Abstract
Background
Intracranial atherosclerotic disease (ICAD) is the most frequent subtype of ischemic stroke globally. It is important to describe the determinants of early ICAD as a strategy to prevent strokes from clinically evident and progressive ICAD. Our objective is to report the determinants of asymptomatic ICAD by linking the presence or absence of ICAD on magnetic resonance angiogram (MRA) with detailed risk assessment in asymptomatic adults.
Methods
This is an observational cross-sectional analytical study. We plan to recruit 200 adult participants from the radiology departments of two tertiary care centers of Karachi, Pakistan. The participants will first be screened for the absence of stroke symptoms via the Questionnaire for Verifying Stroke Free Status (QVSFS). QVSFS negative will be participants will be eligible. After written informed consent, participants will undergo detailed medical, sociodemographic, lifestyle, and anthropometric evaluation by a detailed interview. They will, in addition, undergo MRA to study the presence, degree, and distribution of asymptomatic ICAD. All MRA scans will be reviewed centrally by vascular neurologists blinded to clinical information. These images would be reviewed on DICOM Viewer 3.0 used for calculating the degree of stenosis using Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) study defined criteria employing electronic calipers. A sample size of 200 will achieve 80% power for detecting a minimum difference of 20% in the prevalence of exposure factors (medical and lifestyle) between asymptomatic ICAD positive and ICAD negative persons. This study will generate regional data on risks for ICAD development and prevention in a high-risk susceptible population.
Study ID: NCT02072876
Key Words: Stroke, Asymptomatic Intracranial Stenosis, Developing Countries, Prevention, Sociodemographic Risk Factors, Epidemiology, Radiology
Background
Stroke is the emerging noncommunicable disease epidemic of the developing world. Studies from Pakistan, the sixth most populous nation in the world [1], report that the lifetime prevalence of cerebrovascular disease (CVD) is one in four (25%) over the age of 35 years [2]. Two thirds of all stroke deaths now occur in low- and middle-income countries that report a 100% increase in the incidence of stroke in the last four decades [3].
Intracranial atherosclerotic disease (ICAD), which is progressive narrowing of the arteries at the base of the brain, is a major cause of ischemic stroke, with higher prevalence in Asian, African American, and Hispanic individuals as compared with Caucasians [4]. ICAD causes about 30%–50% of ischemic strokes among patients of Asian ancestry, by contrast, approximately 8%–9% of strokes are attributable to ICAD in Caucasians [5].The Karachi Intracranial Stenosis study (KISS), a hospital-based case–control study for symptomatic ICAD, reported that 80% of vessels studied in stroke patients had significant asymptomatic stenosis (>70%) [6].
Numerically, symptomatic ICAD is the most common mechanism of stroke in the world, given that a large part of the world’s population lives in Asia. Clinical ICAD is likely to be preceded by a period of silent atherosclerotic narrowing and plaque formation. At the point of either critical stenosis, or plaque destabilization, strokes result from loss of blood supply, overlying thrombosis and/or arterial embolization [7].Clinical ICAD is an aggressive disease. The rate of stroke recurrence is high (up to 18%–24% annually) [4], [8]. Current interventions for clinically active ICAD are difficult to implement in regions that bear the brunt of ICAD [9]–[11]. Our overarching rationale is that early detection of asymptomatic ICAD and identification of its risk factors may allow regionally implementable therapeutic interventions at a lower cost and greater efficacy. We feel this is important, since this is a unique population subject to an increased risk of stroke mortality [12] that has increased in the last decade and is a call for action.
Objective
Our objective is to study the clinical, lifestyle, dietary, and psychosocial determinants of asymptomatic ICAD in clinically normal adults. As an additional secondary objective, we will also study the frequency of ICAD-associated findings on magnetic resonance imaging (MRI), e.g., brain volume reduction, silent brain infarcts, and periventricular hyper intensities in patients diagnosed with asymptomatic ICAD on MRI brain. We hypothesized that there is at least a 20% difference in the proportion of clinical, lifestyle (dietary, physical activity, obesity, smoking, and stress/depression), and socioeconomic predictors among asymptomatic ICAD patients than those with no ICAD.
Methods
Study Design
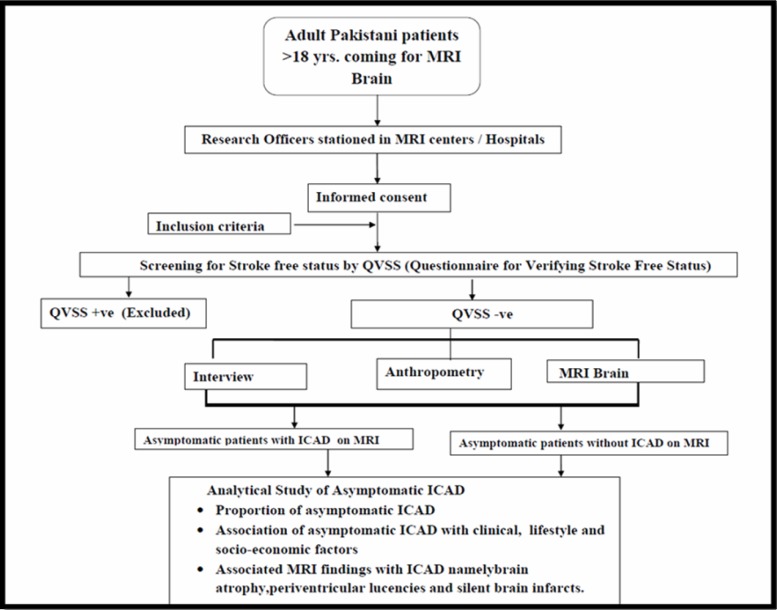
Analytical Cross-Sectional Observational Study (Figure 1, Flow Chart)
Figure 1. Study flow diagram.
Study Setting
The study will be carried out at the radiology departments of two major tertiary care centers in Karachi, Pakistan [Aga Khan University (AKU)], and Dow University of Health Sciences (DUHS). These major MRI centers cover the bulk of MRI referrals from all over Pakistan and cover all socioeconomic and ethnic strata.
AKU is a private not for profit academic international center. The Dow University Radiology Center is a public sector, government funded, subsidized-at-cost center. Both the centers have radiology departments equipped with 1.5 Tesla MRI scanner facilities, which are open 24/7 for all patients (inpatients and outpatients). Since both are based in Karachi, Pakistan’s largest city with a mix of ethnicities, together they represent a microcosm of Pakistan, and provide a wide spectrum of participants that lends representativeness to the sample population.
Study Population
This study aims to recruit adult Pakistani patients > 18 years, with no previous clinical history of stroke (negative on QVSFS), presenting to the two tertiary care centers for MRI brain for indications other than stroke (e.g., nonspecific headache, sinusitis, primary epilepsy, and primary dementia without stroke history) and consenting for MRA, interview, and anthropometry.
Sample Size Estimation
We have calculated that a minimum sample size of 200 people will be required in order to achieve 80% power for detecting a minimum difference of at least 20% in the prevalence of exposure factors (medical and lifestyle) between asymptomatic ICAD positive and ICAD negative persons, with the anticipated prevalence of all exposure factors (medical: diabetes, hypertension, dyslipidemia, and heart disease; and lifestyle: dietary, physical activity, obesity, and stress) ranging from 4%–70%, assuming a 1:3 ratio in patients with ICAD versus no ICAD at a 5% level of significance.
Statistical Analysis
For continuous variables like age, weight, height, waist circumference and body mass index (BMI), mean and standard deviations (SDs) will be calculated. For categorical variables like gender, ethnicity, education, marital status, income, occupation, ICAD, clinical and lifestyle factors (diabetes, hypertension, dyslipidemia, CVD, tobacco use, obesity, diet, and depression), brain atrophy, silent brain infarcts, and perventricular lucencies, proportions will be calculated.
BMI will be calculated by dividing weight in kilogram by the square of height in square meters. BMI was expressed as mean and SD as well as five categories defined using the South Asian cutoffs [13]. Waist circumference was also divided into categories using cutoffs defined specifically for Asians [14].
The independent contribution of any risk factor to ICAD will be examined in the univariate Cox proportional hazards model, which was used to calculate prevalence ratios. Point estimates and 95% confidence intervals (CIs) were calculated from the regression coefficients. The p value of the Wald statistics for each explanatory variable was used for assessing its coefficient with significance of 0.25.
In order to compute the statistically adjusted prevalence ratios (PR), multivariable Cox regression analysis will be done. Variables will be introduced as continuous or categorical variables, as indicated in results. With categorical variables, the lowest or opposite category will serve as the reference group. All the variables significant at univariable level and/or the variables with previously known associations with the outcome (ICAD) will be entered into the model in a block wise fashion. The level of significance of ≤0.05 was set for multivariable analysis. Significance of each individual independent variable was assessed by its Cl, Wald statistic and the change in the −2 log-likelihood. Insignificant variables (p > 0.05) were removed one by one from the model with the most insignificant first after checking their interaction. A final model was recalculated as the most parsimonious version, including only significant variables and interpreted by using adjusted PRs and their 95% CIs. Statistical analyses were conducted using the Statistical Package for Social Sciences (SPSS), Version 19.
Study Inclusion and Exclusion Criteria
Inclusion Criteria:
Adult Pakistani men and women ≥18 years of age getting MRI brain for any indication other than stroke.
Permanent residents of Pakistan for at least 1 year.
No prior history of stroke confirmed by screening with QVSFS. QVSFS negative.
Able to give written informed consent.
Exclusion Criteria:
QVSFS positive
Severe disability, life expectancy less than 6 months at enrollment
Patients in whom MRI prolongation for 5 minutes can be harmful for the patient (Anesthesia, claustrophobia, sedation, and so on)
Any clear contraindications to MRI, e.g., pacemakers, metallic valves, patient is not an MRI candidate
Study Procedures
Prefield Pilot
The questionnaire has been translated from English into Urdu. The aim was not to do a literal word to word translation but to have conceptually equivalent items in the Urdu version of the questionnaires as well as to insert culturally acceptable terms particularly for the diet questionnaire. Already the existing and validated Urdu versions of Short International Physical Activity Questionnaire (IPAQ) and Self-Reporting Questionnaire (SRQ) for depression [15] and tested Urdu version of QVSFS will be used [2], [16], [17]. The rest of the questionnaire has been translated into Urdu by the principal investigator (PI) directly for quality and conceptualization. The Urdu version of the questionnaire will then be pretested in the neurology clinic population (10% of the sample size) to see its acceptability after which any required changes in the phrasing of Urdu version of the questionnaire will be done.
Hiring and Training Data Collectors
Before the start of the data collection process, the data collectors will be given training on communication skills through a two day long workshop conducted by the PI. They will be introduced to the different tools used in the questionnaire and their administration. They will also be given training on how to perform anthropometric measurements. Along with this they will also be given a copy of the Manual of operations (Additional File 1) specifying pictorial methods for anthropometric measurements. A standard method of data reporting will be communicated to all. No box or space in the form is to be left blank and if left blank the reason has to be specified in the form. Training will also include visit to the specified settings to orient the data collectors to the place and introduce them to the relevant people whom they might encounter during the data collection process. The data collectors will be taught to keep records of all patients they approach whether they get enrolled in the study or not. Once training is complete the data collectors will be cross-checked by the PI in the field at the site for audit and feedback.
Data Collection Procedures
Nonprobability purposive sampling will be used to recruit consecutive patients prospectively from the two hospital sites. Data collectors at the two field sites will be responsible for first screening out all outpatients who come for MRI brain, via QVSFS, for presence of stroke and then take informed consent and enroll them in the study. Once the participant is recruited in the study, the research officers will collect history of medical and lifestyle factors on a SRQ consisting of seven sections (as detailed below) followed by height, weight, and waist measurements and imaging study. These images will then be collected on compact disks (CDs) by the designated research officers for centralized image viewing.
All data forms coming from the field shall be checked by the PI and discrepant or unfilled forms will be returned for completion. To ensure data accuracy, we will perform double data entry by a data entry officer who has been trained by the PI in reading the filled data forms using the data code book. (Additional File 2 (Questionnaire)).
Data Collection Tools and Variable Definition
Following data collection tools will be used in this study:
-
Questionnaire (Additional File 2) consisting of eight sections is as follows:
MRI Brain with MRA
Data Collection Form (DCF) (Additional File 3) for detecting intracranial stenosis [24], [25]
Questionnaire
The questionnaire consists of eight sections. Section I is the stroke free status determining questionnaire, which is an important inclusion criterion for the study. It is an eight-item structured questionnaire originally designed to identify study subjects, who are free of symptomatic cerebrovascular disease, with a high sensitivity (0.97) and moderate specificity (0.60) for detecting stroke [26]. It has been used in previous studies on Pakistani population and has a good detection rate [6]. Section II is the demographic and socioeconomic information section. It has 10 items that collect information about age, sex, ethnicity, education, and marital status and five questions regarding income, occupation, and number of household members and household assets. For assessing household assets, the Assets Questionnaire developed by the World Bank for developing countries will be used [18]. The questionnaire has been modified keeping in view the study population. It consists of 19 items inquiring about the presence or absence of household assets, and the number of persons sharing a room. Section III inquires about clinical risk factors, e.g., diabetes, hypertension, dyslipidemia, heart attack, atrial fibrillation, and history of coronary bypass surgery. Hypertension is defined as systolic blood pressure > 140 mmHg or diastolic blood pressure > 90 mmHg, or those with a clear hypertension medical record, or record of antihypertensive drugs usage [27]. Subjects with diabetes mellitus are those with documented diabetes in the medical record, or those having a random glucose level of >11.1 mmol/L (200 mg/dl) or those who are on medicines for diabetes treatment [28]. Dyslipidemia is defined as total cholesterol >200 mg/dl or LDL >160 mg/dl or those taking lipid-lowering drugs [29], [30]. IHD is defined as a known history of myocardial infarction or angina [29].
Section IV has the World Health Organization (WHO) stepwise approach to surveillance (STEPS) questionnaire for tobacco usage [19]. It has 13 items collecting self-reported information of current or past smoking and smokeless tobacco use with durations. Current users are defined as individuals who have been using any kind of tobacco in the previous 12 months. Former users are those who have quit more than a year earlier. Section V is the IPAQ short form. It has seven items that collect information regarding time spent in vigorous, moderate physical activity, walking, and sitting during the last week [31]. Section VI is the dietary questionnaire that collects information on the daily, weekly, and monthly consumption of food items and their quantity using a Food Frequency Questionnaire designed especially for the study keeping in mind the culinary pattern of the common Pakistani. It has 11 sections collecting information on 62 items.(Bread, Cereals, dairy products, Rice, lentils, meat ,Bar BQ, Curries, Fish, Fruits and vegetables and bakery items) [21]. Section VII is the SRQ for depression [15]. This standardized screening instrument measures psychological and somatic symptoms associated with depression through 20 items. Each item has a yes/no answer. The time span refers to the individual's feelings over the past 30 days. Each item is scored 0 or 1. The maximum score is therefore 20. Its validity has been tested in Pakistani population and found to have good psychometric properties with sensitivity of 78% and specificity of 81% [32].
Section VIII is the anthropometric measurements section. This includes measurement of body weight of the individual in kilograms and height and waist measurement in centimeters to calculate BMI. These anthropometric indices are regarded as the measurement of general and central obesity. For categorizing BMI, WHO’s recommendations for Asian population will be used. According to this classification, ≤18.5 kg/m2is underweight, 18.6–22.9 is normal weight, 23–24.9 is pre overweight, 25–29.9 is overweight, and >30 kg/m2 is obese [13].
Waist circumferences of ≥90 cm in men and ≥80 cm in women have been taken as cutoffs for increased risk of metabolic consequences [14].
We presume the questionnaire will take around 30 minutes for administration.
Section II is the phase where the patient will undergo MRI brain along with an additional 5 minutes MRA without contrast administration.
Section III consists of the Questionnaire for documenting intracranial stenosis. It has seven questions regarding brain tissue volume, periventricular lucencies, old vascular lesions, and nonstroke lesions along with a table documenting the degree of stenosis in each cerebral vessel. Information will be collected for a total of 19 vessels per scan. A standardized MRI reading form adapted from the work of J Wardlaw will be used [33]–[38].
MRA Evaluation of Intracranial Stenosis
This is defined as any artery (internal carotid artery, middle cerebral artery, anterior cerebral artery, posterior cerebral artery, basilar artery, and vertebral artery) having >25% stenosis on MRA. An internationally standardized equation will be used to calculate the degree of stenosis. This method has also been used successfully in a Pakistani study [39] to measure symptomatic intracranial stenosis.
Percent stenosis = [1 − (Dstenosis/Dnormal) × 100], where Dstenosis is the diameter of the artery at the site of the most severe stenosis, and Dnormal = diameter of the proximal normal artery [40].
Special freely available software DICOM Viewer 3.1 will be used to save all MRI images. It allows zooming, panning, measurements, annotations, and segmentation of the images. It has the ability to rotate (90°, 180°) and flip (horizontal, vertical) images. It also provides one with angle values, which helps in measuring stenosis around corners. All opened images can be reported simultaneously. All MRI CDs will be reviewed by a vascular neurologist and radiologist team intracranial occlusive lesions will be rated by giving them 1 of 5 grades depending on the narrowness of the arteries [41].
<25%, Atherosclerotic irregularity
25%–49% reduction as mild stenosis
50%–74% reduction as moderate stenosis
75%–99% reduction as severe stenosis
No opening will be graded as complete occlusion
Additionally, the MRI brain will also be viewed for the presence of brain atrophy, periventricular lucencies, old strokes and nonstroke lesions, and coded on a standardized form [33], [41], [42].
Ethical Concerns
The study protocol has been approved by the Ethical Review Committees (ERC) of both participating centers. (ERC number 2327 CHS ERC 12, and IRB 360/DUHs 2012). All participants will provide a written informed consent. All MRA scans are reviewed within 24 hours by radiology teams with a provision in the study to report any critical, life threatening incidental findings like aneurysms to the referring physician and provision of urgent referral to facilitate care. In addition, all key study and field staff received training in Neuroethics, Good Clinical Practice (GCP), informed consent procedures, and human subject’s protection in research.
Discussion
This study will demonstrate the distribution and determinants of ICAD in otherwise normal asymptomatic Pakistanis presenting for MRI without a history of prior stroke. Available international data reports ICAD prevalence of 4%–29% in high-risk asymptomatic subjects [43]–[45]. The range of ICAD varies from 3% to 29.6% depending on the modality used; cutoffs and risk profiles of population. Most of these previous studies [46]–[54] have preferentially selected high-risk individuals to study the prevalence of asymptomatic ICAD, however none have been performed in Pakistan and most have used transcranial doppler (TCD) or a single artery instead of a more holistic approach. Other serious limitations of previous investigations are inappropriate recruitment strategies, evaluating only one artery, or choosing to report only advanced stenosis, or selecting high-risk patients likely to have strokes. Information on all lifestyle predictors like exercise, diet, smoking, obesity, stress of ICAD have to date not been described, although known risks like hypertension, diabetes, and previous stroke have been inconsistently reported.
This study plans to recruit relatively risk free individuals and hopes to uncover social and demographic determinants of ICAD that have not been previously investigated. Regional comparable data for risk free subjects is sparse. Since we are using a sensitive modality like MRI, we hope to accurately detect the presence of ICAD better as compared to studies reported that have employed TCD for the same.
We feel that our study design is valuable for the following reasons. We will not select high-risk participants with hypertension, diabetes, and so on; therefore, biasing our study toward conventional risk factors alone. A detailed review of asymptomatic status shall be done using a questionnaire (QVSFS), which has been used internationally to rule out clinical stroke with 95% sensitivity and specificity [55]. This has been previously used in Pakistan [2]. In addition, all our risk factor assessment tools have been locally validated [15], [24]. Rigorous training will be given to all data collectors, so that the same protocol is implemented at all centers. 24/7 back up was available for any participant intake, where there was a question about stroke, by stroke neurologists further adding clarity to participant eligibility. Same diagnostic MRI, i.e., 1.5 Tesla was used at both the centers thus reducing diagnostic/detection bias. Centralized coding system for MRA and specialized software was used for storing and image viewing with expert back up. This study assessed a number of novel risk factors for ICAD like sociodemographic factors, physical activity, diet, and depression, which have not been explored previously, i.e., a whole person approach rather than focusing on only traditional vascular risk factors like hypertension, diabetes, and so on. This approach for ICAD is comprehensive. This knowledge will inform both global and local stroke preventive strategies of the most common cause of stroke in the world.
List of abbreviations
- ICAD
Intracranial Atherosclerotic Disease
- QVSFS
Questionnaire for Verifying Stroke Free Status
- MRA
Magnetic Resonance Angiogram
- WASID
Warfarin–Aspirin Symptomatic Intracranial Disease
- CVD
Cerebrovascular disease
- MRI
Magnetic Resonance Imaging
- KISS
Karachi Intracranial stenosis study
- AKU
Aga Khan University
- DUHS
Dow University of Health Sciences
- BMI
Body Mass Index
- SD
Standard Deviations
- PR
Prevalence Ratios
- CI
Confidence Interval
- SPSS
Statistical Package for Social Sciences
- IPAQ
International Physical Activity Questionnaire
- SRQ
Self-Reporting Questionnaire
- PI
Principal Investigator
- FFQ
Food frequency Questionnaire
- DCF
Data Collection Form
- WHO
World Health Organization
- STEPS
Stepwise approach to surveillance
- ERC
Ethical Review Committee
- GCP
Good Clinical Practice
- TCD
Transcranial Doppler
Competing Interests: None Declared
Author’s Contributions
AKK and FM jointly conceived the study, performed data analysis and wrote the first draft. OP advised on design issues, MI,IA,BA provided statistical input, MSI,MH,KM assisted and provided intellectual input for the study from the public sector perspective and radiologic collaborative input , SN provided analytical support, ZS provided radiologic support for center, SK provided international intellectual input, perspective and feedback.
Disclosures
Dr Ayeesha Kamran Kamal is the co-director and recipient of grant entitled, “The International Cerebrovascular Translational Clinical Research Training Program” (Fogarty International Center, National Institutes of Health). Dr. Farzin Majeed is a neurovascular research fellow whose mentored research practicum training is currently funded by Award Number D43TW008660 from the Fogarty International Center and the National Institute of Neurologic Disorders and Stroke. Dr Ayeesha Kamran Kamal is also funded by Grand Challenges Canada (GCC), University Research Council (URC), Higher Education Commission, Govt. of Pakistan, (HEC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center, National Institute of Neurologic Disorders and Stroke or the National Institute of Health.
Additional Files:
Please click on the following link:(http://jvin.org/journal/index.php/jvin/issue/archive) and click on Vol 8, No 1 (2015)
Additional File 1: Manual of Operations
Additional File 2: Study Questionnaire for Risk Assessment
Additional File 3: Data Collection Form for Image Analysis
Additional File 4: Short International Physical Activity Questionnaire (IPAQ) short form for physical activity assessment
Acknowledgments
The team would like to thank and appreciate the following: Dennis Fernandes, Salman Karim, LailaLadak at the Clinical Trials Unit for administrative and logistic support. Dr AzizunissaIrumnazat the Clinical Trials Unit for her excellent support for staff training for ethics, Good Clinical Practice for all field and key staff. Abdul Muqeetat the AKDN eHealth Resource center for support and standardization of DICOM on centralized review. Mr. Musa Khan and Mr. Khawaja Mustafa librarian for library assistance, Dr Romaina Iqbal for her intellectual input for Principal Component Analysis, Kawasji Kheswalla in the research office for facilitation and oversight. In addition the stroke team would like to acknowledge the support of the Section of Neurology at AKUH who always facilitates logistically and provides protected time for all research endeavors.
Footnotes
Conflict of Interest
None declared.
References
- Population Census Organization. [3/19/2014];Census 2011. 2011 Available from: http://www.census.gov.pk/.
- Kamal AK, et al. The burden of stroke and transient ischemic attack in Pakistan: a community-based prevalence study. BMC neurology. 2009;9(1):58. doi: 10.1186/1471-2377-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2013;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LKS. Global burden of intracranial atherosclerosis. International Journal of Stroke. 2006;1(3):158–159. doi: 10.1111/j.1747-4949.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- Komotar RJ, et al. Update on the natural history of intracranial atherosclerotic disease: A critical review. World journal of radiology. 2010;2(5):166. doi: 10.4329/wjr.v2.i5.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal AK, et al. The Karachi intracranial stenosis study (KISS) Protocol: an urban multicenter case-control investigation reporting the clinical, radiologic and biochemical associations of intracranial stenosis in Pakistan. BMC Neurol. 2009;9:31. doi: 10.1186/1471-2377-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golledge J, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke. 2000;31(3):774–81. doi: 10.1161/01.str.31.3.774. [DOI] [PubMed] [Google Scholar]
- Feldmann E, et al. Chinese white differences in the distribution of occlusive cerebrovascular disease. Neurology. 1990;40(10):1540. doi: 10.1212/wnl.40.10.1540. [DOI] [PubMed] [Google Scholar]
- Khan M, et al. Intracranial atherosclerotic disease. Stroke research and treatment. 2011;2011 doi: 10.4061/2011/282845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AI, et al. Intracranial atherosclerotic disease: an update. Ann Neurol. 2009;66(6):730–8. doi: 10.1002/ana.21768. [DOI] [PubMed] [Google Scholar]
- Group EBS. Failure of Extracranial-Intracranial Arterial Bypass to Reduce the Risk of Ischemic Stroke: Results of an International Randomized Trial. The Challenge of Epidemiology: Issues and Selected Readings. 2004;1(1):950–964. [Google Scholar]
- Qureshi AI, et al. Paradoxical Increase in stroke mortality among Asian Indians in the United States. Journal of Vascular and Interventional Neurology. 2014. [PMC free article] [PubMed]
- Who EC. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- Kurpad SS, Tandon H, Srinivasan K. Waist circumference correlates better with body mass index than waist-to-hip ratio in Asian Indians. Natl Med J India. 2003;16(4):189–92. [PubMed] [Google Scholar]
- Ahmer S, Faruqui R, Aijaz A. Psychiatric rating scales in Urdu: a systematic review. BMC psychiatry. 2007;7(1):59. doi: 10.1186/1471-244X-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samir N, Mahmud S, Khuwaja AK. Prevalence of physical inactivity and barriers to physical activity among obese attendants at a community health-care center in Karachi, Pakistan. BMC Res Notes. 2011;4:174. doi: 10.1186/1756-0500-4-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain N, et al. Life stress and depression in a tribal area of Pakistan. Br J Psychiatry. 2007;190:36–41. doi: 10.1192/bjp.bp.106.022913. [DOI] [PubMed] [Google Scholar]
- Gwatkin DR, et al. Socio-economic differences in Brazil. HNP/Poverty Thematic Group of the World Bank; Washington, DC: 2000. [Google Scholar]
- World Health, O. WHO STEPS surveillance manual: the WHO STEPwise approach to chronic disease risk factor surveillance. Geneva: World Health Organization; 2005. [Google Scholar]
- Craig CL, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Iqbal R, et al. Refinement and validation of an FFQ developed to estimate macro-and micronutrient intakes in a south Indian population. Public health nutrition. 2009;12(01):12–18. doi: 10.1017/S1368980008001845. [DOI] [PubMed] [Google Scholar]
- Yusuf S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- Husain N, et al. Life stress and depression in a tribal area of Pakistan. The British Journal of Psychiatry. 2007;190(1):36–41. doi: 10.1192/bjp.bp.106.022913. [DOI] [PubMed] [Google Scholar]
- Samuels OB, et al. A standardized method for measuring intracranial arterial stenosis. American journal of neuroradiology. 2000;21(4):643–646. [PMC free article] [PubMed] [Google Scholar]
- Wardlaw J. Image Analysis Tools. [3/20/2014];2014 Available from: http://www.sbirc.ed.ac.uk/research/imageanalysis.html.
- Sung VW, et al. Sensitivity and specificity of stroke symptom questions to detect stroke or transient ischemic attack. Neuroepidemiology. 2011;36(2):100–104. doi: 10.1159/000323951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian AV, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. Jama. 2003;289(19):2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes care. 2010;33(Supplement 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeman JI, et al. Expert panel on detection, evaluation and treatment of high blood cholesterol in adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP III) Jama. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Libby P. What have we learned about the biology of atherosclerosis? The role of inflammation. The American journal of cardiology. 2001;88(7):3–6. doi: 10.1016/s0002-9149(01)01879-3. [DOI] [PubMed] [Google Scholar]
- Macfarlane DJ, et al. Reliability and validity of the Chinese version of IPAQ (short, last 7 days) Journal of Science and Medicine in Sport. 2007;10(1):45–51. doi: 10.1016/j.jsams.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Husain N, Creed F, Tomenson B. Depression and social stress in Pakistan. Psychological medicine. 2000;30(2):395–402. doi: 10.1017/s0033291700001707. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Sellar R. A simple practical classification of cerebral infarcts on CT and its interobserver reliability. American journal of neuroradiology. 1994;15(10):1933–1939. [PMC free article] [PubMed] [Google Scholar]
- Farrell C, et al. Development and initial testing of normal reference MR images for the brain at ages 65-70 and 75-80 years. Eur Radiol. 2009;19(1):177–83. doi: 10.1007/s00330-008-1119-2. [DOI] [PubMed] [Google Scholar]
- Tatu L, et al. Arterial territories of human brain: brainstem and cerebellum. Neurology. 1996;47(5):1125–35. doi: 10.1212/wnl.47.5.1125. [DOI] [PubMed] [Google Scholar]
- Tatu L, et al. Arterial territories of the human brain: cerebral hemispheres. Neurology. 1998;50(6):1699–708. doi: 10.1212/wnl.50.6.1699. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6(7):611–9. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- J C van Swieten AH, Koudstaal PJ, van Gijn J. Grading white matter lesions on CT and MRI: a simple scale. Journal of Neurology, Neurosurgery & Psychiatry. 1990;53(12):1080–1083. doi: 10.1136/jnnp.53.12.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, et al. Stroke radiology and distinguishing characteristics of intracranial atherosclerotic disease in native South Asian Pakistanis. Int J Stroke. 2013;8(Suppl A100):14–20. doi: 10.1111/j.1747-4949.2012.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels OB, et al. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21(4):643–6. [PMC free article] [PubMed] [Google Scholar]
- Uehara T, et al. Detection of occlusive lesions in intracranial arteries by three-dimensional time-of-flight magnetic resonance angiography. Cerebrovascular Diseases. 1994;4(5):365–370. [Google Scholar]
- Uehara T, Tabuchi M, Mori E. Risk factors for silent cerebral infarcts in subcortical white matter and basal ganglia. Stroke. 1999;30(2):378–382. doi: 10.1161/01.str.30.2.378. [DOI] [PubMed] [Google Scholar]
- Uehara T, Tabuchi M, Mori E. Frequency and clinical correlates of occlusive lesions of cerebral arteries in Japanese patients without stroke. Cerebrovascular Diseases. 1998;8(5):267–272. doi: 10.1159/000015864. [DOI] [PubMed] [Google Scholar]
- Park KY, et al. Prevalence and risk factors of intracranial atherosclerosis in an asymptomatic Korean population. Journal of Clinical Neurology. 2006;2(1):29–33. doi: 10.3988/jcn.2006.2.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GN, et al. Increasing severity of cardiovascular risk factors with increasing middle cerebral artery stenotic involvement in type 2 diabetic Chinese patients with asymptomatic cerebrovascular disease. Diabetes care. 2004;27(5):1121–1126. doi: 10.2337/diacare.27.5.1121. [DOI] [PubMed] [Google Scholar]
- Thomas GN, et al. Increasing severity of cardiovascular risk factors with increasing middle cerebral artery stenotic involvement in type 2 diabetic Chinese patients with asymptomatic cerebrovascular disease. Diabetes care. 2004;27(5):1121. doi: 10.2337/diacare.27.5.1121. [DOI] [PubMed] [Google Scholar]
- Uehara T, Tabuchi M, Mori E. Frequency and clinical correlates of occlusive lesions of cerebral arteries in Japanese patients without stroke. Cerebrovascular Diseases. 2000;8(5):267–272. doi: 10.1159/000015864. [DOI] [PubMed] [Google Scholar]
- Uehara T, Tabuchi M, Mori E. Risk factors for occlusive lesions of intracranial arteries in stroke free Japanese. European journal of neurology. 2005;12(3):218–222. doi: 10.1111/j.1468-1331.2004.00959.x. [DOI] [PubMed] [Google Scholar]
- Huang HW, et al. Prevalence and risk factors of middle cerebral artery stenosis in asymptomatic residents in Rongqi County, Guangdong. Cerebrovascular Diseases. 2007;24(1):111–115. doi: 10.1159/000103125. [DOI] [PubMed] [Google Scholar]
- Wong KS, et al. A door-to-door survey of intracranial atherosclerosis in Liangbei County, China. Neurology. 2007;68(23):2031. doi: 10.1212/01.wnl.0000264426.63544.ee. [DOI] [PubMed] [Google Scholar]
- Wong KS, et al. Prevalence of asymptomatic intracranial atherosclerosis in high-risk patients. Neurology. 2007;68(23):2035. doi: 10.1212/01.wnl.0000264427.09191.89. [DOI] [PubMed] [Google Scholar]
- Uehara T, et al. MR angiographic evaluation of carotid and intracranial arteries in Japanese patients scheduled for coronary artery bypass grafting. Cerebrovascular Diseases. 2000;11(4):341–345. doi: 10.1159/000047664. [DOI] [PubMed] [Google Scholar]
- Elmore EM, Mosquera A, Weinberger J. The Prevalence of Asymptomatic Intracranial Large Vessel Occlusive Disease: The Role of Diabetes. Journal of Neuroimaging. 2003;13(3):224–227. [PubMed] [Google Scholar]
- Park KY, et al. Prevalence and risk factors of intracranial atherosclerosis in an asymptomatic Korean population. Journal of Clinical Neurology (Seoul, Korea) 2006;2(1):29. doi: 10.3988/jcn.2006.2.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K, et al. A single question about prior stroke versus a stroke questionnaire to assess stroke prevalence in populations. Neuroepidemiology. 2000;19(5):245–257. doi: 10.1159/000026262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional File 1: Manual of Operations
Additional File 2: Study Questionnaire for Risk Assessment
Additional File 3: Data Collection Form for Image Analysis
Additional File 4: Short International Physical Activity Questionnaire (IPAQ) short form for physical activity assessment