanwhile, anti-OSCAR mAb did not show any effect on the LPS-stimulated cytokine release (data not shown). In contrast to the positive control, namely LPS-matured DCs, IL-12p70 was not secreted by collagen-matured DCs (Col-DCs). Thus, OSCAR signaling leads to activation of DCs characterized by production of a broad range of cytokines and chemokines, mainly of a proinflammatory type.
Table II. Cytokines and chemokines secreted by DCs.
| iDCs | Col-DCs | Col-DCs + Anti-OSCAR | Col-DCs + Isotype mAb | LPS-DCs | |
|---|---|---|---|---|---|
| IL-6 | 2,428.0 ± 1.5 | 8,565.0 ± 175.8* | 1,729.0 ± 97.7** | 6,750.0 ± 501.1ns | 22,547.0 ± 364.4** |
| IL-8 | 11,406.0 ± 485.5 | 20,167.0 ± 523.4** | 11,809.0 ± 457.6** | 19,911.0 ± 699 ns | 20,286.0 ± 508.0** |
| IL-10 | 239.5 ± 48.4 | 2,801.0 ± 188.0* | 85.5 ± 11.0* | 2,330.0 ± 48.4ns | 12,049.0 ± 126.4** |
| IL-12p40 | 536.2 ± 35.0 | 46,361.0 ± 406.0** | 595.6 ± 49.0** | 46,937.0 ± 2,298.0ns | 39,407.0 ± 1771.0* |
| IL-12p70 | 71.5 ± 33.7 | 99.7 ± 6.1ns | 40.1 ± 1.5* | 86.7 ± 0.0ns | 14,287.0 ± 315.8** |
| IL-13 | 21.2 ± 11.2 | 1,034.0 ± 34.3* | 11.5 ± 0.0* | 1,039.0 ± 34.7ns | 1,658.0 ± 6.9** |
| RANTES | 64.0 ± 15.4 | 2,201.0 ± 132.1* | 54.8 ± 0.5* | 2,131.0 ± 52.9ns | 44,79.0 ± 193.4* |
| TNF-α | 366.6 ± 101.2 | 44,763.0 ± 1881.0* | 78.1 ± 0.0* | 28,719.0 ± 2669.0ns | 31,108.0 ± 1961.0* |
| IL-23 | 0.4 ± 0.4 | 272.2 ± 6.5*** | 0.8 ± 3.4*** | 289.3 ± 40.1ns | 696.3 ± 8.6*** |
Multiple cytokines were measured in the supernatants from DCs cultured at the indicated conditions using Luminex technology. Values are shown in pg/ml. Data represent means ± SD of triplicate wells treated independently. Data are shown for 1 representative donor out of >20 donors analyzed with similar results. IL-23 was measured using a sandwich ELISA.
nonsignificant.
p < 0.05, **p < 0.01, ***p < 0.001 by t test for the following conditions: iDCs versus Col-DCs and for Col-DCs versus Col-DCs plus anti-OSCAR.
LPS-DCs, LPS-matured DCs.
Collagen promotes maturation of monocyte-derived DCs
ColI has been shown to induce maturation of monocyte-derived DCs, but the receptors mediating this effect were not identified (21). Cross-linking of OSCAR with specific Abs induced semimaturation of DCs (11). We therefore tested whether collagen-induced DC maturation is mediated by OSCAR. iDCs were generated as described above. The phenotype of these cells (CD14−CD209+) was confirmed by flow cytometry. Treatment with anti-OSCAR mAb did not have an effect on differentiation of iDCs under the conditions used (data not shown). The iDCs were plated onto ColI- (data not shown) or ColII-coated plates and cultured for 1–2 d. The cell surface markers were analyzed by flow cytometry (Fig. 2A). We found that collagen drastically upregulated expression of the maturation markers CD83 and CD86. HLA-DR was upregulated only in donors that had a relatively low basal expression of this marker (Fig. 2B). This effect was completely abolished by anti-OSCAR–blocking mAb in a dose-dependent manner (Fig. 2C), indicating that collagen-induced DC maturation is mediated by OSCAR signaling. A mAb against integrin α2β1, another known collagen receptor, had no inhibitory effect (data not shown), which is in agreement with the data published by Brand et al. (21). LPS, which was used in some experiments as positive control, showed a level of upregulation of the cell surface markers that was comparable to collagen (data not shown). Maturation of DCs can be induced by TNF-α (19). Because we have shown that collagen induces TNF-α release from DCs, we tested whether blocking TNF-α would inhibit ColII-induced DC maturation. Treatment of the cells with Enbrel only partially inhibited DC maturation in two of four donors tested (Fig. 2D). Thus, autocrine TNF-α does not seem to be the major contributor to the collagen-induced DC maturation.
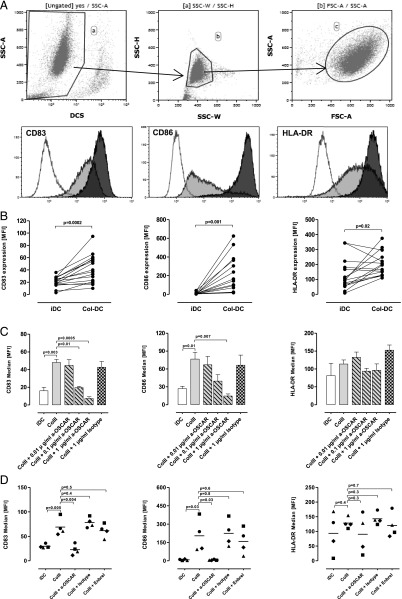
FIGURE 2.
Expression of cell surface markers on DCs analyzed by flow cytometry. Initially, 1 × 106 iDCs/ml were cultured on either uncoated or ColII-coated plates for 18 h. At these conditions, DCs upregulated expression of maturation markers when cultured on collagen. (A) Top panel, Gating strategy for flow cytometry analysis. Only cells negative for the dead cell marker Near-IR were used in the analysis (gate a). Single cells from gate a were further gated on with side scatter height (SSC-H) versus SSC width (SSC-W) (gate b), followed by gating out cell debris with SSC versus forward scatter (FSC) (gate c). Hierarchy of gating is indicated with arrows. Only cells from gate c were further analyzed. Lower panel, Representative histograms are shown for the indicated surface markers. Open histograms represent unstained cells. Gray and black histograms represent iDCs and Col-DCs, respectively. (B) Combined data are shown for 16 donors. (C) ColII-induced expression of maturation markers is downregulated by anti-OSCAR mAb in a dose-dependent manner; 0.01, 0.1, and 1 μg/ml anti-OSCAR mAb or 1 μg/ml isotype control mAb was used. Data are expressed as mean of independently treated triplicate wells ± SD. Data are shown for 1 representative donor out of 10. (D) DCs treated with 10 μg/ml Enbrel or 1 μg/ml isotype control mAb. Data for four donors are shown.
Col-DCs are efficient inducers of T cell proliferation
Triggering of OSCAR with cross-linking mAb was found to potentiate effects of TLR ligands on DC maturation and, as a consequence, to enhance the ability of TLR-matured DCs to induce proliferation of allogeneic naive T cells. In the absence of TLR ligands, the DCs treated with anti-OSCAR cross-linking mAb completely failed to induce T cell proliferation (11). In the present study, we tested whether the Col-DCs can promote proliferation of allogeneic naive CD4+CD45RA+ T cells. The Col-DCs were very efficient in promoting T cell proliferation as monitored either by [3H]thymidine incorporation (Fig. 3A) or by the expression of a proliferation marker, Ki67 (not shown). When the Col-DCs were matured in the presence of anti-OSCAR mAb, this proliferative effect was abolished in a dose-dependent manner.
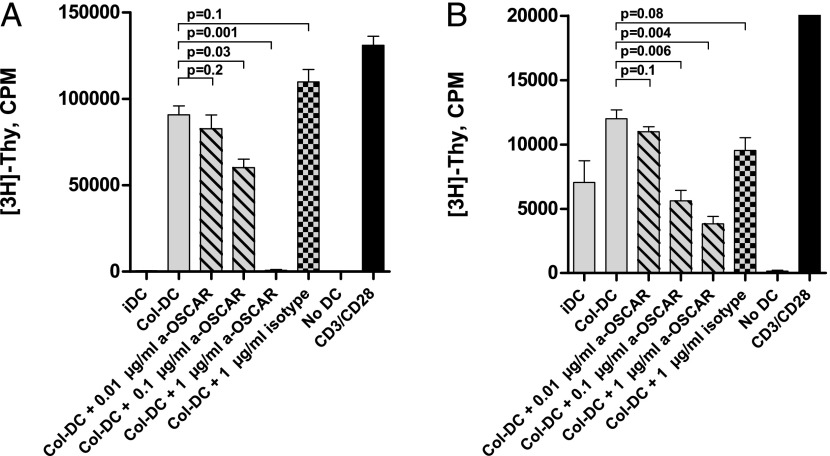
FIGURE 3.
ColII-matured DCs induce proliferation of T cells. (A) Allogeneic MLR. Proliferation of CD3+CD4+CD45+RA+ T cells was estimated by [3H]thymidine incorporation. T cells were incubated for 4 d with the indicated allogeneic DCs: iDCs, ColII-matured DCs, or DCs that were maintained on collagen in the presence of anti-OSCAR mAb (data shown for 0.01, 0.1, and 1 μg/ml mAb) or isotype control mAb (1 μg/ml), as well as T cells alone (no DCs) as negative control and T cells stimulated with CD3/CD28 beads as positive control. (B) Proliferation of autologous CD3+CD4+CD45+RA+ T cells in the presence of the indicated DCs; costimulation of TCR was achieved by treatment with an agonistic OKT-3 mAb; all treatments and controls are as in (A). Data are mean ± SD of triplicate wells from one representative experiment out of seven (allogeneic) or three (autologous) performed with similar results.
Cytokines secreted by T cells, which had been expanded by Col-DCs, were analyzed (Table III). Noticeably, a strong induction of a number of cytokines, including Th2-specific IL-5 and IL-13, as well as proinflammatory IL-6, IL-8, TNF-α, and RANTES (CCL5), was evident in the Col-DC/T cell cocultures, which was not the case when DCs were treated with anti-OSCAR mAb during the maturation step.
Table III. Cytokines and chemokines secreted by T cells cocultured with allogeneic DCs.
| iDCs | Col-DCs | Col-DCs + Anti-OSCAR | Col-DCs + Isotype mAb | |
|---|---|---|---|---|
| IL-2 | 38.4 ± 13.2 | 3,135.0 ± 463.0** | 35.8 ± 18.0** | 2,666.0 ± 543.0ns |
| IL-3 | 811.8 ± 16.4 | 771.7 ± 6.1* | 782.3 ± 62.0ns | 751.7 ± 65.5ns |
| IL-5 | 1.4 ± 0.2 | 2,087.0 ± 234.0** | 1.3 ± 0.0** | 1,919.0 ± 598.9ns |
| IL-6 | 12.0 ± 3.7 | 1,414.0 ± 329.5* | 20.0 ± 10.8* | 1,569.0 ± 163.7ns |
| IL-8 | 1,035.0 ± 761.8 | 10,852.0 ± 859.8** | 1,352.0 ± 458.4** | 10,936.0 ± 274.1ns |
| IL-10 | 13.9 ± 1.4 | 99.0 ± 65.2ns | 14.2 ± 2.5ns | 147.2 ± 54.8ns |
| IL-12p40 | 1,339.0 ± 65.4 | 10,083.0 ± 292.1** | 1,349.0 ± 56.5*** | 13,601.0 ± 403.8ns |
| IL-12p70 | 10.4 ± 0.0 | 13.0 ± 0.0ns | 11.3 ± 1.5ns | 14.3 ± 1.3ns |
| IL-13 | 2.5 ± 0.8 | 3,547.0 ± 142.3*** | 2.5 ± 0.2*** | 3,609.0 ± 321.5ns |
| IL-17 | 46.1 ± 3.2 | 48.9 ± 3.2ns | 48.9 ± 3.2ns | 49.4 ± 5.6ns |
| IL-18 | 25.8 ± 2.5 | 25.2 ± 1.4ns | 24.2 ± 0.7ns | 26.4 ± 1.6ns |
| GM-CSF | 26.1 ± 3.9 | 1,402.0 ± 217.4** | 23.9 ± 2.5** | 1,581.0 ± 135.2ns |
| IFN-γ | 24.5 ± 2.0 | 254.9 ± 69.4* | 23.1 ± 2.4* | 514.0 ± 195.1ns |
| MCP-1 | 992.4 ± 549.3 | 85.6 ± 2.3ns | 1,505.0 ± 654.3ns | 86.0 ± 8.8ns |
| M-CSF | 268.5 ± 12.0 | 347.6 ± 20.0* | 248.4 ± 6.4** | 357.1 ± 30.7ns |
| RANTES | 14.6 ± 6.3 | 2,180.0 ± 230.4** | 18.4 ± 9.6** | 2,287.0 ± 152.2ns |
| TNF-α | 104.3 ± 56.4 | 3,830.0 ± 168.4*** | 101.9 ± 18.1*** | 3,575.0 ± 567.1ns |
Multiple cytokines were measured in the supernatants of T cells cultured with the indicated allogeneic DCs. Supernatants were collected at day 4 of coculture. Anti-OSCAR mAbs or isotype control mAbs were used at 1 μg/ml. Values are shown in pg/ml. Data represent means ± SD of triplicate wells treated independently. Data are shown for one representative experiment out of four independent experiments with similar results.
nonsignificant.
p < 0.05, **p < 0.01, ***p < 0.001 by t test for the following conditions: iDCs versus Col-DCs, Col-DCs versus Col-DCs + anti-OSCAR, and Col-DCs versus Col-DCs + isotype.
We further tested the ability of Col-DCs to induce proliferation of autologous naive T cells. In this setting also, Col-DCs were efficient inducers of T cell proliferation (Fig. 3B). The secretion of cytokines was analyzed as above (Table IV). We detected high levels of IL-13 and IL-5, the cytokines that polarize T cells toward the Th2 lineage. However, the proinflammatory cytokines IL-6, IL-8, and TNF-α as well as Th1-polarizing INF-γ were also induced in an OSCAR-dependent manner. Thus, no clear pattern of T cell polarization was evident.
Table IV. Cytokines and chemokines secreted by T cells cocultured with autologous DCs.
| iDCs | Col-DCs | Col-DCs + Anti-OSCAR | Col-DCs + Isotype mAb | |
|---|---|---|---|---|
| IL-2 | 16.2 ± 0.9 | 18.0 ± 0.6ns | 14.0 ± 1.7ns | 15.6 ± 0.6ns |
| IL-3 | 2397.0 ± 140.9 | 2,399.0 ± 85.2ns | 2,225.0 ± 186.1ns | 2,456.0 ± 47.0ns |
| IL-5 | 52.8 ± 23.9 | 377.9 ± 67.0* | 45.3 ± 22.1* | 302.7 ± 19.4ns |
| IL-6 | 894.0 ± 97.4 | 2,595.0 ± 202.3** | 395.1 ± 88.3** | 3,052.0 ± 1,125.0ns |
| IL-8 | 2,1579.0 ± 17.9 | 22,683.0 ± 270.7* | 18,465.0 ± 1,058* | 21,496.0 ± 328.1ns |
| IL-10 | 213.8 ± 9.0 | 430.7 ± 10.6** | 188.2 ± 23.1** | 419.7 ± 16.1ns |
| IL-12p40 | 20,956.0 ± 3,700.0 | 38,572.0 ± 732.8* | 10,840.0 ± 1,641** | 43,733.0 ± 3518ns |
| IL-12p70 | 28.9 ± 2.7 | 19.3 ± 0.0ns | 16.4 ± 1.4ns | 20.2 ± 2.7ns |
| IL-13 | 207.4 ± 0.2 | 966.1 ± 28.3*** | 158.5 ± 18.4*** | 884.3 ± 114.4ns |
| IL-17 | 52.3 ± 5.5 | 67.8 ± 11.0ns | 42.1 ± 4.8ns | 55.2 ± 1.4ns |
| IL-18 | 20.5 ± 2.5 | 21.0 ± 0.8ns | 20.5 ± 0.8ns | 21.6 ± 2.5ns |
| GM-CSF | 227.5 ± 4.2 | 495.0 ± 58.6* | 230.3 ± 11.0* | 472.3 ± 83.2ns |
| IFN-γ | 1,429.0 ± 352.4 | 3,236.0 ± 954.6ns | 213.3 ± 166.2* | 3,704.0 ± 1,330.0ns |
| MCP-1 | 234.7 ± 52.8 | 8.0 ± 1.1* | 372.9 ± 35.4** | 8.1 ± 1.8ns |
| M-CSF | 893.7 ± 6.0 | 1,873.0 ± 82.6** | 908.0 ± 38.3** | 2,163.0 ± 3.0* |
| RANTES | 1,416.0 ± 0.9 | 1,262.0 ± 85.3ns | 2,408.0 ± 63.6** | 15,12.0 ± 182.8ns |
| TNF-α | 1,706.0 ± 305.0 | 8,297.0 ± 507.3** | 2,016.0 ± 377.2** | 9251.0 ± 855.6ns |
Multiple cytokines were measured in the supernatants of T cells cultured with the indicated autologous DCs. Supernatants were collected at day 4 of coculture. Anti-OSCAR mAbs or isotype control mAbs were used at 1 μg/ml. Values are shown in pg/ml. Data represent means ± SD of triplicate wells treated independently. Data are shown for one representative experiment out of four independent experiments with similar results.
nonsignificant.
p < 0.05, **p < 0.01, ***p < 0.001 by t test for the following conditions: iDCs versus Col-DCs, Col-DCs versus Col-DCs + anti-OSCAR, and Col-DCs versus Col-DCs + isotype.
Signaling by OSCAR–collagen regulates gene expression in DCs
The overall gene expression profile resulting from the OSCAR–collagen interaction in DCs was characterized by Affymetrix gene chip analysis. iDCs were exposed to ColI or ColII for 4 or 20 h in the presence or absence of antagonistic anti-OSCAR mAb or isotype control mAb. The effects of ColI and ColII were very similar (>90% of genes regulated by ColI were also regulated by ColII). Only data for the 4-h ColII treatment are discussed in this section, as it more closely represents the primary response to OSCAR stimulation without following amplification (secondary response). LPS was included as a positive control for DC maturation, and there was a good overlap (∼70%) in gene expression changes in cells treated with LPS and ColI/II. Raw data files for all treatments can be downloaded from the ArrayExpress depository (accession no. E-MTAB-2904).
A marked change in the gene expression level was observed for 709 genes in cells treated with ColII for 4 h (Supplemental Table IA). When comparing the effect of preincubating the iDCs with anti-OSCAR mAb or isotype control mAb prior to plating on ColII, the expression level of 546 genes was significantly different between the two treatments (Supplemental Table IB). No significant differences (FDR of 10%) were found for treatments with ColII in the presence or absence of isotype mAb, negating an effect of the isotype control mAb on OSCAR biology.
When comparing the ColII-regulated genes and genes regulated in the presence of anti-OSCAR mAb, 472 genes were overlapping. Thus, these genes are thought to be the true transcriptional targets of the OSCAR–collagen signaling pathway (Supplemental Table IC). Using Ingenuity Pathway Analysis, the OSCAR-regulated genes were clustered into several different functional pathways. The top 20 canonical pathways are listed in Table V. The OSCAR-dependent genes were mainly associated with immune regulation, maturation, adhesion, apoptosis, and various signal transduction pathways. The data show that OSCAR regulates expression of the major components of the stable immune synapse such as CD40, CD80, CD86, CD83, and ICAM-1. Furthermore, OSCAR signaling induced proinflammatory cytokine and chemokine gene expression as well as the genes encoding cytokine and chemokine receptors. The gene encoding CCL20, a chemokine attracting iDCs, was noticeably the most upregulated gene with a fold change of >150. Of note, chemoattractants for precursors of endothelial cell CXCL1, -2, and -3 mRNA levels were drastically induced (>25-fold) at a transcriptional level (Supplemental Table IC), indicating possible involvement of the OSCAR pathway in the regulation of neoangiogenesis. OSCAR–collagen signaling upregulated genes encoding components of TLR, TREM-1, and uPA/MMP-9 pathways highly relevant for pathogenesis of RA and other autoimmune diseases (22). Of interest is also the downregulation of the Wnt/fzd pathway that programs DCs to induce tolerogenic responses (23). We observed that ColII decreased FZD2 gene expression by 18-fold, which was abrogated by treatment with anti-OSCAR mAb. Taken together, our data indicate that OSCAR signaling in DCs has a profound effect on the induction/maintenance of proinflammatory responses at multiple levels.
Table V. Top 20 canonical pathways regulated by ColII and reversed by anti-OSCAR.
| Ingenuity Canonical Pathways | Molecules | −Log (p Value) |
|---|---|---|
| TLR signaling | MAP3K14, IL1A, MAP3K1, TLR8, TNFAIP3, MAPK13, NFKB1, TLR2, NFKBIA, IL1RN, IL1B, MAP2K3, TNF, TRAF1, IRAK2 | 1.05E+01 |
| Hepatic fibrosis/hepatic stellate cell activation | IL4R, IL1A, ICA, VEGFA, CXCL3, CCL2, CD40, EDN1, CSF1, TNFSF8, IL1B, SERPINE1, IL1RAP, TNF, MMP9, TNFSF14 | 1.04E+01 |
| Atherosclerosis signaling | IL1A, ICAM1, PDGFA, NFKB1, F3, TNFRSF12A, SELPLG, CD40, CCL2, CSF1, IL1RN, LPL, IL1B, SERPINA1, TNF, MMP9, TNFSF14 | 9.06E+00 |
| Type I diabetes mellitus signaling | SOCS3, MAP3K14, IFNGR1, MAPK13, MAP3K5, JAK2, NFKB1, NFKBIA, CD80, IL1B, CD86, BID, MAP2K3, CASP8, IL1RAP, TNF | 8.89E+00 |
| Role of macrophages, fibroblasts, and endothelial cells in RA | SOCS3, IL1A, ICAM1, PDGFA, TLR8, JAK2, NFKB1, VEGFA, NFAT5, NFKBIA, CCL2, OSM, MAPKAPK2, IL1RAP, FZD2, TRAF1, MAP3K14, C5AR1, CREB3, TLR2, CSF1, IL1RN, IL1B, MAP2K3, TNF, IRAK2 | 8.86E+00 |
| Acute phase response signaling | SOCS3, MAP3K14, IL1A, C3, MAP3K1, JAK2, MAPK13, MAP3K5, NFKB1, NFKBIA, SOD2, IL1RN, OSM, IL1B, SERPINA1, MAP2K3, SERPINE1, IL1RAP, TNF | 8.48E+00 |
| Granulocyte adhesion and diapedesis | IL1A, C5AR1, ICAM1, CCL20, CCL22, SDC4, SELPLG, CXCL3, CLDN12, CCL2, CLDN1, IL1RN, IL1B, CXCL1, CXCL2, IL1RAP, TNF, MMP9, CCL1 | 8.14E+00 |
| IL-6 signaling | SOCS3, MAP3K14, IL1A, MAPK13, JAK2, NFKB1, VEGFA, NFKBIA, IL1RN, IL1B, MAP2K3, MAPKAPK2, IL1RAP, TNF, MCL1 | 7.71E+00 |
| IL-10 signaling | SOCS3, MAP3K14, IL1A, IL4R, NFKBIA, IL1RN, IL1B, MAP2K3, MAPK13, NFKB1, TNF, IL1RAP | 7.54E+00 |
| Altered T cell and B cell signaling in RA | MAP3K14, IL1A, SLAMF1, TLR8, NFKB1, TLR2, CD80, CD40, CSF1, IL1RN, CD86, IL1B, TNF | 7.46E+00 |
| TREM1 signaling | TLR2, CXCL3, TREM1, ICAM1, CCL2, CD40, TLR8, CD86, IL1B, JAK2, NFKB1, TNF | 7.27E+00 |
| CD40 signaling | MAP3K14, ICAM1, NFKBIA, CD40, TNFAIP3, MAP2K3, MAPK13, PTGS2, MAPKAPK2, NFKB1, TRAF1 | 7.03E+00 |
| CD27 signaling in lymphocytes | MAP3K14, NFKBIA, MAP3K1, BID, MAP3K8, MAP2K3, MAP3K5, CASP8, NFKB1, MAP3K2 | 6.99E+00 |
| Agranulocyte adhesion and diapedesis | IL1A, C5AR1, ICAM1, CCL20, CCL22, SDC4, SELPLG, CXCL3, CLDN12, CCL2, CLDN1, IL1RN, IL1B, CXCL1, CXCL2, TNF, MMP9, CCL1 | 6.98E+00 |
| NF-κB signaling | MAP3K14, IL1A, FLT1, MAP3K1, TLR8, TNFAIP3, MALT1, NFKB1, TGFBR2, TLR2, NFKBIA, CD40, IL1RN, IL1B, MAP3K8, CASP8, TNF | 6.86E+00 |
| Role of IL-17A in arthritis | CXCL3, NFKBIA, CCL2, CCL20, MAP2K3, CXCL1, MAPK13, PTGS2, MAPKAPK2, NFKB1 | 6.67E+00 |
| DC maturation | MAP3K14, IL1A, ICAM1, CREB3, MAPK13, JAK2, NFKB1, STAT4, TLR2, NFKBIA, CD40, CD80, IL1RN, CD86, IL1B, IRF8, TNF | 6.64E+00 |
| BCR signaling | BLNK, MAP3K14, CREB3, MAP3K1, MAPK13, MALT1, MAP3K5, NFKB1, INPP5D, NFKBIA, NFAT5, SYK, MAP2K3, MAP3K8, RASSF5, BCL2A1, MAP3K2 | 6.57E+00 |
| TWEAK signaling | CASP6, MAP3K14, NFKBIA, BID, CASP8, NFKB1, TNFRSF12A, TRAF1 | 6.42E+00 |
| TNFR1 signaling | CASP6, MAP3K14, NFKBIA, MAP3K1, TNFAIP3, BID, CASP8, NFKB1, TNF | 6.17E+00 |
iDCs were cultured on ColII for 4 h in the presence or absence of GM-CSF (50 ng/ml), anti-OSCAR mAb (1 μg/ml), or isotype control (1 μg/ml). Total cellular RNA was isolated, and genome-wide mRNA levels were determined using Affymetrix GeneChip microarray analysis. The table shows canonical pathways for OSCAR-ColII–regulated genes at an FDR of 5% and a biological criterion of minimum 2-fold change. A complete list of all genes regulated by the OSCAR-ColII signaling at 4 h time point is provided in Supplemental Table IC. Samples from six independent experiments were analyzed.
Discussion
Until recently, the naturally occurring ligand for OSCAR remained unknown. In the present study we have identified OSCAR ligand expressed on murine preosteoblasts as being α1 ColI. OSCAR interaction with native human ColI and ColII was confirmed by the SPR technique. While we were conducting our experiments, the Trowsdale et al. (14) published their discovery of collagens as ligands of OSCAR. In this study, the authors used a synthetic ColII peptide library and identified a number of peptides showing direct binding to human OSCAR. Some of these peptides were efficient stimulators of receptor activator for NF-κB ligand–dependent osteoclastogenesis. The authors confirmed binding of OSCAR to native human ColI, ColII, and ColIII. We neither saw a binding to native ColIII using SPR analysis nor a biological effect on DCs, which might be attributed to differences in qualities of the native protein preparations used in the two studies.
Collagens are broadly distributed in the human and, in addition to their structural function, they play an important role in cell adhesion, migration, proliferation, differentiation, and survival (24). These multiple and diverse effects are highly dependent on the ECM structure and remodeling (25). ColI is the main protein constituent of bone, whereas ColII is the major protein component of cartilage. Under pathological conditions, such as, for example, RA, the ECM undergoes excessive remodeling with drastic upregulation of the ColI–III turnover within the joints (26). We suggest that under such conditions, collagens of bone and cartilage may become available for interaction with infiltrating immune cells and may considerably influence their activation status and viability within RA joints. Indeed, it has been shown that OSCAR-expressing mononuclear cells are in contact with collagens in the joints and the OSCAR-binding sites are expected to be on the surface of collagens exposed to the infiltrating cells (14).
Among the cells expressing the OSCAR protein and potentially capable of entering synovial tissue upon inflammation are monocytes, monocyte-derived DCs, and lineage-negative CD11c+ DC (9). Whereas the latter are thought to represent a rare heterogeneous population of cells found in health and disease, monocytes may serve as precursors of inflammatory DCs, which are only generated in pathological conditions, such as, for example, RA (27). Development of such inflammatory, fully functional DCs is commonly modeled in vitro by treatment of peripheral blood monocytes with GM-CSF and IL-4 (19). The monocyte-derived DCs are potent APCs, which secrete multiple proinflammatory cytokines and express CD1a, a surface marker that was shown to be specific for this cell type (28). CD1a+ cells were found in RA synovial tissue, where they are thought to be functional (29). In the present study, we chose to focus on this population of inflammatory DCs to mechanistically address the outcome of OSCAR–collagen signaling with relevance to the RA pathogenesis, although we cannot exclude an impact of the OSCAR–collagen interaction on the Ag-presenting function in subpopulations of the CD11c+ cells, which should further be addressed in clinical studies.
Merck et al. (9, 11) have shown that cross-linking of OSCAR with the receptor-specific Abs can provide survival, activation, and maturation signals to human monocyte-derived DCs. The use of cross-linking mAbs to stimulate the ITAM-bearing receptors via aggregation is an efficient way to address the signaling potential of a receptor when its natural ligand is unknown. However, an endogenous ligand may induce a signal of different magnitude and quality, depending on the affinity of interaction and cross-talking signaling pathways modulating the ITAM signal. Therefore, it is essential to investigate the outcome of the OSCAR signaling in the presence of the native ligand in the cells where other collagen receptors are coexpressed.
OSCAR expression in human monocyte-derived DCs (11) and monocytes (30) was suggested to support cell survival in the absence of growth factors via induction of antiapoptotic molecules Bcl-2 and Bcl-xL through PI3K and ERK signaling. In our study, the DCs plated on ColI/II showed prolonged viability in the absence of the essential growth factor, GM-CSF, indicating that OSCAR could play an important role in cell survival. Expression of genes encoding multiple components of the ERK pathway (including MAP3K1 and MAP3K8), as well as BCL2A1 encoding a Bcl-2 related protein, was strongly upregulated by the OSCAR–Col signaling. Autocrine TNF-α was previously shown to provide survival signals to DCs (20). Blocking of TNF-α with either soluble p55TNFR-Fc or anti–TNF-α mAb (infliximab) significantly reduced survival of LPS-matured DCs (31). We show in the present study that survival of Col-DCs was impaired by treatment with Enbrel, although to a much lower degree than by treatment with anti-OSCAR mAb. These data indicate that collagen supports survival of DCs at least in part via an autocrine TNF-α signaling.
DCs plated on ColI/II secreted a number of cytokines with a proinflammatory function. In contrast to the OSCAR cross-linking data (9, 11), we observed a profound induction of IL-6, IL-1β, and TNF-α by ColI/II. The discrepancy might be due to a higher sensitivity of the detection method used in the present study (Luminex versus ELISA) or due to the differences in biological set-up, where exposure to ColI/II may provide additional signals necessary for more efficient performance of the OSCAR pathway. Most of the cytokines and their receptors were found to be transcriptional targets of OSCAR based on the microarray analysis. A number of chemokines were also induced by OSCAR–collagen signaling, including CCL20 (recruiting iDCs), IL-8 (chemoattractant for granulocytes), and RANTES (CCL5, chemoattractant for T cells) as well as attractants for endothelial cell precursors CXCL1, -2, and -3. The components of the pathways regulating expression of these proinflammatory mediators (e.g., NF-κB pathway members, STAT pathway members, MAPKs) were also upregulated by collagen in an OSCAR-dependent manner at the transcriptional level. Merck et al. (11) have shown potentiation of TLR signaling by OSCAR cross-linking characterized by secretion of proinflammatory cytokines and maturation of DCs. In the present study, we provide evidence that the OSCAR signaling pathway upregulates transcription of the genes encoding receptors and multiple components of the TLR signaling machinery.
Earlier studies indicated that ColI can stimulate maturation of human monocyte-derived DCs in an integrin-independent manner (21), the mechanism for which so far remained undiscovered. Our data show that the OSCAR signaling pathway controls Col-induced DC maturation. The iDCs plated on ColI/II showed fast and drastic upregulation of maturation markers. Microarray analysis has shown that most of the major components of the stable immune synapse, including CD40, CD80, CD86, and ICAM-1, are transcriptional targets of the OSCAR–collagen signaling. Treatment of cells with anti-OSCAR mAb completely blocked this collagen-induced maturation process. The cell surface expression of HLA-DR was upregulated in an OSCAR-dependent manner. However, expression of the MHC class II genes was not influenced by collagen exposure, indicating regulation at a posttranscriptional level. These results are in line with the previously published studies showing posttranscriptional regulation of the surface expression of MHC class II (32), particularly via protein recycling/trafficking to and from the plasma membrane (33, 34).
In contrast to the OSCAR cross-linking data (11), in our study triggering of the OSCAR signaling in DCs with a native ligand led to formation of matured cells, which efficiently stimulated proliferation of allogeneic and autologous naive T cells. Thus, even in the absence of microbial or inflammatory stimulation, OSCAR engagement by collagen was sufficient to induce a full DC maturation program. The T cells expanded by Col-DCs produced multiple cytokines mainly of the proinflammatory type, but with no clear signs of T cell polarization. Additional studies on phenotyping of these cells (e.g., microarray and transcription factor profiling) are needed to fully understand the T cell polarizing effect of the Col-DCs.
Upregulation of OSCAR expression on the surface of blood monocytes was found in RA patients with active disease, and OSCAR-expressing mononuclear cells were detected in perivascular areas of the synovium (13). The RA synovium is considered an ectopic lymphoid organ (35). Both immature and mature DCs are present in the RA synovium in about a 1:1 ratio. The immature cells are primarily located in the sublining or lining layer of the synovium and in the perivascular infiltrates, whereas mature DCs are associated with perivascular lymphocytic infiltrates (36, 37). These perivascular DCs were suggested to originate from blood-derived precursors that migrated through the activated endothelium and received differentiation signals, such as GM-CSF and IL-13, within the joint (reviewed in Ref. 38). It is thought that functional DC/T cell interaction can take place locally (39, 40), and the perivascular region may act as a site for cell activation (41). Intriguingly, surgical removal of cartilage in RA patients led to reduced inflammation and suppression of functional differentiation of DCs in the synovium (42). Because ColII makes up to 60% of dry weight of articular cartilage (43), we speculate that this observed effect might at least partially be attributed to interference with the OSCAR signaling in DCs.
Taken together, our data indicate that the principal role of OSCAR in DCs is proinflammatory. Although OSCAR is expressed in resting myeloid cells (9, 13), it may not be functional because the ligand accessibility is limited. We hypothesize that the OSCAR–collagen signaling plays a pivotal role in pathology, such as in RA, where ligands become available due to ongoing tissue remodeling. OSCAR may act at multiple levels contributing to perpetuation of local synovial inflammation and may impact systemic inflammation in RA via expansion of proinflammatory T cells.
Supplementary Material
Acknowledgments
We thank Lise F. W. Jensen, Jette W. Platou, Jing Wang, Bing Zhang, Helene Bregnbak, Jian Zhou, Wenjuan Xia, Xiaoai Wu, Li Yang, and Chunyan Ma for excellent technical assistance.
H.S.S. and L.M.N. received Ph.D. fellowships from the Danish Agency for Science, Technology, Innovation and Research.
The microarray dataset presented in this article has been submitted to the Array Express database (http://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-2904/) under accession number E-MTAB-2904.
The online version of this article contains supplemental material.
- Col
- collagen type
- Col-DC
- collagen-matured DC
- DC
- dendritic cell
- ECD
- extracellular domain
- ECM
- extracellular matrix
- FDR
- false discovery rate
- iDC
- immature DC
- LC-MS
- liquid chromatography–mass spectrometry
- MS
- mass spectrometry
- NP-40
- Nonidet P-40
- OSCAR
- osteoclast-associated receptor
- RA
- rheumatoid arthritis
- SPR
- surface plasmon resonance.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.McInnes I. B., Schett G. 2011. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365: 2205–2219. [DOI] [PubMed] [Google Scholar]
- 2.Holmdahl R., Malmström V., Burkhardt H. 2014. Autoimmune priming, tissue attack and chronic inflammation—the three stages of rheumatoid arthritis. Eur. J. Immunol. 44: 1593–1599. [DOI] [PubMed] [Google Scholar]
- 3.Thomas R., Davis L. S., Lipsky P. E. 1994. Rheumatoid synovium is enriched in mature antigen-presenting dendritic cells. J. Immunol. 152: 2613–2623. [PubMed] [Google Scholar]
- 4.Radstake T. R., van Lieshout A. W., van Riel P. L., van den Berg W. B., Adema G. J. 2005. Dendritic cells, Fcγ receptors, and Toll-like receptors: potential allies in the battle against rheumatoid arthritis. Ann. Rheum. Dis. 64: 1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebre M. C., Tak P. P. 2008. Dendritic cell subsets: their roles in rheumatoid arthritis. Acta Reumatol. Port. 33: 35–45. [PubMed] [Google Scholar]
- 6.Radstake T. R., van der Voort R., ten Brummelhuis M., de Waal Malefijt M., Looman M., Figdor C. G., van den Berg W. B., Barrera P., Adema G. J. 2005. Increased expression of CCL18, CCL19, and CCL17 by dendritic cells from patients with rheumatoid arthritis, and regulation by Fcγ receptors. Ann. Rheum. Dis. 64: 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivollier A., Mazzorana M., Tebib J., Piperno M., Aitsiselmi T., Rabourdin-Combe C., Jurdic P., Servet-Delprat C. 2004. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood 104: 4029–4037. [DOI] [PubMed] [Google Scholar]
- 8.Alnaeeli M., Penninger J. M., Teng Y. T. 2006. Immune interactions with CD4+ T cells promote the development of functional osteoclasts from murine CD11c+ dendritic cells. J. Immunol. 177: 3314–3326. [DOI] [PubMed] [Google Scholar]
- 9.Merck E., Gaillard C., Gorman D. M., Montero-Julian F., Durand I., Zurawski S. M., Menetrier-Caux C., Carra G., Lebecque S., Trinchieri G., Bates E. E. 2004. OSCAR is an FcRγ-associated receptor that is expressed by myeloid cells and is involved in antigen presentation and activation of human dendritic cells. Blood 104: 1386–1395. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa S., Arase N., Suenaga T., Saita Y., Noda M., Kuriyama T., Arase H., Saito T. 2004. Involvement of FcRγ in signal transduction of osteoclast-associated receptor (OSCAR). Int. Immunol. 16: 1019–1025. [DOI] [PubMed] [Google Scholar]
- 11.Merck E., de Saint-Vis B., Scuiller M., Gaillard C., Caux C., Trinchieri G., Bates E. E. 2005. Fc receptor γ-chain activation via hOSCAR induces survival and maturation of dendritic cells and modulates Toll-like receptor responses. Blood 105: 3623–3632. [DOI] [PubMed] [Google Scholar]
- 12.Tenca C., Merlo A., Merck E., Bates E. E., Saverino D., Simone R., Zarcone D., Trinchieri G., Grossi C. E., Ciccone E. 2005. CD85j (leukocyte Ig-like receptor-1/Ig-like transcript 2) inhibits human osteoclast-associated receptor-mediated activation of human dendritic cells. J. Immunol. 174: 6757–6763. [DOI] [PubMed] [Google Scholar]
- 13.Herman S., Müller R. B., Krönke G., Zwerina J., Redlich K., Hueber A. J., Gelse H., Neumann E., Müller-Ladner U., Schett G. 2008. Induction of osteoclast-associated receptor, a key osteoclast costimulation molecule, in rheumatoid arthritis. Arthritis Rheum. 58: 3041–3050. [DOI] [PubMed] [Google Scholar]
- 14.Barrow A. D., Raynal N., Andersen T. L., Slatter D. A., Bihan D., Pugh N., Cella M., Kim T., Rho J., Negishi-Koga T., et al. 2011. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J. Clin. Invest. 121: 3505–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canfield S. M., Morrison S. L. 1991. The binding affinity of human IgG for its high affinity Fc receptor is determined by multiple amino acids in the CH2 domain and is modulated by the hinge region. J. Exp. Med. 173: 1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider C., Newman R. A., Sutherland D. R., Asser U., Greaves M. F. 1982. A one-step purification of membrane proteins using a high efficiency immunomatrix. J. Biol. Chem. 257: 10766–10769. [PubMed] [Google Scholar]
- 17.Shevchenko A., Wilm M., Vorm O., Mann M. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68: 850–858. [DOI] [PubMed] [Google Scholar]
- 18.Kim N., Takami M., Rho J., Josien R., Choi Y. 2002. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J. Exp. Med. 195: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sallusto F., Lanzavecchia A. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179: 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludewig B., Graf D., Gelderblom H. R., Becker Y., Kroczek R. A., Pauli G. 1995. Spontaneous apoptosis of dendritic cells is efficiently inhibited by TRAP (CD40-ligand) and TNF-α, but strongly enhanced by interleukin-10. Eur. J. Immunol. 25: 1943–1950. [DOI] [PubMed] [Google Scholar]
- 21.Brand U., Bellinghausen I., Enk A. H., Jonuleit H., Becker D., Knop J., Saloga J. 1998. Influence of extracellular matrix proteins on the development of cultured human dendritic cells. Eur. J. Immunol. 28: 1673–1680. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z., Li N., Diaz L. A., Shipley M., Senior R. M., Werb Z. 2005. Synergy between a plasminogen cascade and MMP-9 in autoimmune disease. J. Clin. Invest. 115: 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulendran B., Tang H., Manicassamy S. 2010. Programming dendritic cells to induce TH2 and tolerogenic responses. Nat. Immunol. 11: 647–655. [DOI] [PubMed] [Google Scholar]
- 24.Hynes R. O. 2009. The extracellular matrix: not just pretty fibrils. Science 326: 1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daley W. P., Peters S. B., Larsen M. 2008. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 121: 255–264. [DOI] [PubMed] [Google Scholar]
- 26.Karsdal M. A., Woodworth T., Henriksen K., Maksymowych W. P., Genant H., Vergnaud P., Christiansen C., Schubert T., Qvist P., Schett G., et al. 2011. Biochemical markers of ongoing joint damage in rheumatoid arthritis—current and future applications, limitations and opportunities. Arthritis Res. Ther. 13: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shortman K., Naik S. H. 2007. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 7: 19–30. [DOI] [PubMed] [Google Scholar]
- 28.León B., López-Bravo M., Ardavín C. 2005. Monocyte-derived dendritic cells. Semin. Immunol. 17: 313–318. [DOI] [PubMed] [Google Scholar]
- 29.Steenbakkers P. G., Baeten D., Rovers E., Veys E. M., Rijnders A. W., Meijerink J., De Keyser F., Boots A. M. 2003. Localization of MHC class II/human cartilage glycoprotein-39 complexes in synovia of rheumatoid arthritis patients using complex-specific monoclonal antibodies. J. Immunol. 170: 5719–5727. [DOI] [PubMed] [Google Scholar]
- 30.Merck E., Gaillard C., Scuiller M., Scapini P., Cassatella M. A., Trinchieri G., Bates E. E. 2006. Ligation of the FcRγ chain-associated human osteoclast-associated receptor enhances the proinflammatory responses of human monocytes and neutrophils. J. Immunol. 176: 3149–3156. [DOI] [PubMed] [Google Scholar]
- 31.Baldwin H. M., Ito-Ihara T., Isaacs J. D., Hilkens C. M. 2010. Tumour necrosis factor alpha blockade impairs dendritic cell survival and function in rheumatoid arthritis. Ann. Rheum. Dis. 69: 1200–1207. [DOI] [PubMed] [Google Scholar]
- 32.Pai R. K., Askew D., Boom W. H., Harding C. V. 2002. Regulation of class II MHC expression in APCs: roles of types I, III, and IV class II transactivator. J. Immunol. 169: 1326–1333. [DOI] [PubMed] [Google Scholar]
- 33.Shin J. S., Ebersold M., Pypaert M., Delamarre L., Hartley A., Mellman I. 2006. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature 444: 115–118. [DOI] [PubMed] [Google Scholar]
- 34.Chow A., Toomre D., Garrett W., Mellman I. 2002. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature 418: 988–994. [DOI] [PubMed] [Google Scholar]
- 35.Kratz A., Campos-Neto A., Hanson M. S., Ruddle N. H. 1996. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J. Exp. Med. 183: 1461–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page G., Lebecque S., Miossec P. 2002. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J. Immunol. 168: 5333–5341. [DOI] [PubMed] [Google Scholar]
- 37.Manzo A., Bugatti S., Caporali R., Prevo R., Jackson D. G., Uguccioni M., Buckley C. D., Montecucco C., Pitzalis C. 2007. CCL21 expression pattern of human secondary lymphoid organ stroma is conserved in inflammatory lesions with lymphoid neogenesis. Am. J. Pathol. 171: 1549–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas R., MacDonald K. P., Pettit A. R., Cavanagh L. L., Padmanabha J., Zehntner S. 1999. Dendritic cells and the pathogenesis of rheumatoid arthritis. J. Leukoc. Biol. 66: 286–292. [DOI] [PubMed] [Google Scholar]
- 39.Baeten D., Steenbakkers P. G., Rijnders A. M., Boots A. M., Veys E. M., De Keyser F. 2004. Detection of major histocompatibility complex/human cartilage gp-39 complexes in rheumatoid arthritis synovitis as a specific and independent histologic marker. Arthritis Rheum. 50: 444–451. [DOI] [PubMed] [Google Scholar]
- 40.Timmer T. C., Baltus B., Vondenhoff M., Huizinga T. W., Tak P. P., Verweij C. L., Mebius R. E., van der Pouw Kraan T. C. 2007. Inflammation and ectopic lymphoid structures in rheumatoid arthritis synovial tissues dissected by genomics technology: identification of the interleukin-7 signaling pathway in tissues with lymphoid neogenesis. Arthritis Rheum. 56: 2492–2502. [DOI] [PubMed] [Google Scholar]
- 41.Krenn V., Schalhorn N., Greiner A., Molitoris R., König A., Gohlke F., Müller-Hermelink H. K. 1996. Immunohistochemical analysis of proliferating and antigen-presenting cells in rheumatoid synovial tissue. Rheumatol. Int. 15: 239–247. [DOI] [PubMed] [Google Scholar]
- 42.Li T. F., Mandelin J., Hukkanen M., Lassus J., Sandelin J., Santavirta S., Virtanen I., Konttinen Y. T. 2002. Dendritic cells in rheumatoid synovial membrane after total removal of the hyaline articular cartilage. Rheumatology (Oxford) 41: 319–323. [DOI] [PubMed] [Google Scholar]
- 43.Sophia Fox A. J., Bedi A., Rodeo S. A. 2009. The basic science of articular cartilage: structure, composition, and function. Sports Health 1: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.