Abstract
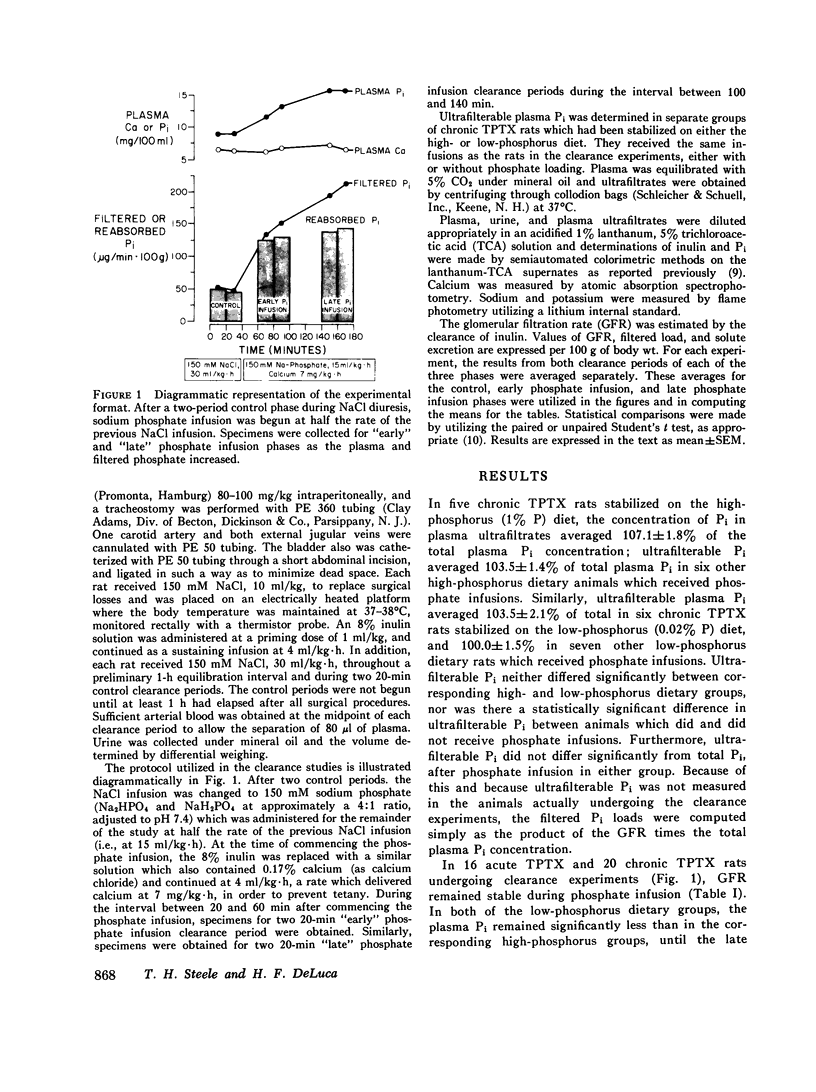
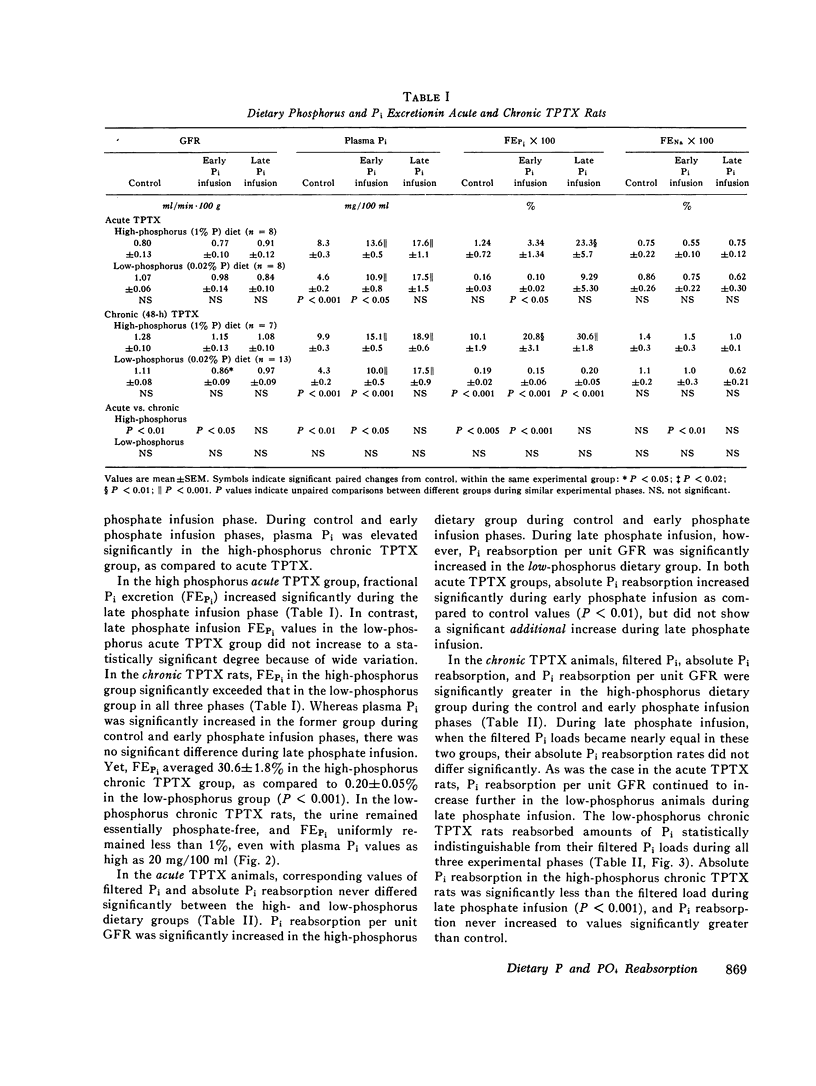
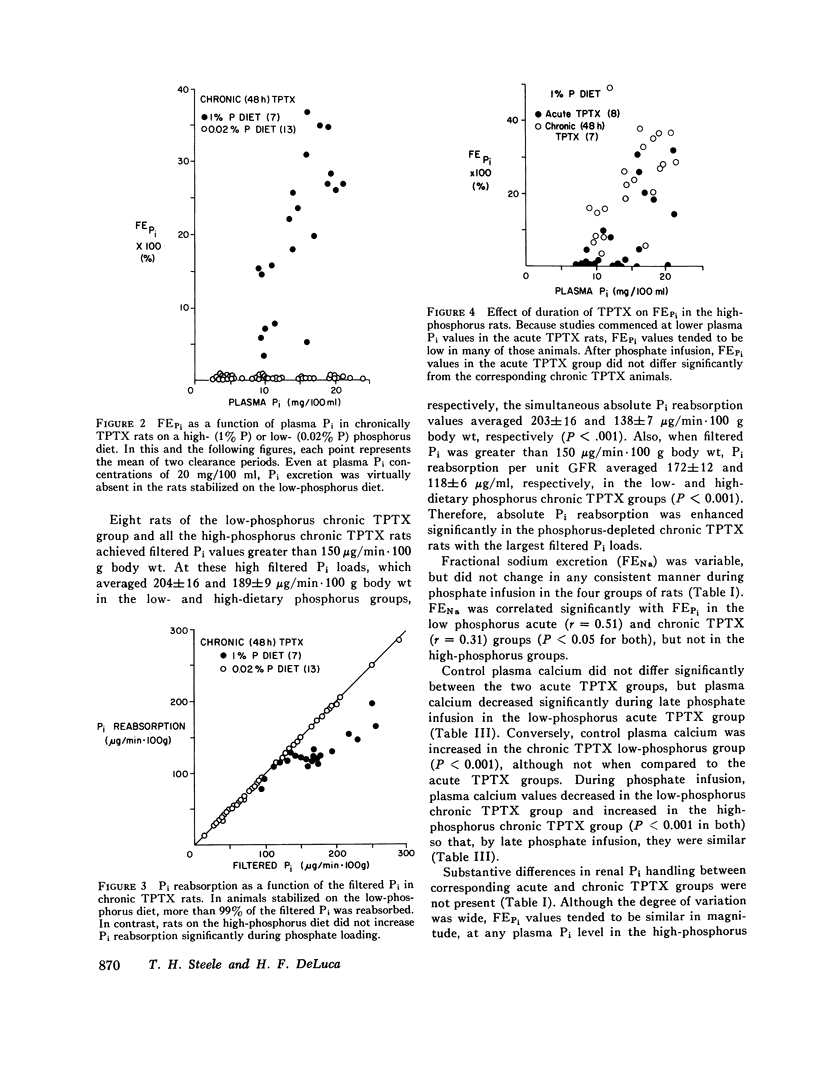
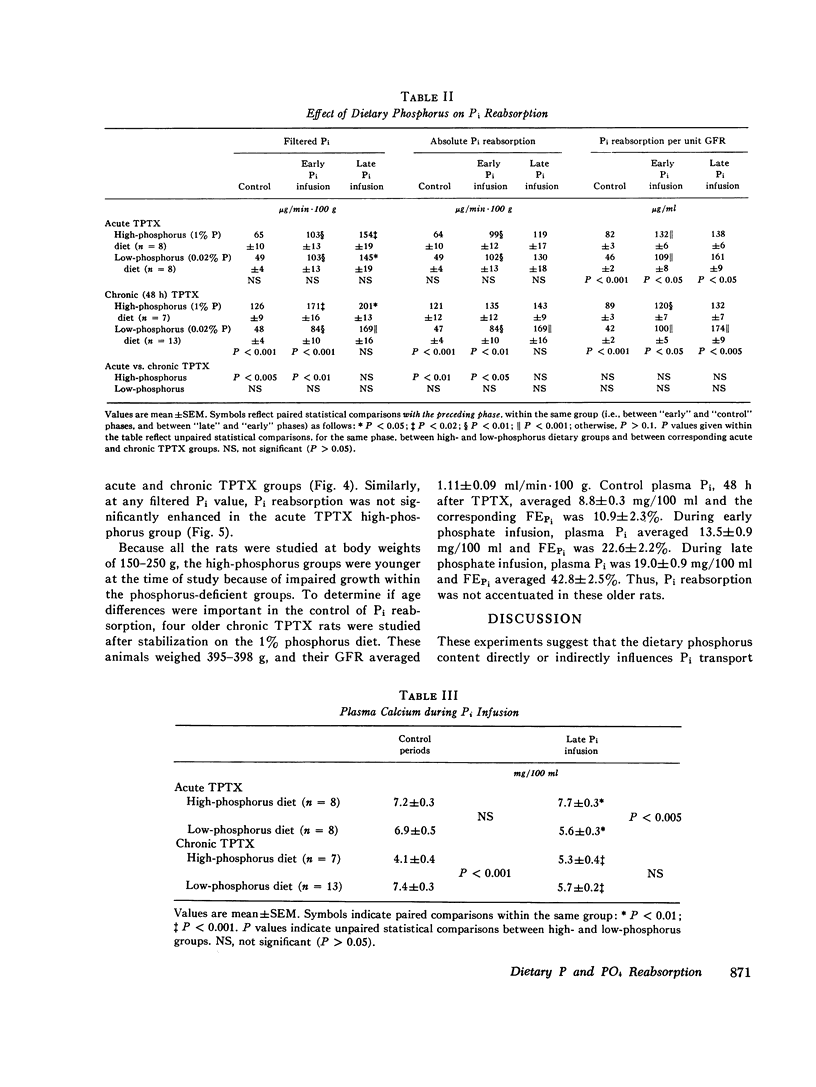
Inorganic phosphate (Pi) reabsorption was studied during Pi infusion, after acute or chronic thyroparathyroidectomy (TPTX), in rats stabilized on a high-phosphorus (1% P) or a low-phosphorus (0.02% P) diet. After acute TPTX, there were no consistent differences in Pi reabsorption between the high- and low-phosphorus dietary groups. After chronic TPTX, the rats stabilized on the low-phosphorus diet exhibited nearly complete Pi reabsorption at every plasma Pi level, while the animals receiving the high-phosphorus diet manifested a marked phosphaturic response to Pi infusion. In addition, Pi reabsorption was significantly increased in the chronic TPTX low-phosphorus rats which achieved the highest filtered Pi loads, while their urine remained essentially phosphate-free. Dietary phosphorus-dependent alterations in Pi reabsorption may play a significant role in establishing the rate of Pi excretion per nephron under certain circumstances and should be considered in the interpretation of studies investigating renal Pi handling. The ability of phosphorus-depleted animals to maintain a phosphate-free urine during Pi loading would favor the rapid repletion of body phosphorus stores.
Full text
PDF
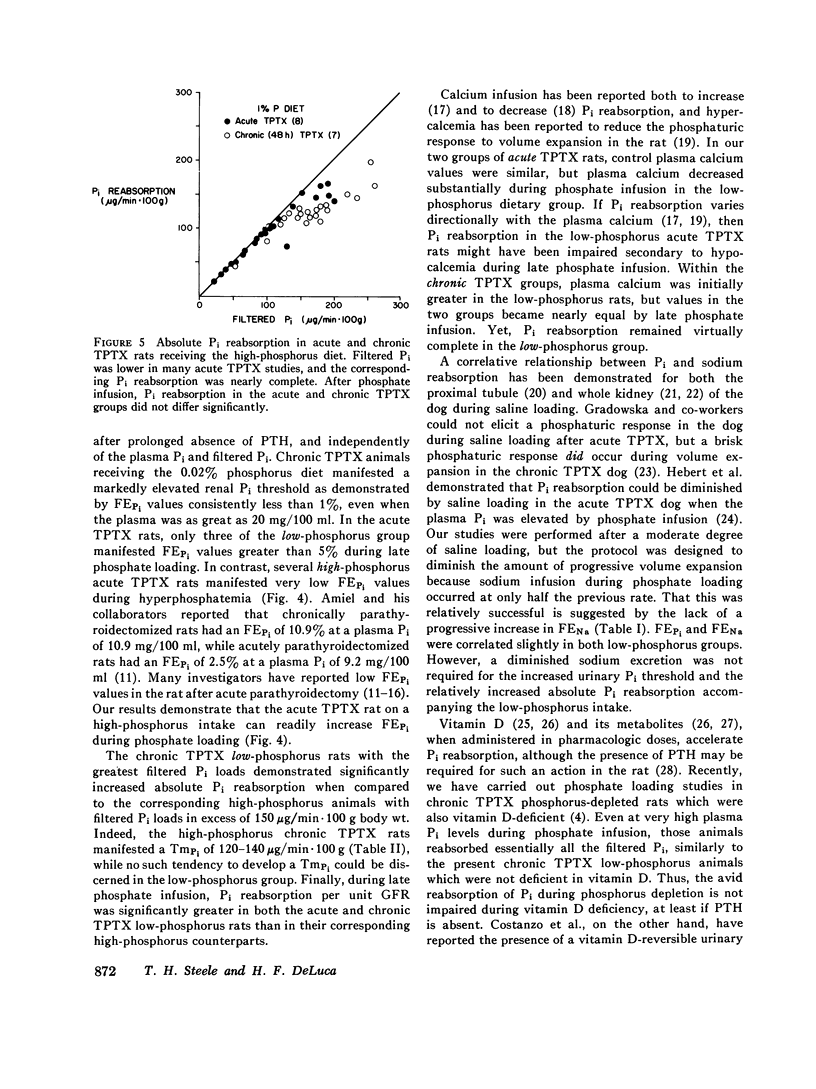


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiel C., Kuntziger H., Richet G. Micropuncture study of handling of phosphate by proximal and distal nephron in normal and parathyroidectomized rat. Evidence for distal reabsorption. Pflugers Arch. 1970;317(2):93–109. doi: 10.1007/BF00592495. [DOI] [PubMed] [Google Scholar]
- Brunette M. G., Taleb L., Carriere S. Effect of parathyroid hormone on phosphate reabsorption along the nephron of the rat. Am J Physiol. 1973 Nov;225(5):1076–1081. doi: 10.1152/ajplegacy.1973.225.5.1076. [DOI] [PubMed] [Google Scholar]
- Costanzo L. S., Sheehe P. R., Weiner I. M. Renal actions of vitamin D in D-deficient rats. Am J Physiol. 1974 Jun;226(6):1490–1495. doi: 10.1152/ajplegacy.1974.226.6.1490. [DOI] [PubMed] [Google Scholar]
- EISENBERG E. EFFECTS OF SERUM CALCIUM LEVEL AND PARATHYROID EXTRACTS ON PHOSPHATE AND CALCIUM EXCRETION IN HYPOPARATHYROID PATIENTS. J Clin Invest. 1965 Jun;44:942–946. doi: 10.1172/JCI105211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick A. Mechanism of inorganic phosphate diuresis secondary to saline infusions in the rat. Excretion of sodium, inorganic phosphate, and calcium in normal and in parathyroidectomized rats. Pflugers Arch. 1969;313(2):106–122. doi: 10.1007/BF00586239. [DOI] [PubMed] [Google Scholar]
- Frick A. Parathormone as a mediator of inorganic phosphate diuresis during saline infusion in the rat. Pflugers Arch. 1971;325(1):1–13. doi: 10.1007/BF00587487. [DOI] [PubMed] [Google Scholar]
- Frick A. Proximal tubular reabsorption of inorganic phosphate during saline infusion in the rat. Am J Physiol. 1972 Nov;223(5):1034–1040. doi: 10.1152/ajplegacy.1972.223.5.1034. [DOI] [PubMed] [Google Scholar]
- Gradowska L., Caglar S., Rutherford E., Harter H., Slatopolsky E. On the mechanism of the phosphaturia of extracellular fluid volume expansion in the dog. Kidney Int. 1973 Apr;3(4):230–237. doi: 10.1038/ki.1973.36. [DOI] [PubMed] [Google Scholar]
- Hebert C. S., Rouse D., Eknoyan G., Martinez-Maldonado M., Suki W. N. Decreased phosphate reabsorption by volume expansion in the dog. Kidney Int. 1972 Nov;2(5):247–252. doi: 10.1038/ki.1972.103. [DOI] [PubMed] [Google Scholar]
- Knox F. G., Schneider E. G., Willis L. R., Strandhoy J. W., Ott C. E. Editorial: Site and control of phosphate reabsorption by the kidney. Kidney Int. 1973 Jun;3(6):347–353. doi: 10.1038/ki.1973.56. [DOI] [PubMed] [Google Scholar]
- Lavender A. R., Pullman T. N. Changes in inorganic phosphate excretion induced by renal arterial infusion of calcium. Am J Physiol. 1963 Nov;205(5):1025–1032. doi: 10.1152/ajplegacy.1963.205.5.1025. [DOI] [PubMed] [Google Scholar]
- Maesaka J. K., Levitt M. F., Abramson R. G. Effect of saline infusion on phosphate transport in intact and thyroparathyroidectomized rats. Am J Physiol. 1973 Dec;225(6):1421–1429. doi: 10.1152/ajplegacy.1973.225.6.1421. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Coburn J. W., Kleeman C. R. The influence of extracellular volume expansion on renal phosphate reabsorption in the dog. J Clin Invest. 1969 Jul;48(7):1237–1245. doi: 10.1172/JCI106088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovtzer M. M., Robinette J. B., DeLuca H. F., Holick M. F. The acute effect of 25-hydroxycholecalciferol on renal handling of phosphorus. Evidence for a parathyroid hormone-dependent mechanism. J Clin Invest. 1974 Mar;53(3):913–921. doi: 10.1172/JCI107632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschett J. B., Agus Z. S., Senesky D., Goldberg M. Effects of saline loading and aortic obstruction on proximal phosphate transport. Am J Physiol. 1972 Oct;223(4):851–857. doi: 10.1152/ajplegacy.1972.223.4.851. [DOI] [PubMed] [Google Scholar]
- Puschett J. B., Fernandez P. C., Boyle I. T., Gray R. W., Omdahl J. L., DeLuca H. F. The acute renal tubular effects of 1,25-dihydroxycholecalciferol. Proc Soc Exp Biol Med. 1972 Oct;141(1):379–384. doi: 10.3181/00379727-141-36781. [DOI] [PubMed] [Google Scholar]
- Puschett J. B., Moranz J., Kurnick W. S. Evidence for a direct action of cholecalciferol and 25-hydroxycholecalciferol on the renal transport of phosphate, sodium, and calcium. J Clin Invest. 1972 Feb;51(2):373–385. doi: 10.1172/JCI106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss E., Canterbury J. M., Bercovitz M. A., Kaplan E. L. The role of phosphate in the secretion of parathyroid hormone in man. J Clin Invest. 1970 Nov;49(11):2146–2149. doi: 10.1172/JCI106432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIKITA M., TSURUFUJI S., ITO Y. Adaptation in renal phosphorus excretion under the influence of parathyroids; a study in ureterally catheterized rats. Endocrinol Jpn. 1962 Sep;9:171–180. doi: 10.1507/endocrj1954.9.171. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E., Caglar S., Gradowska L., Canterbury J., Reiss E., Bricker N. S. On the prevention of secondary hyperparathyroidism in experimental chronic renal disease using "proportional reduction" of dietary phosphorus intake. Kidney Int. 1972 Sep;2(3):147–151. doi: 10.1038/ki.1972.84. [DOI] [PubMed] [Google Scholar]
- Slatopolsky E., Caglar S., Pennell J. P., Taggart D. D., Canterbury J. M., Reiss E., Bricker N. S. On the pathogenesis of hyperparathyroidism in chronic experimental renal insufficiency in the dog. J Clin Invest. 1971 Mar;50(3):492–499. doi: 10.1172/JCI106517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatopolsky E., Gradowska L., Kashemsant C., Keltner R., Manley C., Bricker N. S. The control of phosphate excretion in uremia. J Clin Invest. 1966 May;45(5):672–677. doi: 10.1172/JCI105382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele T. H., Dudgeon K. L. Reabsorption of lithium and phosphate by the rat kidney: role of the parathyroids. Kidney Int. 1974 Mar;5(3):196–203. doi: 10.1038/ki.1974.24. [DOI] [PubMed] [Google Scholar]
- Steele T. H., Engle J. E., Tanaka Y., Lorenc R. S., Dudgeon K. L., DeLuca H. F. Phosphatemic action of 1,25-dihydroxyvitamin D3. Am J Physiol. 1975 Aug;229(2):489–495. doi: 10.1152/ajplegacy.1975.229.2.489. [DOI] [PubMed] [Google Scholar]
- Suki W. N., Martinez-Maldonado M., Rouse D., Terry A. Effect of expansion of extracellular fluid volume on renal phosphate handling. J Clin Invest. 1969 Oct;48(10):1888–1894. doi: 10.1172/JCI106155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. S., Weisinger J. R., Ruggeri J. L., Reaven G. M. Evidence that parathyroid hormone is not required for phosphate homeostasis in renal failure. Metabolism. 1975 Feb;24(2):199–204. doi: 10.1016/0026-0495(75)90021-9. [DOI] [PubMed] [Google Scholar]
- THOMPSON D. D., HIATT H. H. Effects of phosphate loading and depletion on the renal excretion and reabsorption of inorganic phosphate. J Clin Invest. 1957 Apr;36(4):566–572. doi: 10.1172/JCI103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMPSON D. D., HIATT H. H. Renal reabsorption of phosphate in normal human subjects and in patients with parathyroid disease. J Clin Invest. 1957 Apr;36(4):550–556. doi: 10.1172/JCI103453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Deluca H. F. Role of 1,25-dihydroxyvitamin D3 in maintaining serum phosphorus and curing rickets. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1040–1044. doi: 10.1073/pnas.71.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Stone J. C., Hano J. E. Phosphate excretion in the parathyroidectomized rat receiving parathyroid hormone. Metabolism. 1972 Sep;21(9):849–854. doi: 10.1016/0026-0495(72)90008-x. [DOI] [PubMed] [Google Scholar]



