Abstract
Intraarticular (IA) administration of viral vectors expressing a therapeutic transgene is an attractive treatment modality for osteoarthritis (OA) as the joint can be treated as a contained unit. Humoral and cell-mediated immune responses in vivo can limit vector effectiveness. Transduction of articular tissues has been investigated; however, the immune response to IA vectors remains largely unknown. We hypothesized that IA rAAV2 and rAAV5 overexpressing insulin-like growth factor-I (IGF-I) would result in long-term IGF-I formation but would also induce neutralizing antibodies (NAb) and anti-capsid effector T cells. Twelve healthy horses were assigned to treatment (rAAV2 or rAAV5) or control (saline) groups. Middle carpal joints were injected with 5×1011 vector genomes/joint. Synovial fluid was analyzed for changes in composition, NAb titers, immunoglobulin isotypes, proinflammatory cytokines, and IGF-I. Serum was analyzed for antibody titers and cytokines. A T cell restimulation assay was used to assess T cell responses. Injection of rAAV2- or rAAV5-IGF-I did not induce greater inflammation compared with saline. Synovial fluid IGF-I was significantly increased in both rAAV2- and rAAV5-IGF-I joints by day 14 and remained elevated until day 56; however, rAAV5 achieved the highest concentrations. A capsid-specific T cell response was not noted although all virus-treated horses had increased NAbs in serum and synovial fluid after treatment. Taken together, our data show that IA injection of rAAV2- or rAAV5-IGF-I does not incite a clinically detectable inflammatory or cell-mediated immune response and that IA gene therapy using minimally immunogenic vectors represents a clinically relevant tool for treating articular disorders including OA.
Introduction
Traumatic joint injury is common, and because of the poor intrinsic healing capabilities of cartilage, often precipitates osteoarthritis (OA).1 At present there are no disease-modifying treatments for cartilage damage or OA, with end-stage arthritic joints requiring total joint replacement surgery. Gene therapy approaches to enhance cartilage repair and treat OA have great potential. as vectors carrying transgenes can be injected intraarticularly for concentrated, local therapeutic protein production. Previous studies have shown that articular tissues, including synoviocytes and chondrocytes, can be transduced through direct intraarticular (IA) injection.2–4 Gene therapy techniques, which provide long-term in situ expression of repair-enhancing genes, would be superior to repeated injections or depots of peptide that are transient.
Insulin-like growth factor-I (IGF-I) has been shown to have anabolic and mitogenic effects on chondrocytes with increased production of extracellular matrix (ECM) proteins including collagen type II and aggrecan.5 IGF-I has also been shown to enhance the repair potential of chondrocytes6 and aids in the protection and recovery of the ECM after damage.7 Intraarticular overexpression of IGF-I after cartilage damage could aid in chondrocyte-mediated repair of the articular surface, thereby limiting joint degeneration and OA.
Many different viral vectors including retrovirus,8 lentivirus,9 and adenovirus2 have been investigated for use in OA; however, a significant impediment has been the substantial immune response, which limits transduction and transgene expression.2 Adeno-associated virus (AAV) may be a promising alternative as it is nonpathogenic, transduces dividing and nondividing cells and enables long-term transgene expression.10 Both rAAV2 and rAAV5 have been shown to effectively transduce equine synoviocytes and chondrocytes in our laboratory,11 and by others.12
Although AAV has great potential as a viral vector for gene therapy, the immune response to capsid proteins has surfaced as a major obstacle to its success. The humoral response has been well documented and it is estimated that 20–40% of humans have neutralizing antibody (NAb) titers against any given serotype.13,14 The prevalence of NAbs depends on serotype with 80% of humans having NAbs to AAV2.15 Several animal studies have shown that titers as low as 1:2–1:4 can prevent successful transduction.16–18 Although the immune response to AAV appears to be primarily humoral, a cellular immune response to epitopes present on the AAV capsid has been elucidated in several studies.19–22 Cytotoxic CD8+ T cells that recognize capsid proteins loaded into MHC-I would be of particular concern as they would be able to eliminate transduced cells.
In this study, we aimed to investigate the humoral and T cell response to IA rAAV2 and rAAV5, quantify the production of transgene IGF-I, and correlate the immune response with transgene expression. We hypothesized that rAAV2- and rAAV5-IGF-I would lead to minimal joint inflammation with increased levels of IGF-I in the synovial fluid. However, IGF-I concentrations would be limited by a humoral response against AAV2 and AAV5. In addition, we hypothesized that a T cell response would be invoked by restimulation of T cells with the AAV serotype used for the intraarticular injection.
Materials and Methods
Adeno-associated viral vector preparation
Full-length equine IGF-I cDNA was amplified by PCR and subcloned into the rAAV transfer plasmid pHpa-trs-SK, using SacII and NotI sites. The transgene was flanked by inverted terminal repeats (ITRs) and under the control of the cytomegalovirus (CMV) promoter. Self-complementary rAAV2-IGF-I and rAAV5-IGF-I vectors were generated by the Research Vector Core at the Children's Hospital of Philadelphia (CHOP, Philadelphia, PA) in HEK293 cells, using a triple-plasmid transfection method as described.23 AAV vectors were purified by density gradient centrifugation and viral titers were determined by quantitative dot-blot.
Intraarticular injection
All animal procedures were approved by the Institutional Laboratory Animal Care and Use Committee at Cornell University (Ithaca, NY). Twelve healthy adult horses between the ages of 5 and 19 years (median age, 12.5 years) were assigned to one of three treatment groups: (1) injection with Dulbecco's phosphate-buffered saline (DPBS), (2) injection with rAAV2-IGF-I, or (3) injection with rAAV5-IGF-I, with four horses per group. One middle carpal (MC) joint of each horse was randomly assigned for injection with rAAV2-IGF-I, rAAV5-IGF-I, or DPBS control. There were seven Thoroughbreds, one Quarter Horse, and three Warmbloods.
Intraarticular injection was performed in horses sedated with xylazine hydrochloride (0.4 mg/kg, intravenous) and butorphanol (0.01 mg/kg, intravenous). Middle carpal joints were clipped and prepared aseptically, held in flexion, and a 21-gauge, 1.5-inch needle was inserted into the dorsolateral aspect of the joint. Synovial fluid was collected from the joint before injection of 5×1011 viral genomes (VG) in 3 ml of sterile DPBS or 3 ml of sterile DPBS on day 0.
Clinical response to joint injection
Before injection with the viral vector, horses were evaluated for lameness according to the American Association of Equine Practitioners (AAEP) grading system (0–5),24 range of motion of the carpi (0–4), joint effusion (0–4), and response to sustained joint flexion (0–4). The circumferences of both MC joints were measured with a flexible measuring tape. Lameness evaluations and joint circumference measurements were repeated on days 4, 7, 14, 28, and 56 postinjection.
Whole blood and synovial fluid from both MC joints were collected immediately before viral vector injection and then on days 4, 7, 14, 28, and 56 postinjection. Cytological analysis of the synovial fluid was performed with total and differential leukocyte counts and smear analysis performed by Coulter (Beckman Coulter, Fullerton, CA). Smear evaluation was performed on Wright-stained smears (Hematek; Siemens Healthcare, Erlangen, Germany). Synovial fluid was analyzed for IGF-I concentration by ELISA (Quantikine; R&D Systems, Minneapolis, MN). Synovial fluid was also analyzed for cytokine concentration by fluorescent bead-based multiplex assays (interleukin [IL]-10, interferon [IFN]-γ, IFN-α, soluble CD14 [sCD14], and CCL2 [chemokine, C-C motif, ligand 2]).25,26
Synovial membrane analysis
Synovial tissue was collected from the dorsal joint space 56 days postinjection. Biopsies were performed with horses standing and sedated. A scalpel was used to incise the skin and joint capsule and Ferris-Smith rongeurs were used to collect synovium. Samples were fixed in 4% paraformaldehyde or placed in a −80°C freezer for RNA analysis. Synovial membrane was sectioned and stained with hematoxylin and eosin (H&E) for scoring of inflammatory changes (Supplementary Table S1; supplementary data are available online at www.liebertpub.com/hum).
Frozen synovial membrane samples were pulverized with a mortar and pestle, mechanically homogenized, and then RNA was isolated with a PerfectPure RNA tissue kit (5 Prime, Gaithersburg, MD). Purity and concentration of the RNA were assessed by ultraviolet spectrophotometry. Gene expression was quantified by real-time PCR, using the TaqMan One-Step RT-PCR technique (Absolute Quantitative PCR; ABI PRISM 7900 HT sequence detection system; Applied Biosystems, Foster City, CA). Primer Express software version 2.0 (Applied Biosystems) was used to design equine primers and dual-labeled fluorescent probes (6-carboxyfluorescein [FAM] as the 5′ label [reporter dye] and 5-carboxymethylrhodamine [TAMRA] as the 3′ label [quenching dye]) (Supplementary Table S2). Gene expression of IGF-I was measured with all samples being run in duplicate. The total copy number of mRNA was determined by comparison with a validated standard curve, and these values were normalized to the housekeeping gene 18S.
Neutralizing antibody assay
AAV vectors carrying the lacZ gene driven by the CMV promoter were generated by the Vector Core of the University of Pennsylvania (Philadelphia, PA), as described elsewhere.13 Recombinant AAV genomes with AAV2 ITRs were packaged in triple transfection 293 cells with cis-plasmid, adenovirus helper plasmid, and a chimeric packaging construct in which the AAV2 rep gene was fused with cap genes of AAV2 and AAV5 serotypes, as described.13 All vectors were purified by CsCl sedimentation and titers were determined by qPCR, using probes and primers targeting a bovine growth hormone polyadenylation signal, as described elsewhere.27
Serum and synovial fluid samples collected on days 0, 28, and 56 were heat inactivated at 56°C for 35 min and analyzed by an in vitro neutralizing antibody assay.13 Recombinant rAAV-LacZ (109 VG/well) was diluted in serum-free Dulbecco's modified Eagle's medium (DMEM) and incubated with 2-fold serial dilutions (initial dilution, 1:5) of samples for 1 hr at 37°C. The mixture was added to 96-well plates seeded with 1×105 Huh7 cells per well infected 2 hr previously with wild-type HAdV5 (50 viral particles/cell). After 1 hr, wells were supplemented with an equal volume of 20% fetal bovine serum–DMEM and incubated for 18–22 hr at 37°C. Cells were then washed with PBS and lysed, and lysate was developed with a β-galactosidase (β-Gal) assay kit for bioluminescence (Applied Biosystems). Bioluminescence was measured in a microplate luminometer (Clarity; BioTek, Winooski, VT). Neutralizing antibody titers were reported as the highest sample dilution that inhibited rAAV-LacZ transduction (β-Gal expression) by ≥50%, compared with mouse serum control. Titers to AAV2 were evaluated in serum and synovial fluid from rAAV2-injected (n=4) and DPBS-injected (n=4) horses, and AAV5 titers were evaluated in serum and synovial fluid from rAAV5-injected (n=4) and DPBS-injected (n=4) horses.
Immunoglobulin isotyping
Immunoglobulin isotyping assays were performed as previously described with some modifications.28,29 ELISA plates (Costar; Corning, Corning, NY) were coated with rAAV2 or rAAV5 virions at 1 μg/ml and incubated at 4°C overnight. Plates were then incubated with blocking buffer (4% bovine serum albumin [BSA]–PBS) for 1 hr. Plates were washed five times between all subsequent steps. Equine serum or synovial fluid samples were diluted 1:100 and incubated for 1 hr at 37°C. Serum and synovial fluid samples with known high antibody titers from previous testing were serially diluted and used to establish a standard curve. The highest dilution was assigned an ELISA unit of 200 or 400 depending on the isotype being tested. Serum and synovial fluid samples with known negative titers were used as a negative reference sample. Plates were incubated for 1 hr with primary monoclonal mouse anti-equine antibodies including anti-IgM (antibody 1-22; diluted 1:20), anti-IgG1 (antibody CVS 45; diluted 1:10), anti-IgG4 (antibody CVS 39; diluted 1:20), and anti-IgG5 (antibody 416-2; diluted 1:10). A secondary peroxidase-conjugated anti-mouse IgG (H+L) antibody (Jackson ImmunoResearch, West Grove, PA) diluted 1:200,000 was added, followed by tetramethylbenzidine substrate for chromogen development. Optical density was measured with a Tecan plate reader for absorbance at 450 nm.
T cell intracellular cytokine assay
Whole blood was collected from horses 7 days before virus injection and on days 14 and 56 postinjection. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (Ficoll-Paque Plus; GE Healthcare, Piscataway, NJ). PBMCs (3×106) were cultured in 24-well plates in 1000 μl of cell culture medium (DMEM, 10% [v/v] fetal calf serum, 1% [v/v] nonessential amino acids, 2 mM l-glutamine, 50 μM 2-mercaptoethanol, gentamicin [50 μg/ml]; all from Gibco, Invitrogen, Grand Island, NY). Stimulated cells were treated with phorbol myristate acetate (PMA)–ionomycin (Sigma, St. Louis, MO) or with rAAV serotype 2 or 5. Nonstimulated cells were cultured in medium only. At 24 hr of culture, brefeldin A (Sigma) was added to all wells. After 48 hr of culture, cells were fixed, permeabilized, and stained for CD4 or CD8, and for IFN-γ expression, by using directly labeled antibodies and isotype-matched controls as previously described.30 A FACSCanto II flow cytometer (BD Biosciences, San Diego, CA) was used to analyze cells with fluorescence gates set according to isotype control staining.
PBMC cytokine secretion assay
PBMCs (6×105) were cultured in 96-well plates in 200 μl of culture medium as described previously. Stimulated cells were treated with PMA–ionomycin or with rAAV serotype 2 or 5. Nonstimulated cells were cultured in medium only. After 48 hr of culture at 37°C supernatants were collected and analyzed for IL-10, IFN-γ, and IFN-α concentration, using a fluorescent bead-based multiplex assay.25 IL-6 concentrations were quantified by ELISA.31
Statistics
A Kruskal–Wallis test was used to compare differences between groups. A mixed effects model, with horse as a random effect, was constructed and least-square means were used to compare changes in synovial fluid composition and IGF-I concentration over time. Day was treated as a categorical variable to allow for the nonlinear effect of time. Multiple comparisons for each interaction were made with a Tukey post hoc test. Specific linear contrasts were fitted to the model where appropriate. Continuous data that did not appear to have a Gaussian distribution were transformed using the natural logarithm. A mixed effects model was also used for analysis of associations between NAb titers, immunoglobulins, and IGF-I concentrations. Statistical analysis was performed with JMP. The level of significance was set at p<0.05.
Results
Intraarticular rAAV injection does not incite a clinically significant inflammatory response in the joint
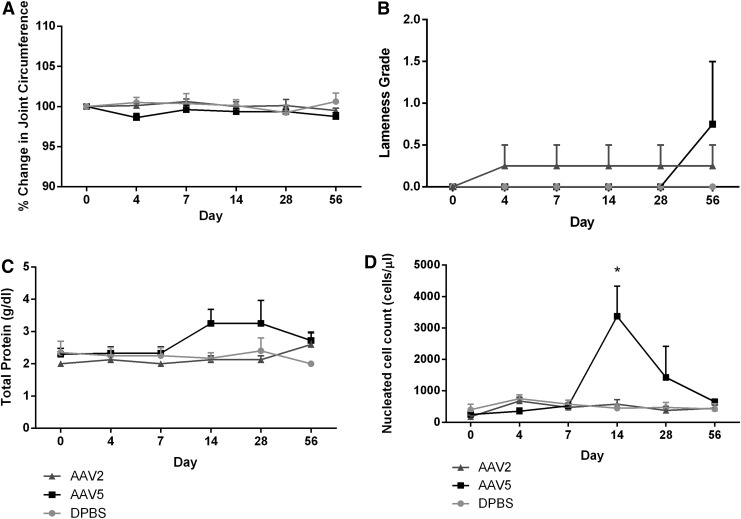
The clinical response of injected MC joints to IA injection was determined by evaluating horses for joint pain (lameness), changes in joint circumference, joint effusion, range of motion, and response to sustained flexion of injected joints over the 56-day study period. Vector injection was well tolerated by all horses with no adverse reactions noted. rAAV2-IGF-I or rAAV5-IGF-I did not induce greater articular inflammation compared with DPBS-injected control joints as lameness grades, percent change in joint circumference, joint effusion, range of motion, and response to sustained flexion were not different among any of the treatment groups at any time point (Fig. 1A and B).
FIG. 1.
Joint response to middle carpal joint intraarticular administration of rAAV2-IGF-I, rAAV5-IGF-I, or DPBS. (A) Percent change in joint circumference was measured after vector injection to quantify joint swelling. (B) Lameness grading was performed serially after injection, using the AAEP scoring system.24 (C and D) Synovial fluid total protein concentration and total nucleated cell count, respectively, were quantified to evaluate general inflammatory changes in synovial fluid composition. Graphs illustrate mean values±SEM averaged from four horses. *p<0.05.
Synovial fluid was serially collected and its composition was analyzed (total protein and nucleated cell count) to further quantify the local articular response to vector injection. Synovial total protein (TP) levels were slightly increased over baseline in rAAV5-IGF-I-treated joints on days 14 (3.25±0.44 g/dl) and 28 (3.25±0.72 g/dl); however, these increases were not statistically or clinically significant, and returned to baseline by day 56 (Fig. 1C). Total protein did not increase in rAAV2-IGF-I-treated joints (Fig. 1C). Synovial fluid total nucleated cell count (NCC) was significantly increased in rAAV5-IGF-I-treated joints on day 14 (3375±957.73 cells/μl; p<0.0001) only (Fig. 1D). NCCs in rAAV5-IGF-I-treated joints decreased after this time point and were not significantly different from baseline on day 28 or 56 (Fig. 1D).
To further assess the inflammatory response to rAAV injection, serum and synovial fluid were evaluated for several cytokines and chemokines, all of which are indicative of viral infection or inflammation in horses. A multiplex fluorescent bead assay was used to quantify IL-10, IFN-γ, IFN-α, sCD14, and CCL2 with no significant differences observed in serum or synovial fluid at any time point, suggesting that vector injection did not cause a significant inflammatory response.
Further assessment of the joint response to rAAV injection was performed by collecting synovial membrane biopsies 56 days postinjection. Synovium was stained with H&E and scored for inflammatory changes including degree of villi clubbing, subintimal thickening, fibrosis, vasculature, and inflammatory cell infiltration (Supplementary Table S1). Joints injected with rAAV2 or rAAV5 did not have significantly increased inflammatory scores compared with saline-injected joints, further supporting lack of a detectable inflammatory response (Supplementary Fig. S1).
IGF-I is significantly increased after direct IA injection
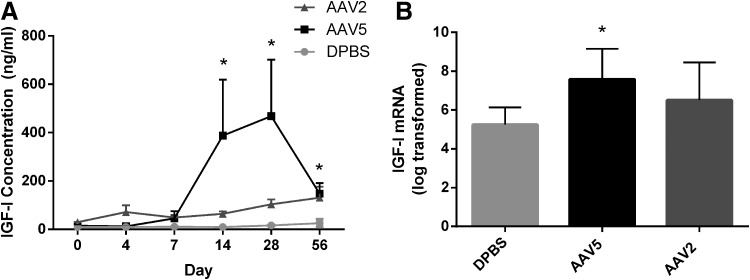
Synovial fluid, collected from injected joints over time, was assayed for IGF-I concentration by ELISA to quantify transgene expression. IGF-I concentrations in the synovial fluid from both rAAV2 and rAAV5 joints began to increase on day 14 postinjection. Although IGF-I concentrations in joints injected with rAAV2-IGF-I and rAAV5-IGF-I were both significantly increased over joints treated with DPBS on days 14, 28, and 56 (Fig. 2A), rAAV5-IGF-I injection led to much higher IGF-I concentrations than did rAAV2-IGF-I injection.
FIG. 2.
Synovial fluid concentration and mRNA expression of IGF-I from rAAV2 and rAAV5 vectors after direct intraarticular (IA) injection. (A) IGF-I concentration (ng/ml) in the synovial fluid over time in joints injected with either rAAV2-IGF-I, rAAV5-IGF-I, or DPBS. IGF-I concentration was measured by ELISA. Graphs illustrate mean values±SEM averaged from four horses per group. *IGF-I concentration significantly increased in rAAV5- and rAAV2-injected horses compared with DPBS-injected horses (p<0.05). (B) IGF-I mRNA expression in the synovial membrane 56 days after IA injection of either rAAV2-IGF-I, rAAV5-IGF-I, or DPBS. IGF-I values were transformed, using natural log transformation. mRNA was measured by qPCR and expressed as total copy number, using a validated standard curve. *p<0.05.
To further determine IA transgene expression, synovial membrane biopsies collected on day 56 were analyzed for IGF-I mRNA expression by qPCR. IGF-I mRNA expression was 25 times higher in synovial membrane tissue from joints treated with rAAV5-IGF-I and 10 times higher in synovial membrane tissue from joints treated with rAAV2-IGF-I than in joints treated with saline (Fig. 2B). IGF-I expression in the synovial membrane from rAAV5-IGF-I-treated joints was significantly increased over DPBS treatment (p=0.03) at 56 days posttransduction. Overall, IGF-I mRNA expression and synthesis were higher in rAAV5-IGF-I-treated joints compared with rAAV2-IGF-I-treated joints.
Capsid-specific CD4+and CD8+ effector T cells are not present in restimulated PBMC cultures
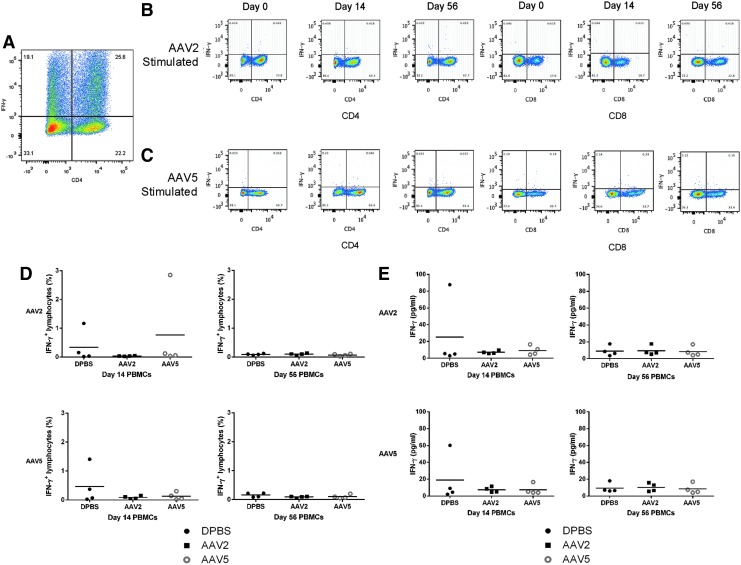
To characterize the T cell response to rAAV2 and rAAV5, PBMCs were isolated from the three treatment groups, before and after exposure, and then restimulated with rAAV2 or rAAV5. Cells were stained for CD4 and CD8, and intracellular IFN-γ, as a marker of T cell activation, and then quantified by fluorescence-activated cell sorting (FACS) (Fig. 3).
FIG. 3.
Flow cytometric analysis and IFN-γ secretion after restimulation assay of PBMCs with rAAV2 or rAAV5. PBMCs were isolated from horses injected intraarticularly with DPBS, rAAV2-IGF-I, or rAAV5-IGF-I before viral exposure (day 0) and on day 14 and day 56 postinjection. After PBMC restimulation, cells were stained for CD4, CD8, and intracellular IFN-γ. An isotype control was used to establish the quadrant gates for IFN-γ. Supernatant IFN-γ concentration was quantified in a multiplex assay. (A) Representative plot of PBMCs stimulated with PMA–ionomycin to validate cell viability and T cell IFN-γ production in response to stimulation. (B) Representative plots of rAAV2-restimulated PBMCs collected on days 0, 14, and 56 from rAAV2-IGF-I-injected horses. CD4+ and CD8+ T cells are shown. (C) Representative plots of rAAV5-restimulated PBMCs collected on days 0, 14, and 56 from rAAV5-IGF-I-injected horses. CD4+ and CD8+ T cells are shown. (D) Total percentages of IFN-γ-producing T cells from horses injected with DPBS, rAAV2-IGF-I, or rAAV5-IGF-I and restimulated with either rAAV2 or rAAV5 on day 14 and day 56. (E) IFN-γ (ng/ml) secretion measured in the supernatants of rAAV2- or rAAV5-restimulated PBMCs of three treatment groups on days 14 and 56.
A T cell response to rAAV2 or rAAV5 was not noted in any treatment group, whether previous exposure to the specific serotype had occurred or not. As shown in Fig. 3, IFN-γ+ lymphocytes in either CD4+ or CD8+ populations were not significantly increased after restimulation in any treatment group, other than PMA–ionomycin treatment, indicating lack of a capsid-specific T cell response (Fig. 3A–D). More specifically, T cells from horses injected with rAAV2 did not have a significant response to restimulation with rAAV2 14 or 56 days after initial exposure, and T cells collected from horses injected with rAAV5 did not have a response to restimulation with rAAV5. Cross-reactivity to either serotype was not noted at any time point.
Further evaluation of the T cell response was performed by analyzing PBMC culture supernatants for production of IL-10, IFN-γ, IFN-α, and IL-6, all of which are characteristic of T cell activation, viral infection, or inflammation. Stimulation of cells from all three treatment groups with either AAV serotype did not induce significant amounts of IFN-γ (Fig. 3E), which correlates with the results obtained by flow cytometric analysis. In addition, there were no significant changes in IL-10, IFN-α, or IL-6 in any of the treatment groups.
All horses had preexisting AAV5 NAbs, and rAAV5 injection led to a more robust and diverse immunoglobulin response
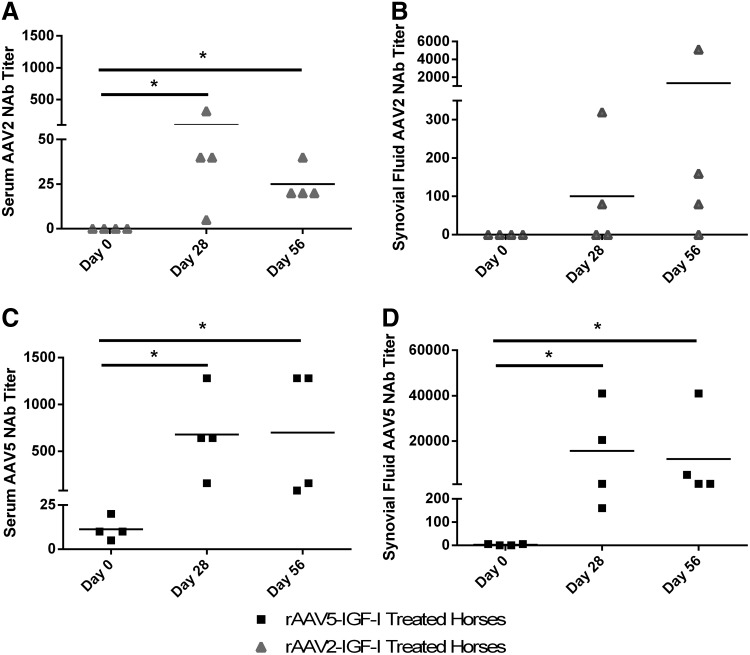
To quantify preexisting NAb titers and evaluate the NAb response, serum and synovial fluid were serially collected during the 56-day period and an in vitro transduction inhibition assay was performed (Fig. 4). All tested horses had preexisting NAb titers to AAV5 in serum and five of eight horses had NAb titers in synovial fluid. This is in contrast to AAV2, to which no tested horses had preexisting NAb titers in either serum or synovial fluid. The NAb response on days 28 and 56 was more robust to AAV5 than AAV2 in both serum and synovial fluid (Fig. 4A–D). There was a significant correlation between serum and synovial fluid AAV5 NAb titers (r2=0.54, p=0.007); however, there was no significant correlation between serum and synovial fluid AAV2 NAb titers.
FIG. 4.
Reciprocal neutralizing antibody titers (NAbs) to AAV2 and AAV5 were measured in serum and synovial fluid before and after IA injection. NAb titers were determined in a transduction inhibition assay. (A) Serum NAbs to AAV2 in horses injected with AAV2 (n=4). (B) Synovial fluid NAbs to AAV2 in horses injected with AAV2 (n=4). (C) Serum NAbs to AAV5 in horses injected with AAV5 (n=4). (D) Synovial fluid NAbs to AAV5 in horses injected with AAV5 (n=4). A titer ≥1:5 was considered positive. *p<0.05.
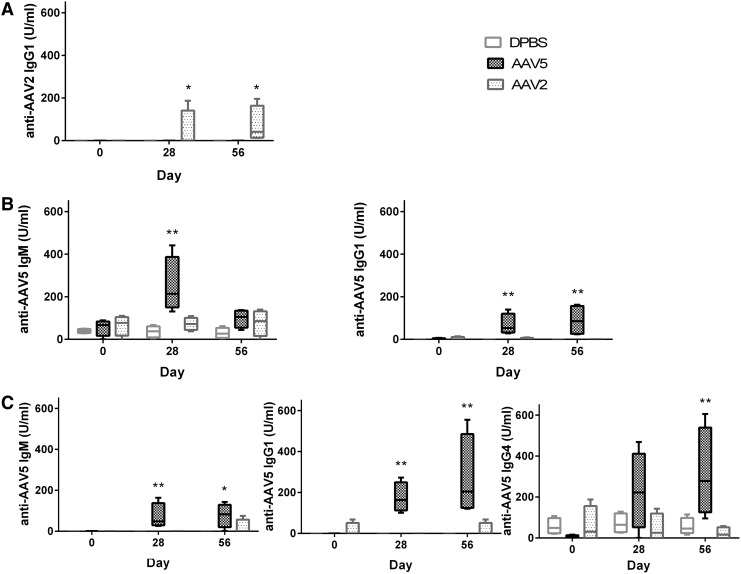
To further characterize the NAb response to rAAV injection, we performed immunoglobulin isotyping assays, using serum and synovial fluid taken from treated horses before and after vector injection. The main immunoglobulin isotypes of horses were assessed, including IgM, IgG1, IgG4, and IgG5. Before rAAV joint injection, 11 of 12 horses had serum IgM against AAV2 and 10 of 12 horses had IgM against AAV5. All horses had detectable IgG5 to both AAV2 and AAV5 whereas 6 of 12 horses had detectable IgG4 to both serotypes. No horses had IgG1 against AAV2 or AAV5 in the serum before injection. Figure 5 shows only the statistically significant changes for the four different immunoglobulin isotypes after injection of rAAV2 or rAAV5 in the three treatment groups. The humoral response to rAAV5 injection appeared to be more diverse as there were more significant changes in immunoglobulin isotypes postinjection (Fig. 5B and D).
FIG. 5.
Immunoglobulin isotype results from horses injected with DPBS, rAAV2, or rAAV5. Serum and synovial fluid were collected and analyzed for anti-AAV2 or anti-AAV5 IgM, IgG1, IgG4, and IgG5 by ELISA, using equine-specific antibodies. Only serotypes and isotypes with significant results are shown. (A) Synovial fluid anti-AAV2 IgG1 significantly increased in rAAV2-injected joints on days 28 and 56. (B) Serum anti-AAV5 IgM was significantly increased on day 28 in horses injected with rAAV5, whereas serum anti-AAV5 IgG1 was significantly increased on days 28 and 56 in horses injected with rAAV5. (C) Synovial fluid anti-AAV5 IgM and IgG1 were significantly increased on days 28 and 56 in horses injected with rAAV5, whereas synovial fluid anti-AAV5 IgG4 was significantly increased on day 56 only. *p<0.05; **p<0.01.
Correlation between humoral response and IGF-I concentrations in synovial fluid
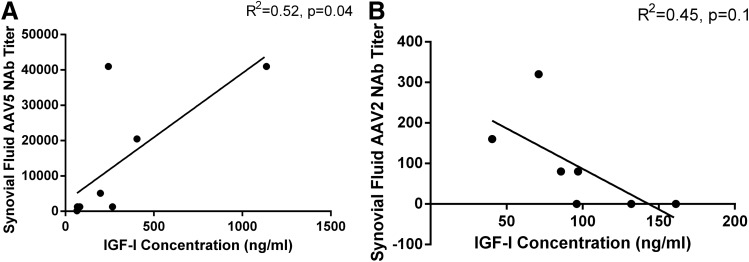
To investigate the effect of NAbs in the synovial fluid on transgene expression, we examined the relationship between NAb titers and IGF-I concentration. Interestingly, in rAAV5-treated horses there was a significant positive correlation between IGF-I concentration and AAV5 NAb titers in the synovial fluid (r2=0.52, p=0.04), suggesting that horses with higher synovial fluid NAb titers had higher transgene expression (Fig. 6A). The relationship between AAV2 NAb titers in the synovial fluid and IGF-I concentration in rAAV2-treated horses was not significant (Fig. 6B).
FIG. 6.
Correlation between synovial fluid AAV5 NAb titers or AAV2 NAb titers and synovial fluid IGF-I concentration in horses after IA injection of rAAV5-IGF-I (n=4) or rAAV2-IGF-I (n=4). (A) A significant, positive correlation between AAV5 NAb titer and IGF-I concentration in the synovial fluid was noted such that horses with higher titers appeared to have higher articular transgene expression (p=0.04). (B) The relationship between AAV2 NAb titer and IGF-I concentration in the synovial fluid was not significant (p=0.1).
Discussion
In this study, we evaluated the immune response and transgene expression, after direct IA injection of rAAV2 and rAAV5 in the equine middle carpal joint. Both vectors were able to effectively transduce articular tissues of the middle carpal joints with expression of the therapeutic transgene, IGF-I, throughout the 8-week study period (Fig. 2). Encouragingly, injection of either vector did not cause any adverse effects or clinically significant articular inflammation. There were no statistically significant changes in joint effusion or circumference, lameness grades, or inflammatory cytokine and chemokine concentrations (Fig. 1). Synovial membrane histology showed no evidence of inflammatory infiltrates 56 days postinjection (Supplementary Fig. S1). Although both serotypes successfully transduced host tissues, rAAV5-IGF-I showed superior transgene expression with higher synovial fluid concentrations of IGF-I and increased gene expression of IGF-I in the synovial tissue (Fig. 2). This was in spite of all horses having preexisting NAb titers to AAV5 and a more robust humoral immune response to rAAV5, compared with rAAV2 (Fig. 4C and D). Synovial fluid IGF-I was not significantly increased in rAAV2- or rAAV5-injected joints, compared with DPBS-injected joints, until day 14 postinjection, consistent with a lag period between time of injection and functional transgene production by the host cells.
IGF-I concentrations remained significantly increased until day 56 postinjection. Previous studies have shown that synoviocytes are the predominant cell type transduced after IA injection, as chondrocytes are largely sequestered in a thick extracellular matrix.32,33 Although synoviocytes appear to be easily transduced, the half-life of these cells is less than 1 month,33 which can limit longevity of transgene expression in joints. Intraarticular transgene expression at 56 days suggests transduction of longer lasting cell types such as chondrocytes or fibroblasts in the joint capsule and supporting ligaments, as seen in murine joints injected with AAV.3
Although the immune response to AAV appears to be significantly less than to similar vectors, such as adenovirus, several studies have shown limited transduction efficiency and longevity of transgene expression in animals, with titers as low as 1:4.16,34 Few studies have evaluated the immune response, and its impact on transgene expression, after direct IA injection of AAV vectors. Before treatment with either AAV serotype, all horses had low NAb titers to AAV5, whereas no horses had NAb titers to AAV2. After injection, there was a more robust humoral response to rAAV5 than rAAV2, as noted by the high NAb titers both in the serum and especially in the synovial fluid. In addition, the humoral response to rAAV5 injection was more diverse, with statistically significant increases in serum IgM and IgG1 and synovial fluid IgM, IgG1, and IgG4 levels.
Despite the presence of preexisting NAbs in all rAAV5-injected horses and the robust humoral response to this serotype, rAAV5 appeared to be a more effective IA vector with much higher levels of the transgene being expressed on days 14 and 28, compared with rAAV2-injected joints. It is interesting that IGF-I concentration in the synovial fluid of rAAV5-IGF-I-treated joints peaked on day 28, with a sharp decline noted on day 56. This may suggest loss of transduced cells due to an activated humoral immune system. Indeed, there were higher NAb titers in the rAAV5-injected joint compared with the contralateral saline-injected joint, which could support recruitment of NAbs to the transduced joint. Alternatively, induction of transgene-specific CD8+ T cells may have led to decreased IGF-I expression, although this response was not examined in this study.
To the authors' knowledge, no studies have examined the cell-mediated immune response to AAV in the horse. On the basis of flow cytometric analysis of IFN-γ+ T cells, restimulation of PBMCs with either AAV serotype did not elicit an AAV-specific effector T cell response. These findings were supported by lack of IFN-γ in the supernatants of restimulated cells. Memory CD8+ T cell responses have been most commonly reported in humans,18,19 although these responses are seemingly variable and dose dependent. AAV capsid presentation by MHC-I could result in activation and expansion of capsid-specific CD8+ memory T cells with subsequent removal of transduced cells. The lack of AAV-specific CD8+ T cells in this study may indicate that horses do not mount a significant cell-mediated response to AAV vectors. However, T cell responses may have been limited because of the relatively small dose of vector (5×1011 VG/joint) and/or the IA nature of vector administration.
CD4+ T cell responses to AAV are less commonly reported, possibly because of the poor transduction efficiency of antigen-presenting cells (APCs) by AAV.35 T-regulatory responses were not evaluated in this study, although their role in AAV-mediated transgene expression was described by Mueller and colleagues,36 in which long-term M-type α1-antitrypsin (M-ATT) expression was seen in the muscle of patients despite a T cell response. Immunophenotyping revealed approximately 10% of the T cell population to be T-regulatory cells, which likely play a role in modulating the immune response and permitting transgene expression.
Supernatants from restimulated PBMCs, serum, and synovial fluid were evaluated for changes in proinflammatory cytokines (IFN-γ, IFN-α, IL-6, IL-10). Both type I and II interferons have been shown to increase in mice treated with AAV vectors; increases in IFN-α may be upregulated by AAV after activation of Toll-like receptor-9 (TLR9) in response to intracellular viral genomes, while increases in IFN-γ appear to be associated with activated T cells.37 In this study, there was no evidence of an IFN-α or IFN-γ response in restimulated PBMCs, serum, or synovial fluid at any time point. In addition, no significant increases in IL-6 or IL-10 were seen after rAAV2 or rAAV5 administration. The lack of significant changes in several proinflammatory and antiinflammatory cytokines in these horses is encouraging, as it provides further evidence that these viral vectors are not inducing a significant innate or cell-mediated immune response.
Although serum or synovial fluid immunoglobulin concentrations did not limit transgene expression in this group of horses, it is possible that the humoral response observed after injection would limit effectiveness of readministration of the vector, as a large dose of virus would again be presented to the primed adaptive immune system. Cottard and colleagues38 demonstrated that the IgG antibodies in the synovial fluid of human patients are primarily responsible for limiting transduction efficiency; therefore, increased IgG concentrations after the primary AAV exposure could greatly complicate vector readministration. If readministration is required, a heterologous prime–boost strategy in which serologically distinct AAV serotypes are used will be crucial for successful treatment.
Limitations of this study include the small sample size in each treatment group, especially given the variability in the humoral response observed. Increasing the number of horses per group would allow greater detection of differences. In addition, it would have been more ideal to continue the study past 56 days given that IGF-I concentrations in the rAAV2-IGF-I administered joints peaked on day 56. A longer term study would allow a more comprehensive understanding of transgene expression and persistence. Finally, readministration of the vector would have shed light on the in vivo memory immune response and potential consequences of increasing AAV-specific IgG antibodies on transduction efficiency.
In conclusion, direct IA injection of rAAV2-IGF-I or rAAV5-IGF-I into the MC joints of horses led to sustained, high transgene expression without significant local inflammation or AAV-specific T cell responses. The presence of preexisting NAb to AAV5 did not appear to affect in vivo transduction and transgene expression. A humoral immune response was noted, especially in rAAV5-treated horses, although higher NAb titers in synovial fluid were actually correlated with increased IGF-I concentrations. Neutralizing antibodies to several AAV serotypes in serum and synovial fluid have been demonstrated in humans39,40; however, the impact of this on intraarticular gene transfer and the humoral response in human patients after AAV injection has not been investigated. A significant cell-mediated response was not observed after IA injection in this group of horses. Although no cell-mediated response was noted in this experiment, it is possible that horses, like humans, could have a cell-mediated response to systemic AAV injection. Further investigations into the equine immune response to systemic AAV administration are indicated. Overall, it is possible that human patients would have similar immune responses to IA AAV administration; however, caution is needed when translating these data to human IA gene therapy, as there appear to be significant differences in the nature of the immune response between species.
Supplementary Material
Acknowledgments
The authors acknowledge Ms. Bethany Austin for assistance with animal care, Ms. Mary Lou Norman for assistance with histology, Ms. Heather Freer for assistance with cytokine analysis, and Ms. Susanna Babasyan for assistance with flow cytometry. This work was supported by NIH grant 5R01-AR055373 (A.N.) and NIH grant P30-DK047757 (J.W.).
Author Disclosure Statement
James M. Wilson is a consultant to ReGenX Holdings, and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings; in addition, relevant to this work, he is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings.
References
- 1.Strauss EJ, Goodrich LR, Chen CT, et al. Biochemical and biomechanical properties of lesion and adjacent articular cartilage after chondral defect repair in an equine model. Am J Sports Med 2005;33:1647–1653 [DOI] [PubMed] [Google Scholar]
- 2.Goodrich LR, Brower-Toland BD, Warnick L, et al. Direct adenovirus-mediated IGF-I gene transduction of synovium induces persisting synovial fluid IGF-I ligand elevations. Gene Ther 2006;13:1253–1262 [DOI] [PubMed] [Google Scholar]
- 3.Lee HH, O'Malley MJ, and Friel NA. Persistence, localization, and external control of transgene expression after single injection of adeno-associated virus into injured joints. Hum Gene Ther 2013;24:457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne KA, Lee HH, Haleem AM, et al. Single intra-articular injection of adeno-associated virus results in stable and controllable in vivo transgene expression in normal rat knees. Osteoarthritis Cartilage 2011;19:1058–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortier LA, Lust G, Mohammed HO, et al. Coordinate upregulation of cartilage matrix synthesis in fibrin cultures supplemented with exogenous insulin-like growth factor-I. J Orthop Res 1999;17:467–474 [DOI] [PubMed] [Google Scholar]
- 6.Fortier LA, Lust G, Mohammed HO, et al. Insulin-like growth factor-I enhances cell-based articular cartilage repair. J Bone Joint Surg Br 2002;84-B:276–288 [DOI] [PubMed] [Google Scholar]
- 7.Fosang AJ, Tyler JA, and Hardingham TE. Effect of interleukin-1 and insulin like growth factor-1 on the release of proteoglycan components and hyaluronan from pig articular cartilage in explant culture. Matrix 1991;11:17–24 [DOI] [PubMed] [Google Scholar]
- 8.Pelletier JP, Caron JP, Evans C, et al. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum 1997;40:1012–1019 [DOI] [PubMed] [Google Scholar]
- 9.Gouze E, Pawliuk R, Gouze JN, et al. Lentiviral-mediated gene delivery to synovium: potent intra-articular expression with amplification by inflammation. Mol Ther 2003;7:460–466 [DOI] [PubMed] [Google Scholar]
- 10.Daya S, and Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev 2008;21:583–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begum L, Ortved KF, and Nixon AJ. AAV-5 provides more efficient transgene expression in chondrocytes grown in adherent and suspension culture. In: Orthopaedic Research Society 56th Annual Meeting, 2010, Seattle, WA [Google Scholar]
- 12.Goodrich LR, Choi VW, Carbone BA, et al. Ex vivo serotype-specific transduction of equine joint tissue by self-complementary adeno-associated viral vectors. Hum Gene Ther 2009;20:1697–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calcedo R, Vandenberghe LH, Gao G, et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009;199:381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boutin S, Monteilhet V, Veron P, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 2010;21:704–712 [DOI] [PubMed] [Google Scholar]
- 15.Erles K, Sebokova P, and Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J Med Virol 1999;59:406–411 [DOI] [PubMed] [Google Scholar]
- 16.Petry H, Brooks A, Orme A, et al. Effect of viral dose on neutralizing antibody response and transgene expression after AAV1 vector re-administration in mice. Gene Ther 2008;15:54–60 [DOI] [PubMed] [Google Scholar]
- 17.Scallan CD, Jiang H, Liu T, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood 2006;107:1810–1817 [DOI] [PubMed] [Google Scholar]
- 18.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347 [DOI] [PubMed] [Google Scholar]
- 19.Mingozzi F, Maus MV, Hui DJ, et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat Med 2007;13:419–422 [DOI] [PubMed] [Google Scholar]
- 20.Mingozzi F, Meulenberg JJ, Hui DJ, et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood 2009;114:2077–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veron P, Leborgne C, Monteilhet V, et al. Humoral and cellular capsid-specific immune responses to adeno-associated virus type 1 in randomized healthy donors. J Immunol 2012;188:6418–6424 [DOI] [PubMed] [Google Scholar]
- 22.Li H, Lasaro MO, Jia B, et al. Capsid-specific T-cell responses to natural infections with adeno-associated viruses in humans differ from those of nonhuman primates. Mol Ther 2011;19:2021–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsushita T, Elliger S, Elliger C, et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther 1998;5:938–945 [DOI] [PubMed] [Google Scholar]
- 24.American Association of Equine Practitioners. Guide for Veterinary Service and Judging of Equestrian Events [AAEP Monograph]. (AAEP, Lexington, KY: ). 1991; pp. 19–27 [Google Scholar]
- 25.Wagner B, and Freer H. Development of a bead-based multiplex assay for simultaneous quantification of cytokines in horses. Vet Immunol Immunopathol 2009;127:242–248 [DOI] [PubMed] [Google Scholar]
- 26.Wagner B, Ainsworth DM, and Freer H. Analysis of soluble CD14 and its use as a biomarker in neonatal foals with septicemia and horses with recurrent airway obstruction. Vet Immunol Immunopathol 2013;155:124–128 [DOI] [PubMed] [Google Scholar]
- 27.Gao G, Qu G, Burnham MS, et al. Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum Gene Ther 2000;11:2079–2091 [DOI] [PubMed] [Google Scholar]
- 28.Goodman LB, Wagner B, Flaminio MJ, et al. Comparison of the efficacy of inactivated combination and modified-live virus vaccines against challenge infection with neuropathogenic equine herpesvirus type 1 (EHV-1). Vaccine 2006;24:3636–3645 [DOI] [PubMed] [Google Scholar]
- 29.Wagner B, Radbruch A, Rohwer J, et al. Monoclonal anti-equine IgE antibodies with specificity for different epitopes on the immunoglobulin heavy chain of native IgE. Vet Immunol Immunopathol 2003;92:45–60 [DOI] [PubMed] [Google Scholar]
- 30.Wagner B, Burton A, and Ainsworth D. Interferon-γ, interleukin-4 and interleukin-10 production by T helper cells reveals intact Th1 and regulatory TR1 cell activation and a delay of the Th2 cell response in equine neonates and foals. Vet Res 2010;41:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burton AB, Wagner B, Erb HN, et al. Serum interleukin-6 (IL-6) and IL-10 concentrations in normal and septic neonatal foals. Vet Immunol Immunopathol 2009;132:122–128 [DOI] [PubMed] [Google Scholar]
- 32.Goater J, Muller R, Kollias G, et al. Empirical advantages of adeno associated viral vectors in vivo gene therapy for arthritis. J Rheumatol 2000;27:983–989 [PubMed] [Google Scholar]
- 33.Gouze E, Gouze JN, Palmer GD, et al. Transgene persistence and cell turnover in the diarthrodial joint: implications for gene therapy of chronic joint diseases. Mol Ther 2007;15:1114–1120 [DOI] [PubMed] [Google Scholar]
- 34.Jiang H, Couto LB, Patarroyo-White S, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood 2006;108:3321–3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pien GC, Basner-Tschakarjan E, Hui DJ, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest 2009;119:1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mueller C, Chulay JD, Trapnell BC, et al. Human Treg responses allow sustained recombinant adeno-associated virus-mediated transgene expression. J Clin Invest 2013;12:5310–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martino AT, Suzuki M, Markusic DM, et al. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood 2011;117:6459–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cottard V, Valvason C, Falgarone G, et al. Immune response against gene therapy vectors: influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J Clin Immunol 2004;24:162–169 [DOI] [PubMed] [Google Scholar]
- 39.Mingozzi F, Chen Y, Edmonson SC, et al. Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther 2013;20:417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boissier MC, Lemeiter D, Clavel C, et al. Synoviocyte infection with adeno-associated virus (AAV) is neutralized by human synovial fluid from arthritis patients and depends on AAV serotype. Hum Gene Ther 2007;18:525–535 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.