Abstract
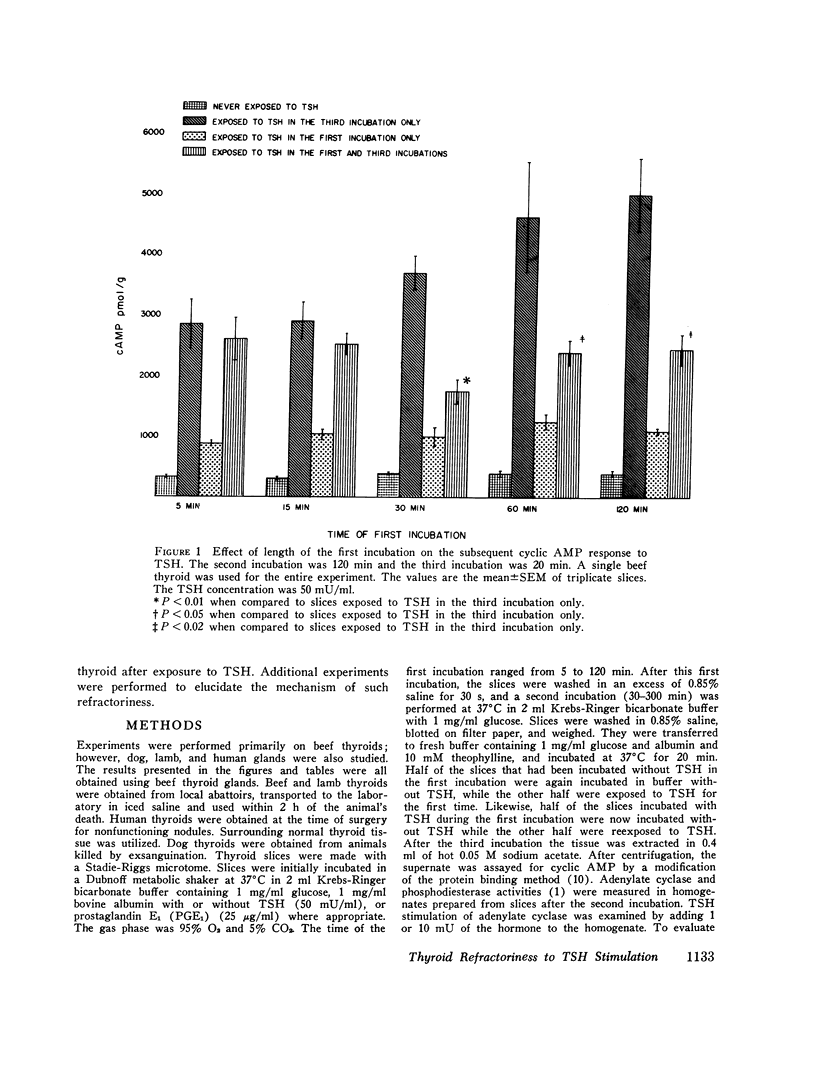
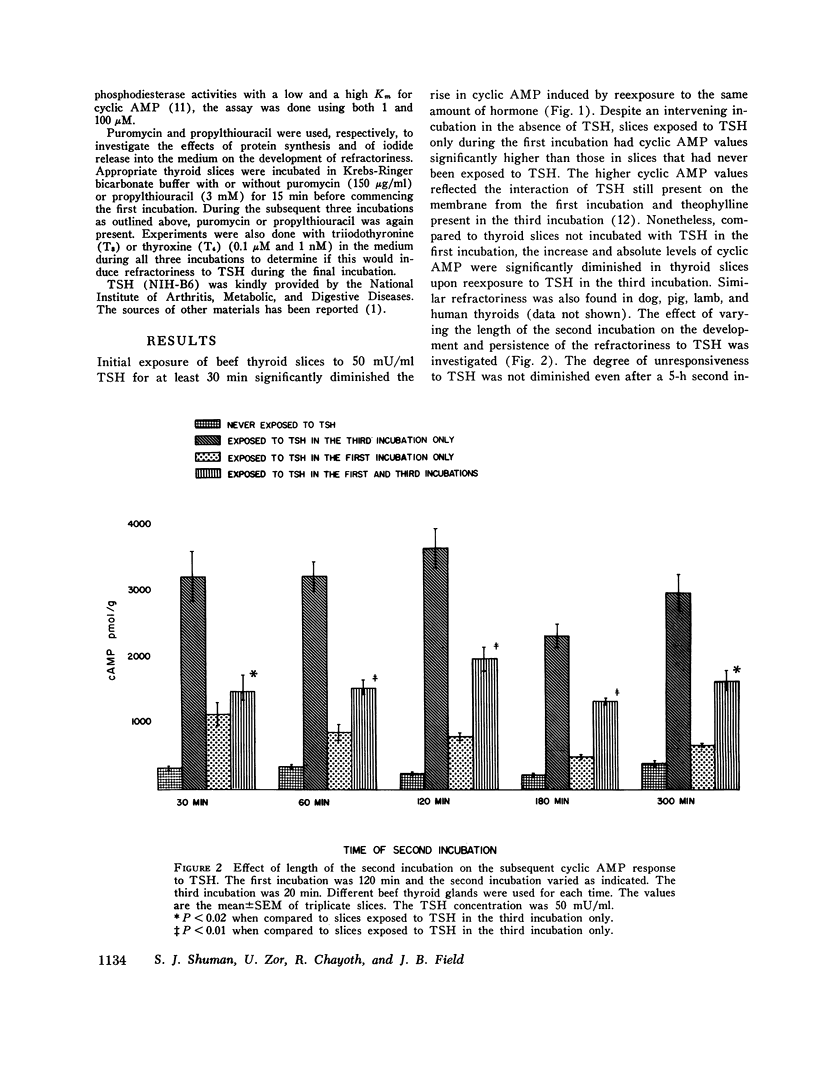
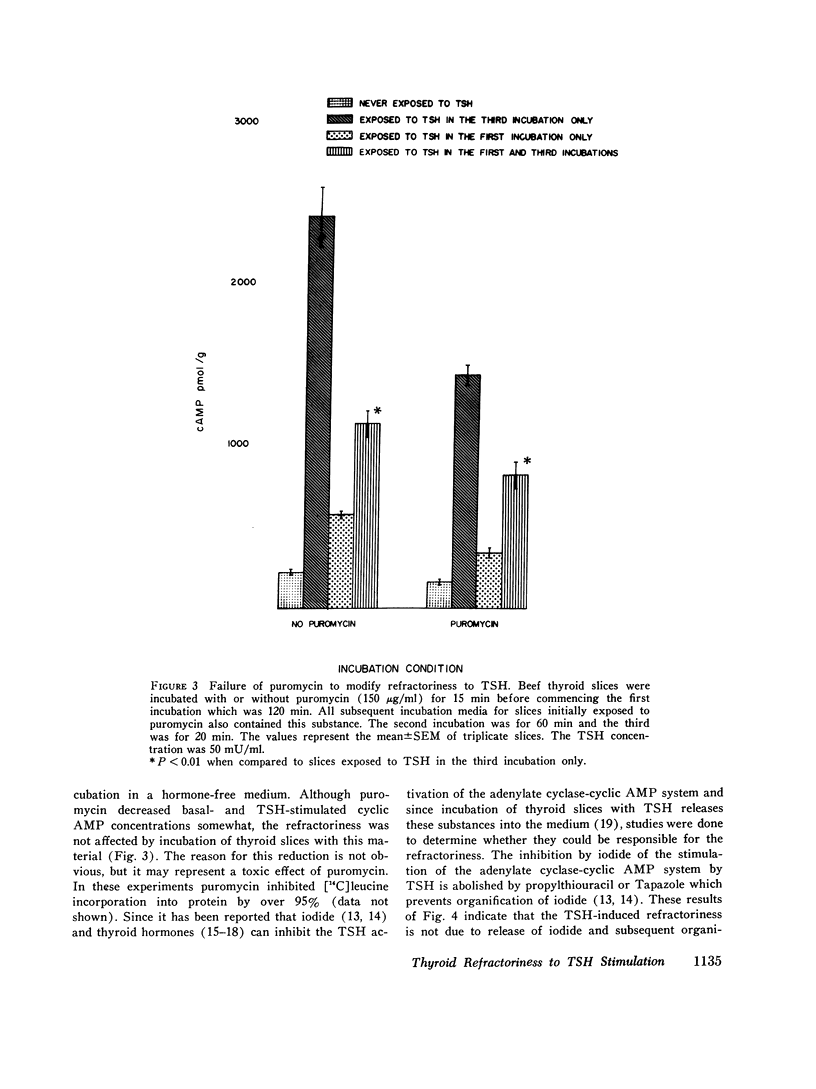
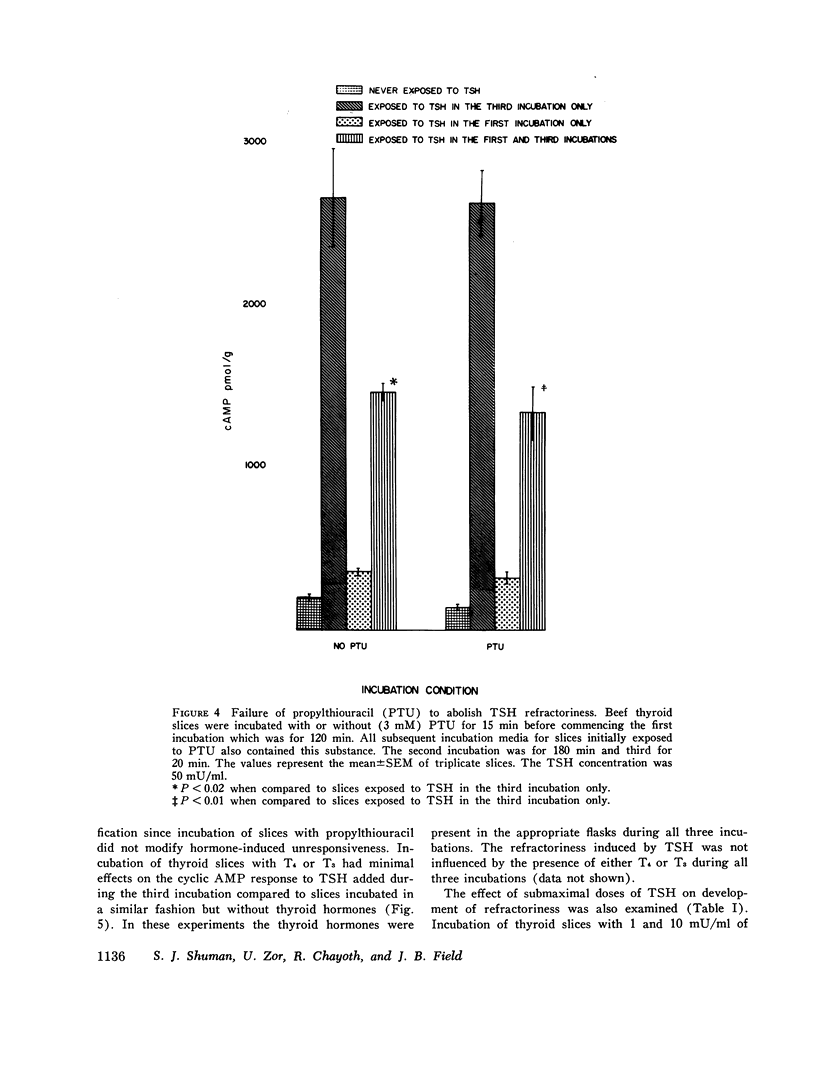
These studies evaluated the influence of an initial exposure of thyroid slices to thyroid-stimulating hormone (TSH) on the subsequent responsiveness to the hormone. Bovine thyroid slices were incubated with or without 50 mU/ml TSH for varying periods and then incubated in hormone-free medium for varying periods. Subsequently, slices were incubated for 20 min with 10 mM theophylline and with or without TSH. Cylic AMP was measured after the third incubation. Phosphodiesterase and adenylate cylase were assayed in homogenates prepared from slices after the second incubation. In some experiments prostaglandin E1, puromycin, thyroxine, and triiodothyronine and propylthiouracil were included in the media. In other experiments, low does of TSH (1 AND 10 mU/ml) were used instead of 50 mU/ml. Slices previously exposed to TSH have decreased responsiveness of the adenylate cyclase-cylic AMP system. Such refractoriness is hormone specific since initial exposure to prostaglandin E1 decreases the subsequent response to this substance but not to TSH. Refractoriness to TSH develops only when the first incubation is at least 30 min. It is not reversed by 5 h of incubation without hormone. Incubation of thyroid slices with puromycin does not eliminate refractoriness. The decreased response to TSH cannot be explained by release of thyroxine, triiodothyronine, or iodide from the slices. Phosphodiesterase activity is not increased during the refractory period. The decreased cyclic AMP response to TSH is associated with diminished response of adenylate cyclase activity to the hormone. Guanosine triphosphate (1 mM) increased adenylate cyclase activity in both control and TSH treated tissue, but the effect was significantly less in the latter. Although with guanosine triphosphate, TSH increased adenylate cyclase activity in TSH treated tissue, the enzyme activity was still less than that present in control tissue incubated with guanosine triphosphate and TSH. NaF caused an equivalent stimulation of adenylate cyclase in both control and TSH treated tissue. These results suggest that the refractoriness represents an alteration in hormone binding or the coupling of the bound hormone to the adenylate cyclase activity rather than any modification of the catalytic site of the enzyme.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn C. S., Rosenberg I. N. Iodine metabolism in thyroid slices: effects of TSH, dibutyryl cyclic 3',5'-AMP, NaF and prostaglandin E-1. Endocrinology. 1970 Feb;86(2):396–405. doi: 10.1210/endo-86-2-396. [DOI] [PubMed] [Google Scholar]
- Brooker G., Thomas L. J., Jr, Appleman M. M. The assay of adenosine 3',5'-cyclic monophosphate and guanosine 3',5'-cyclic monophosphate in biological materials by enzymatic radioisotopic displacement. Biochemistry. 1968 Dec;7(12):4177–4181. doi: 10.1021/bi00852a006. [DOI] [PubMed] [Google Scholar]
- Burke G. Effects of iodide on thyroid stimulation. J Clin Endocrinol Metab. 1970 Jan;30(1):76–84. doi: 10.1210/jcem-30-1-76. [DOI] [PubMed] [Google Scholar]
- D'Armiento M., Johnson G. S., Pastan I. Regulation of adenosine 3',5'-cyclic monophosphate phosphodiesterase activity in fibroblasts by intracellular concentrations of cyclic adenosine monophosphate (3T3-dibutyryl cyclic AMP-SV40-transformed cells-michaelis constants-L cells-prostaglandin E 1 ). Proc Natl Acad Sci U S A. 1972 Feb;69(2):459–462. doi: 10.1073/pnas.69.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubertis F. R., Chayoth R., Zor U., Field J. B. Evidence for persistent binding of biologically active thyrotropin to thyroid in vitro. Endocrinology. 1975 Jun;96(6):1579–1586. doi: 10.1210/endo-96-6-1579. [DOI] [PubMed] [Google Scholar]
- DeVellis J., Brooker G. Reversal of catecholamine refractoriness by inhibitors of RNA and protein synthesis. Science. 1974 Dec 27;186(4170):1221–1223. doi: 10.1126/science.186.4170.1221. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Robison G. A., Sutherland E. W., Park C. R. Studies on the role of adenosine 3',5'-monophosphate in the hepatic actions of glucagon and catecholamines. J Biol Chem. 1971 Oct 25;246(20):6166–6177. [PubMed] [Google Scholar]
- Fain J. N. Inhibition of adenosine cyclic 3', 5'-monophosphate accumulation in fat cells by adenosine, N6-(phenylisopropyl) adenosine, and related compounds. Mol Pharmacol. 1973 Sep;9(5):595–604. [PubMed] [Google Scholar]
- Franklin T. J., Foster S. J. Hormone-induced desensitisation of hormonal control of cyclic AMP levels in human diploid fibroblasts. Nat New Biol. 1973 Dec 5;246(153):146–148. doi: 10.1038/newbio246146a0. [DOI] [PubMed] [Google Scholar]
- Ho R. J., Russell T., Asakawa T. The last conversation with Dr. Earl W. Sutherland, Jr: the feedback regulation of cyclic nucleotides. Metabolism. 1975 Mar;24(3):257–264. doi: 10.1016/0026-0495(75)90107-9. [DOI] [PubMed] [Google Scholar]
- Ho R. J., Sutherland E. W. Formation and release of a hormone antagonist by rat adipocytes. J Biol Chem. 1971 Nov 25;246(22):6822–6827. [PubMed] [Google Scholar]
- Kakiuchi S., Rall T. W. The influence of chemical agents on the accumulation of adenosine 3',5'-Phosphate in slices of rabbit cerebellum. Mol Pharmacol. 1968 Jul;4(4):367–378. [PubMed] [Google Scholar]
- Kotani M., Kariya T., Field J. B. Studies of thyroid-stimulating hormone binding to bovine thyroid plasma membranes. Metabolism. 1975 Aug;24(8):959–971. doi: 10.1016/0026-0495(75)90088-8. [DOI] [PubMed] [Google Scholar]
- Mashiter K., Mashiter G. D., Hauger R. L., Field J. B. Effects of cholera and E. coli enterotoxins on cyclic adenosine 3',5'-monophosphate levels and intermediary metabolism in the thyroid. Endocrinology. 1973 Feb;92(2):541–549. doi: 10.1210/endo-92-2-541. [DOI] [PubMed] [Google Scholar]
- Moore W. V., Wolff J. Thyroid-stimulating hormone binding to beef thyroid membranes. Relation to adenylate cyclase activity. J Biol Chem. 1974 Oct 10;249(19):6255–6263. [PubMed] [Google Scholar]
- Salomon Y., Lin M. C., Londos C., Rendell M., Rodbell M. The hepatic adenylate cyclase system. I. Evidence for transition states and structural requirements for guanine nucloetide activiation. J Biol Chem. 1975 Jun 10;250(11):4239–4245. [PubMed] [Google Scholar]
- Schimmel R. J. Responses of adipose tissue to sequential lipolytic stimuli. Endocrinology. 1974 May;94(5):1372–1380. doi: 10.1210/endo-94-5-1372. [DOI] [PubMed] [Google Scholar]
- Schultz J., Daly J. W. Cyclic adenosine 3',5'-monophosphate in guinea pig cerebral cortical slices. 3. Formation, degradation, and reformation of cyclic adenosine 3',5'-monophosphate during sequential stimulations by biogenic amines and adenosine. J Biol Chem. 1973 Feb 10;248(3):860–866. [PubMed] [Google Scholar]
- Schwabe U., Ebert R., Erbler H. C. Adenosine release from isolated fat cells and its significance for the effects of hormones on cyclic 3',5'-AMP levels and lipolysis. Naunyn Schmiedebergs Arch Pharmacol. 1973;276(2):133–148. doi: 10.1007/BF00501186. [DOI] [PubMed] [Google Scholar]
- Takasu N., Sato S., Tsukui T., Yamada T., Furihata R. Inhibitory action of thyroid hormone of the activation of adenyl cyclase-cyclic AMP system by thyroid-stimulating hormone in human thyroid tissues from euthyroid subjects and thyrotoxic patients. J Clin Endocrinol Metab. 1974 Oct;39(4):772–778. doi: 10.1210/jcem-39-4-772. [DOI] [PubMed] [Google Scholar]
- Van Sande J., Dumont J. E. Effects of thyrotropin, prostaglandin E1 and iodide on cyclic 3',5'-AMP concentration in dog thyroid slices. Biochim Biophys Acta. 1973 Jul 28;313(2):320–328. doi: 10.1016/0304-4165(73)90031-7. [DOI] [PubMed] [Google Scholar]
- Wolff J., Cook G. H. Activation of thyroid membrane adenylate cyclase by purine nucleotides. J Biol Chem. 1973 Jan 10;248(1):350–355. [PubMed] [Google Scholar]
- Zor U., Kaneko T., Lowe I. P., Bloom G., Field J. B. Effect of thyroid-stimulating hormone and prostaglandins on thyroid adenyl cyclase activation and cyclic adenosine 3',5',-monophosphate. J Biol Chem. 1969 Oct 10;244(19):5189–5195. [PubMed] [Google Scholar]
- Zor U., Lamprecht S. A., Kaneko T., Schneider H. P., McCann S. M., Field J. B., Tsafriri A., Lindner H. R. Functional relations between cyclic AMP, prostaglandins, and luteinizing hormone in rat pituitary and ovary. Adv Cyclic Nucleotide Res. 1972;1:503–520. [PubMed] [Google Scholar]


