Abstract
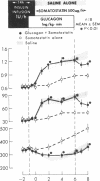
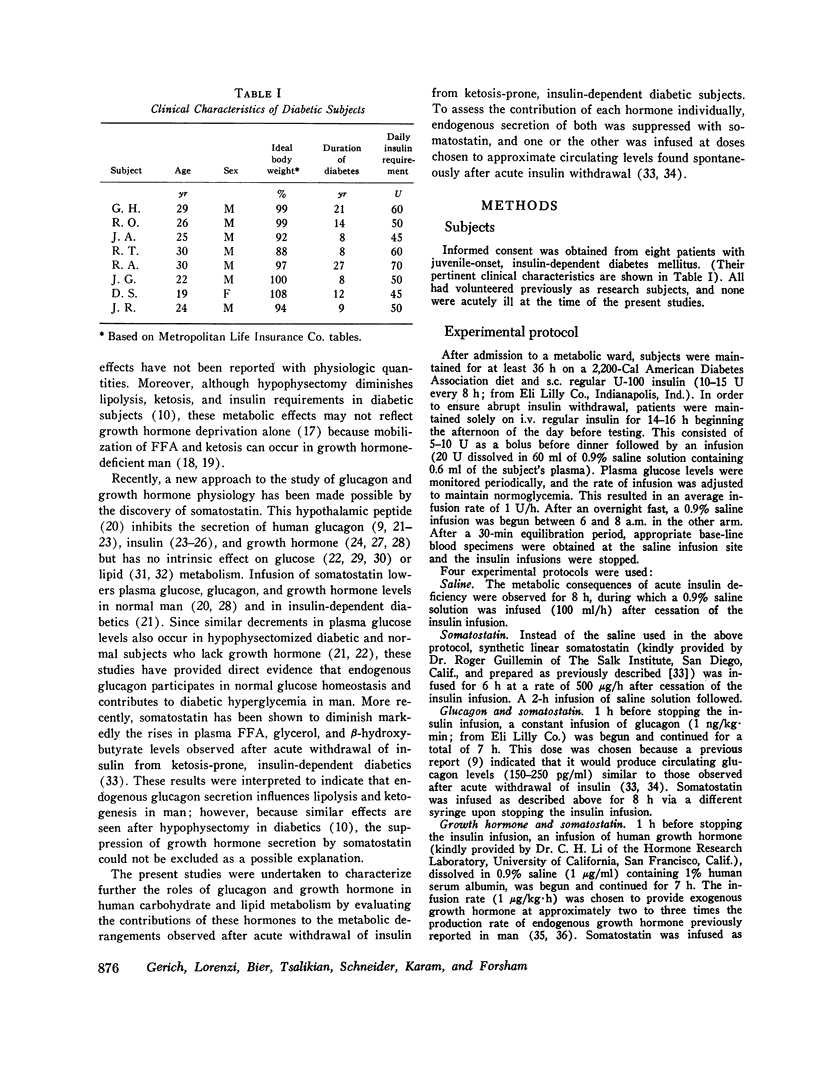
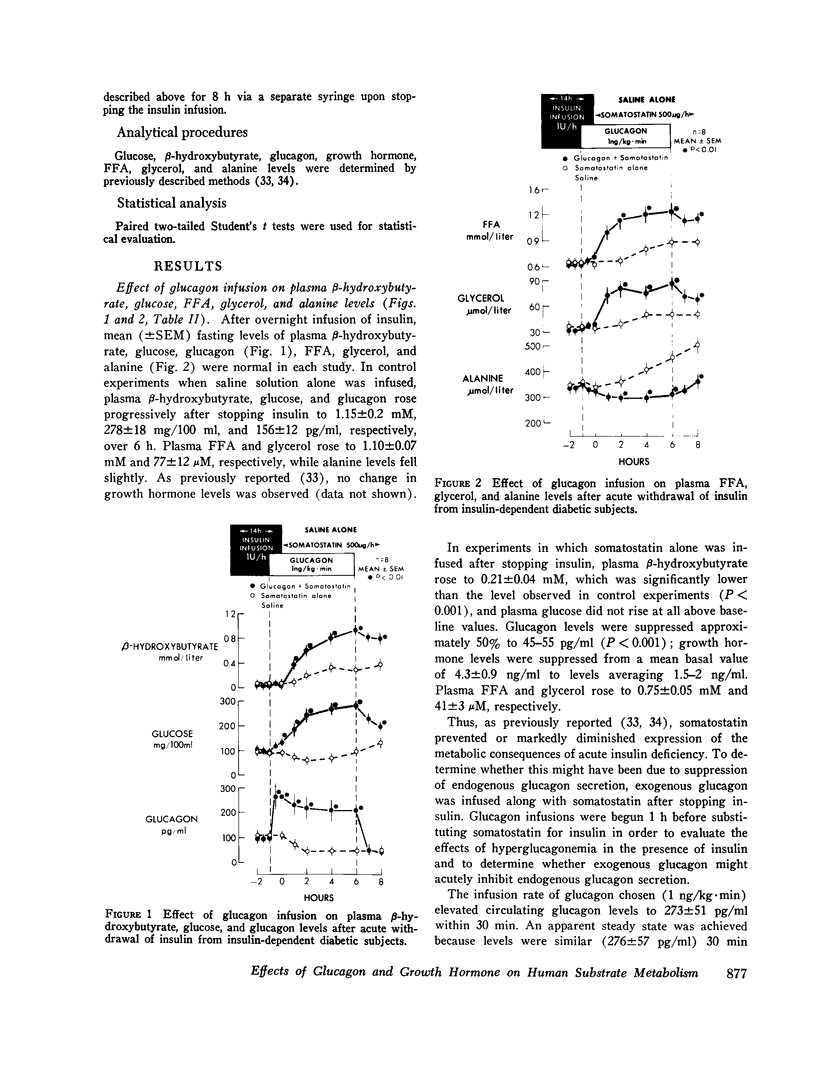
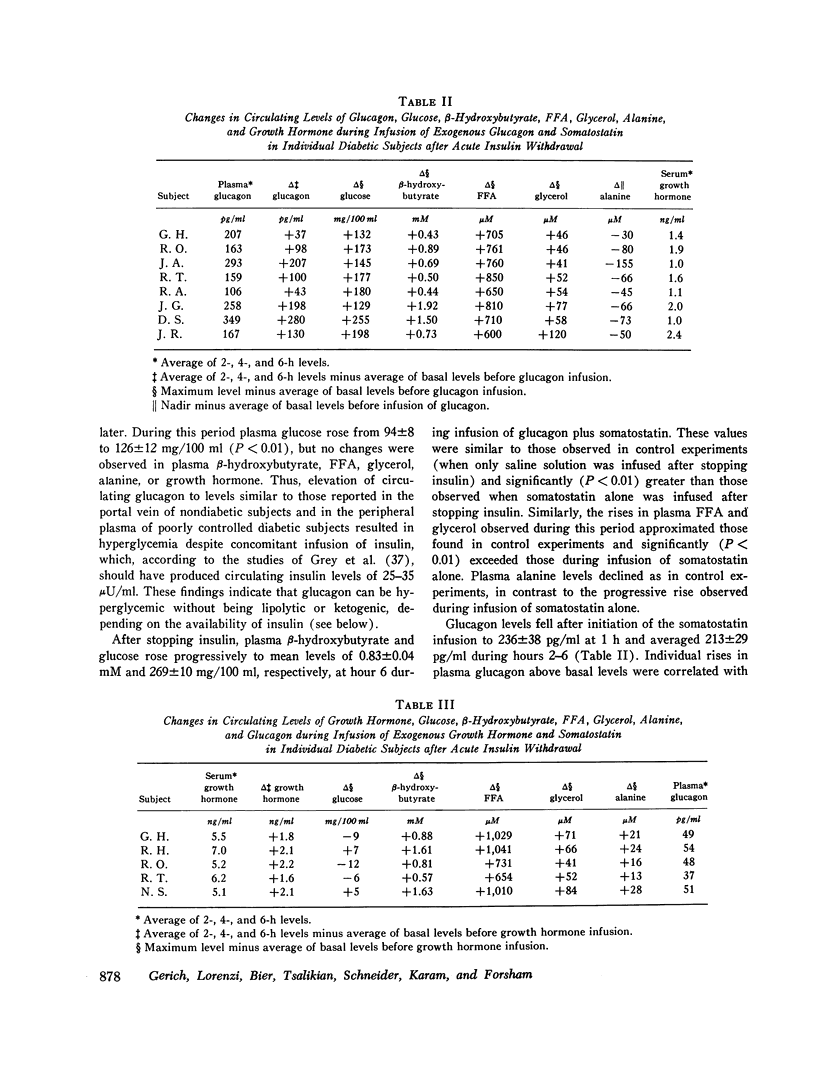
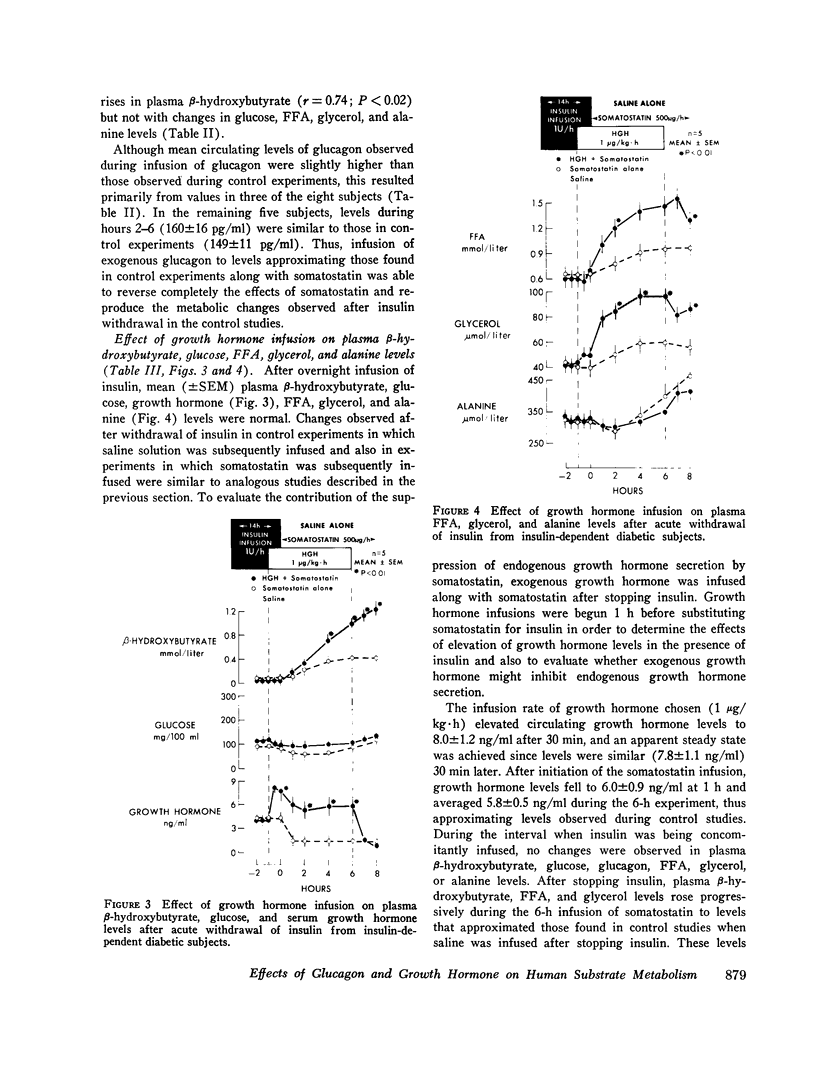
To study the individual effects of glucagon and growth hormone on human carbohydrate and lipid metabolism, endogenous secretion of both hormones was simultaneously suppressed with somatostatin and physiologic circulating levels of one or the other hormone were reproduced by exogenous infusion. The interaction of these hormones with insulin was evaluated by performing these studies in juvenile-onset, insulin-deficient diabetic subjects both during infusion of insulin and after its withdrawal. Infusion of glucagon (1 ng/kg-min) during suppression of its endogenous secretion with somatostatin produced circulating hormone levels of approximately 200 pg/ml. When glucagon was infused along with insulin, plasma glucose levels rose from 94 +/- 8 to 126 +/- 12 mg/100 ml over 1 h (P less than 0.01); growth hormone, beta-hydroxy-butyrate, alanine, FFA, and glycerol levels did not change. When insulin was withdrawn, plasma glucose, beta-hydroxybutyrate, FFA, and glycerol all rose to higher levels (P less than 0.01) than those observed under similar conditions when somatostatin alone had been infused to suppress glucagon secretion. Thus, under appropriate conditions, physiologic levels of glucagon can stimulate lipolysis and cause hyperketonemia and hyperglycemia in man; insulin antagonizes the lipolytic and ketogenic effects of glucagon more effectively than the hyperglycemic effect. Infusion of growth hormone (1 mug/kg-h) during suppression of its endogenous secretion with somastostatin produced circulating hormone levels of approximately 6 ng/ml. When growth hormone was administered along with insulin, no effects were observed. After insulin was withdrawn, plasma beta-hydroxybutyrate, glycerol, and FFA all rose to higher levels (P less than 0.01) than those observed during infusion of somatostatin alone when growth hormone secretion was suppressed; no difference in plasma glucose, alanine, and glucagon levels was evident. Thus, under appropriate conditions, physiologic levels of growth hormone can augment lipolysis and ketonemia in man, but these actions are ordinarily not apparent in the presence of physiologic levels of insulin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti K. G., Christensen N. J., Christensen S. E., Hansen A. P., Iversen J., Lundbaek K., Seyer-Hansen K., Orskov H. Inhibition of insulin secretion by somatostatin. Lancet. 1973 Dec 8;2(7841):1299–1301. doi: 10.1016/s0140-6736(73)92873-0. [DOI] [PubMed] [Google Scholar]
- Alford F. P., Bloom S. R., Nabarro J. D., Hall R., Besser G. M., Coy D. H., Kastin A. J., Schally A. V. Glucagon control of fasting glucose in man. Lancet. 1974 Oct 26;2(7887):974–977. doi: 10.1016/s0140-6736(74)92071-6. [DOI] [PubMed] [Google Scholar]
- Blackard W. G., Nelson N. C., Andrews S. S. Portal and peripheral vein immunoreactive glucagon concentrations after arginine or glucose infusions. Diabetes. 1974 Mar;23(3):199–202. doi: 10.2337/diab.23.3.199. [DOI] [PubMed] [Google Scholar]
- Cheng J. S., Kalant N. Effects of insulin and growth hormone on the flux rates of plasma glucose and plasma free fatty acids in man. J Clin Endocrinol Metab. 1970 Dec;31(6):647–653. doi: 10.1210/jcem-31-6-647. [DOI] [PubMed] [Google Scholar]
- Chernick S. S., Clark C. M., Jr, Gardiner R. J., Scow R. O. Role of lipolytic and glucocorticoid hormones in the development of diabetic ketosis. Diabetes. 1972 Sep;21(9):946–954. doi: 10.2337/diab.21.9.946. [DOI] [PubMed] [Google Scholar]
- Chideckel E. W., Palmer J., Koerker D. J., Ensinck J., Davidson M. B., Goodner C. J. Somatostatin blockade of acute and chronic stimuli of the endocrine pancreas and the consequences of this blockade on glucose homeostasis. J Clin Invest. 1975 Apr;55(4):754–762. doi: 10.1172/JCI107986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRaimondo V. C., Earll J. M. Remarkable sensitivity to insulin in a patient with hypopituitarism and diabetic acidosis. Diabetes. 1968 Mar;17(3):147–151. doi: 10.2337/diab.17.3.147. [DOI] [PubMed] [Google Scholar]
- Dobbs R., Sakurai H., Sasaki H., Faloona G., Valverde I., Baetens D., Orci L., Unger R. Glucagon: role in the hyperglycemia of diabetes mellitus. Science. 1975 Feb 14;187(4176):544–547. doi: 10.1126/science.1089999. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Kovacev V. P., Scow R. O. Effect of growth hormone and dexamethasone on lipolysis and metabolism in isolated fat cells of the rat. J Biol Chem. 1965 Sep;240(9):3522–3529. [PubMed] [Google Scholar]
- Felig P., Gusberg R., Hendler R., Gump F. E., Kinney J. M., Mulrow P. J. Concentrations of glucagon and the insulin:glucagon ratio in the portal and peripheral circulation. Proc Soc Exp Biol Med. 1974 Oct;147(1):88–90. doi: 10.3181/00379727-147-38286. [DOI] [PubMed] [Google Scholar]
- Felig P., Marliss E. B., Cahill G. F., Jr Metabolic response to human growth hormone during prolonged starvation. J Clin Invest. 1971 Feb;50(2):411–421. doi: 10.1172/JCI106508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P. The glucose-alanine cycle. Metabolism. 1973 Feb;22(2):179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Fineberg S. E., Merimee T. J. Acute metabolic effects of human growth hormone. Diabetes. 1974 Jun;23(6):499–504. doi: 10.2337/diab.23.6.499. [DOI] [PubMed] [Google Scholar]
- Frohman L. A., MacGillivray M. H., Aceto T., Jr Acute effects of human growth hormone on insulin secretion and glucose utilization in normal and growth hormone deficient subjects. J Clin Endocrinol Metab. 1967 Apr;27(4):561–567. doi: 10.1210/jcem-27-4-561. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Bier D. M., Schneider V., Tsalikian E., Karam J. H., Forsham P. H. Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N Engl J Med. 1975 May 8;292(19):985–989. doi: 10.1056/NEJM197505082921901. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Hane S., Gustafson G., Guillemin R., Forsham P. H. Evidence for a physiologic role of pancreatic glucagon in human glucose homeostasis: studies with somatostatin. Metabolism. 1975 Feb;24(2):175–182. doi: 10.1016/0026-0495(75)90018-9. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Schneider V., Forsham P. H. Effect of somatostatin on plasma glucose and insulin responses to glucagon and tolbutamide in man. J Clin Endocrinol Metab. 1974 Dec;39(6):1057–1060. doi: 10.1210/jcem-39-6-1057. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Schneider V., Karam J. H., Rivier J., Guillemin R., Forsham P. H. Effects of somatostatin on plasma glucose and glucagon levels in human diabetes mellitus. Pathophysiologic and therapeutic implications. N Engl J Med. 1974 Sep 12;291(11):544–547. doi: 10.1056/NEJM197409122911102. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Schneider V., Kwan C. W., Karam J. H., Guillemin R., Forsham P. H. Inhibition of pancreatic glucagon responses to arginine by somatostatin in normal man and in insulin-dependent diabetics. Diabetes. 1974 Nov;23(11):876–880. doi: 10.2337/diab.23.11.876. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Tsalikian E., Lorenzi M., Karam J. H., Bier D. M. Plasma glucagon and alanine responses to acute insulin deficiency in man. J Clin Endocrinol Metab. 1975 Mar;40(3):526–529. doi: 10.1210/jcem-40-3-526. [DOI] [PubMed] [Google Scholar]
- Grey N. J., Karl I., Kipnis D. M. Physiologic mechanisms in the development of starvation ketosis in man. Diabetes. 1975 Jan;24(1):10–16. doi: 10.2337/diab.24.1.10. [DOI] [PubMed] [Google Scholar]
- Hansen A. P., Orskov H., Seyer-Hansen K., Lundbaek K. Some actions of growth hormone release inhibiting factor. Br Med J. 1973 Sep 8;3(5879):523–524. doi: 10.1136/bmj.3.5879.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowarski A., Thompson R. G., Migeon C. J., Blizzard R. M. Determination of integrated plasma concentrations and true secretion rates of human growth hormone. J Clin Endocrinol Metab. 1971 Mar;32(3):356–360. doi: 10.1210/jcem-32-3-356. [DOI] [PubMed] [Google Scholar]
- Liljenquist J. E., Bomboy J. D., Lewis S. B., Sinclair-Smith B. C., Felts P. W., Lacy W. W., Crofford O. B., Liddle G. W. Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men. J Clin Invest. 1974 Jan;53(1):190–197. doi: 10.1172/JCI107537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marliss E. B., Aoki T. T., Unger R. H., Soeldner J. S., Cahill G. F., Jr Glucagon levels and metabolic effects in fasting man. J Clin Invest. 1970 Dec;49(12):2256–2270. doi: 10.1172/JCI106445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Foà P. P. Plasma glucose, insulin, pancreatic, and enteroglucagon levels in normal and depancreatized dogs. Proc Soc Exp Biol Med. 1974 Oct;147(1):97–102. doi: 10.3181/00379727-147-38288. [DOI] [PubMed] [Google Scholar]
- McGarry J., Wright P. H., Foster D. W. Hormonal control of ketogenesis. Rapid activation of hepatic ketogenic capacity in fed rats by anti-insulin serum and glucagon. J Clin Invest. 1975 Jun;55(6):1202–1209. doi: 10.1172/JCI108038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merimee T. J., Felig P., Marliss E., Fineberg S. E., Cahill G. G., Jr Glucose and lipid homeostasis in the absence of human growth hormone. J Clin Invest. 1971 Mar;50(3):574–582. doi: 10.1172/JCI106527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merimee T. J., Rabin D. A survey of growth hormone secretion and action. Metabolism. 1973 Sep;22(9):1235–1251. doi: 10.1016/0026-0495(73)90211-4. [DOI] [PubMed] [Google Scholar]
- Mitchell M. L., Suvunrungsi P., Sawin C. T. Effect of propranolol on the response of serum growth hormone to glucagon. J Clin Endocrinol Metab. 1971 Apr;32(4):470–475. doi: 10.1210/jcem-32-4-470. [DOI] [PubMed] [Google Scholar]
- Mortimer C. H., Tunbridge W. M., Carr D., Yeomans L., Lind T., Coy D. H., Bloom S. R., Kastin A., Mallinson C. N., Besser G. M. Effects of growth-hormone release-inhibiting hormone on circulating glucagon, insulin, and growth hormone in normal, diabetic, acromegalic, and hypopituitary patients. Lancet. 1974 Apr 20;1(7860):697–701. doi: 10.1016/s0140-6736(74)92903-1. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. Hyperglucagonemia in diabetic ketoacidosis. Its prevalence and significance. Am J Med. 1973 Jan;54(1):52–57. doi: 10.1016/0002-9343(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Nilsson L. H. Liver glycogen content in man in the postabsorptive state. Scand J Clin Lab Invest. 1973 Dec;32(4):317–323. doi: 10.3109/00365517309084354. [DOI] [PubMed] [Google Scholar]
- PEARSON O. H., DOMINGUEZ J. M., GREENBERG E., PAZIANOS A., RAY B. S. Diabetogenic and hypoglycemic effects of human growth hormone. Trans Assoc Am Physicians. 1960;73:217–226. [PubMed] [Google Scholar]
- Penhos J. C., Wu C. H., Lemberg A., Daunas J., Brodoff B., Levine R. The effect of growth hormone on the metabolism of lipids and on urea formation by the perfused rat liver. Metabolism. 1966 Dec;15(12):1109–1119. doi: 10.1016/0026-0495(66)90101-6. [DOI] [PubMed] [Google Scholar]
- RABEN M. S., HOLLENBERG C. H. Effect of growth hormone on plasma fatty acids. J Clin Invest. 1959 Mar;38(3):484–488. doi: 10.1172/JCI103824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABINOWITZ D., KLASSEN G. A., ZIERLER K. L. EFFECT OF HUMAN GROWTH HORMONE ON MUSCLE AND ADIPOSE TISSUE METABOLISM IN THE FOREARM OF MAN. J Clin Invest. 1965 Jan;44:51–61. doi: 10.1172/JCI105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier J., Brazeau P., Vale W., Ling N., Burgus R., Gilon C., Yardley J., Guillemin R. Synthèse totale par phase solide d'un tétradécapepide ayant les propriétés chimiques et biologiques de la somatostaine. C R Acad Sci Hebd Seances Acad Sci D. 1973 May 9;276(19):2737–2740. [PubMed] [Google Scholar]
- SALTER J. M., EZRIN C., LAIDLAW J. C., GORNALL A. G. Metabolic effects of glucagon in human subjects. Metabolism. 1960 Aug;9:753–768. [PubMed] [Google Scholar]
- SARCIONE E. J., BACK N., SOKAL J. E., MEHLMAN B., KNOBLOCK E. Elevation of plasma epinephrine levels produced by glucagon in vivo. Endocrinology. 1963 Apr;72:523–526. doi: 10.1210/endo-72-4-523. [DOI] [PubMed] [Google Scholar]
- Samols E., Marri G., Marks V. Interrelationship of glucagon, insulin and glucose. The insulinogenic effect of glucagon. Diabetes. 1966 Dec;15(12):855–866. doi: 10.2337/diab.15.12.855. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Rubalcava B., Baetens D., Blazquez E., Srikant C. B., Orci L., Unger R. H. Identification of glucagon in the gastrointestinal tract. J Clin Invest. 1975 Jul;56(1):135–145. doi: 10.1172/JCI108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade D. S., Eaton R. P. Modulation of fatty acid metabolism by glucagon in man. I. Effects in normal subjects. Diabetes. 1975 May;24(5):502–509. doi: 10.2337/diab.24.5.502. [DOI] [PubMed] [Google Scholar]
- Schade D. S., Eaton R. P. Modulation of fatty acid metabolism by glucagon in man. II. Effects in insulin-deficient diabetics. Diabetes. 1975 May;24(5):510–515. doi: 10.2337/diab.24.5.510. [DOI] [PubMed] [Google Scholar]
- Sperling M. A., Wollesen F., DeLamater P. V. Daily production and metabolic clearance of growth hormone in juvenile diabetes mellitus. Diabetologia. 1973 Oct;9(5):380–383. doi: 10.1007/BF01239431. [DOI] [PubMed] [Google Scholar]
- Unger R. H., Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet. 1975 Jan 4;1(7897):14–16. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- Vranic M., Pek S., Kawamori R. Increased "glucagon immunoreactivity" in plasma of totally depancreatized dogs. Diabetes. 1974 Nov;23(11):905–912. doi: 10.2337/diab.23.11.905. [DOI] [PubMed] [Google Scholar]
- Yen S. S., Siler T. M., DeVane G. W. Effect of somatostatin in patients with acromegaly: suppression of growth hormone, prolactin, insulin and glucose levels. N Engl J Med. 1974 Apr 25;290(17):935–938. doi: 10.1056/NEJM197404252901704. [DOI] [PubMed] [Google Scholar]