Abstract
In the absence of vitamin E deficiency, red cell lipid peroxidation has not been clearly demonstrated in freshly drawn blood obtained from patients with various hemolytic anemias despite indirect evidence that oxidative decomposition of cell membrane unsaturated fatty acids occurs in these particular hemolytic states. Recent studies have indicated that malonaldehyde, a decomposition product of oxidized polyunsaturated fatty acids, is able to covalently cross-link the amino groups of protein or lipid resulting in a fluorescent compound. In the present study we have utilized spectrofluorescent technique to assess whether such fluorescence is present in red cell lipid extracts in association with lipid peroxidation. In vitro red cell lipid peroxidation produced by ultraviolet radiation or the oxidant gas ozone was associated with the development of a fluorescent peak (excitation maximum 360 nm; emission maximum 440 nm) in lipid-containing red cell extracts Similar fluorescence was observed after incubation of red cells with malonaldehyde or with malonaldehyde-containing extracts of peroxidized red cell lipid. Spectrofluorescent evaluation of chloroform: isopropanol extracts obtained from the freshly drawn red cells of six patients receiving the oxidant hemolytic drug diaminodiphenylsulfone also revealed a peak at 440 nm which ranged from 39 to 78 U. In contrast, the levels in samples obtained from 11 hematologically normal subjects were 17-27 fluorescence U. No evidence for an increase in blood levels of free malomaldehyde was observed using the 2-thiobarbituric acid test which is the most commonly performed assay of lipid peroxidation. Serum vitamin E levels were within the normal range. Density separation indicated that the bulk of the fluorescence was present in older red cells. A similar fluorescent peak was also observed in lipid-containing extracts of red cells obtained from rabbits repeatedly injected with phenylhydrazine. The finding of fluorescent spectra consistent with the cross-linking of aminolid by malonaldehyde in the red cells of patients receiving diaminodiphenylsulfone indicates that in vivo red cell lipid peroxidation does occur in the absence of vitamin E deficiency.
Full text
PDF

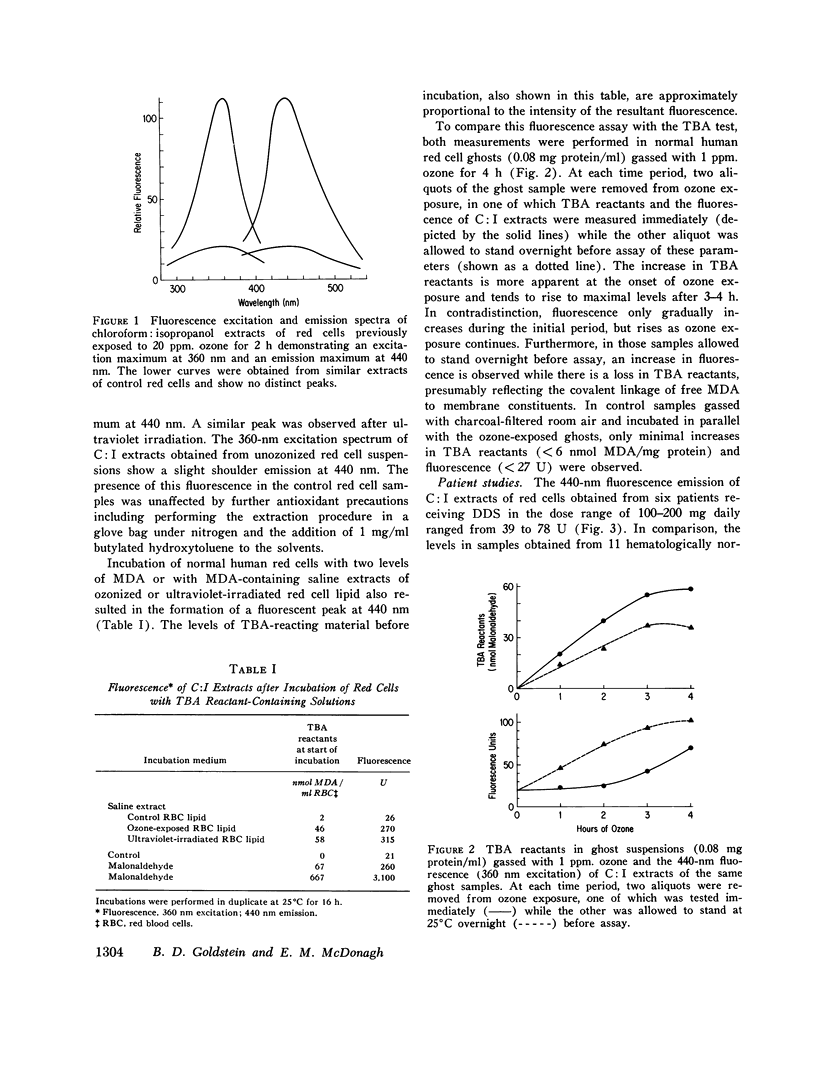
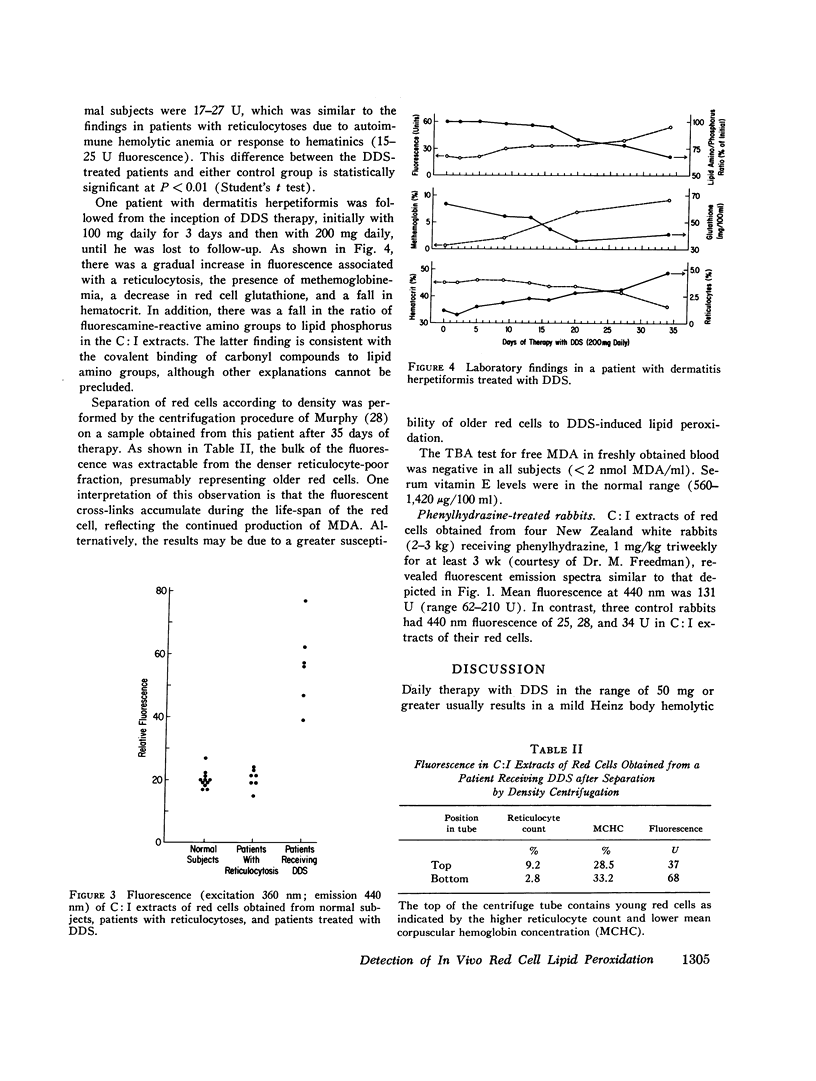


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- Bidlack W. R., Tappel A. L. Fluorescent products of phospholipids during lipid peroxidation. Lipids. 1973 Apr;8(4):203–207. doi: 10.1007/BF02544636. [DOI] [PubMed] [Google Scholar]
- Brooks B. R., Klamerth O. L. Interaction of DNA with bifunctional aldehydes. Eur J Biochem. 1968 Jul;5(2):178–182. doi: 10.1111/j.1432-1033.1968.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Chio K. S., Reiss U., Fletcher B., Tappel A. L. Peroxidation of subcellular organelles: formation of lipofuscinlike fluorescent pigments. Science. 1969 Dec 19;166(3912):1535–1536. doi: 10.1126/science.166.3912.1535. [DOI] [PubMed] [Google Scholar]
- Chio K. S., Tappel A. L. Inactivation of ribonuclease and other enzymes by peroxidizing lipids and by malonaldehyde. Biochemistry. 1969 Jul;8(7):2827–2832. doi: 10.1021/bi00835a020. [DOI] [PubMed] [Google Scholar]
- Chio K. S., Tappel A. L. Synthesis and characterization of the fluorescent products derived from malonaldehyde and amino acids. Biochemistry. 1969 Jul;8(7):2821–2826. doi: 10.1021/bi00835a019. [DOI] [PubMed] [Google Scholar]
- Cohen R. J., Sachs J. R., Wicker D. J., Conrad M. E. Methemoglobinemia provoked by malarial chemoprophylaxis in Vietnam. N Engl J Med. 1968 Nov 21;279(21):1127–1131. doi: 10.1056/NEJM196811212792102. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Shattil S. J. Mechanisms of hemolysis--the minimal red-cell defect. N Engl J Med. 1971 Dec 30;285(27):1514–1520. doi: 10.1056/NEJM197112302852706. [DOI] [PubMed] [Google Scholar]
- DeGowin R. L. A review of therapeutic and hemolytic effects of dapsone. Arch Intern Med. 1967 Aug;120(2):242–248. [PubMed] [Google Scholar]
- Degowin R. L., Eppes R. B., Powell R. D., Carson P. E. The haemolytic effects of diaphenylsulfone (DDS) in normal subjects and in those with glucose-6-phosphate-dehydrogenase deficiency. Bull World Health Organ. 1966;35(2):165–179. [PMC free article] [PubMed] [Google Scholar]
- Dillard C. J., Tappel A. L. Fluorescent products from reaction of peroxidizing polyunsaturated fatty acids with phosphatidyl ethanolamine and phenylalanine. Lipids. 1973 Apr;8(4):183–189. doi: 10.1007/BF02544632. [DOI] [PubMed] [Google Scholar]
- Dodge J. T., Cohen G., Kayden H. J., Phillips G. B. Peroxidative hemolysis of red blood cells from patients with abetalipoproteinemia (acanthocytosis). J Clin Invest. 1967 Mar;46(3):357–368. doi: 10.1172/JCI105537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glader B. E., Conrad M. E. Hemolysis by diphenylsulfones: comparative effects of DDS and hydroxylamine-DDS. J Lab Clin Med. 1973 Feb;81(2):267–272. [PubMed] [Google Scholar]
- Goldstein B. D., Harber L. C. Erythropoietic protoporphyria: lipid peroxidation and red cell membrane damage associated with photohemolysis. J Clin Invest. 1972 Apr;51(4):892–902. doi: 10.1172/JCI106884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B. D. Production of paroxysmal nocturnal haemoglobinuria-like red cells by reducing and oxidizing agents. Br J Haematol. 1974 Jan;26(1):49–58. doi: 10.1111/j.1365-2141.1974.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Gross S., Melhorn D. K. Vitamin E, red cell lipids and red cell stability in prematurity. Ann N Y Acad Sci. 1972 Dec 18;203:141–162. doi: 10.1111/j.1749-6632.1972.tb27868.x. [DOI] [PubMed] [Google Scholar]
- HUTCHISON H. E., JACKSON J. M., CASSIDY P. Drug-induced haemolytic anaemia in dermatitis herpetiformis. The importance of Heinz bodies in its recognition. Br J Dermatol. 1963 Apr;75:161–166. doi: 10.1111/j.1365-2133.1963.tb13960.x. [DOI] [PubMed] [Google Scholar]
- Hamberg M. Decomposition of unsaturated fatty acid hydroperoxides by hemoglobin: Structures of major products of 13L-hydroperoxy-9,11-octadecadienoic acid. Lipids. 1975 Feb;10(2):87–92. doi: 10.1007/BF02532161. [DOI] [PubMed] [Google Scholar]
- Hegesh E., Gruener N., Cohen S., Bochkovsky R., Shuval H. I. A sensitive micromethod for the determination of methemoglobin in blood. Clin Chim Acta. 1970 Dec;30(3):679–682. doi: 10.1016/0009-8981(70)90260-3. [DOI] [PubMed] [Google Scholar]
- Hjelm M., de Verdier C. H. Biochemical effects of aromatic amines. I. Methaemoglobinaemia, haemolysis and Heinz-body formation induced by 4,4'-diaminodiphenylsulphone. Biochem Pharmacol. 1965 Jul;14(7):1119–1128. doi: 10.1016/0006-2952(65)90041-9. [DOI] [PubMed] [Google Scholar]
- Israili Z. H., Cucinell S. A., Vaught J., Davis E., Lesser J. M., Dayton P. G. Studies of the metabolism of dapsone in man and experimental animals: formation of N-hydroxy metabolites. J Pharmacol Exp Ther. 1973 Oct;187(1):138–151. [PubMed] [Google Scholar]
- Kayden H. J., Silber R., Kossmann C. E. The role of vitamin E deficiency in the abnormal autohemolysis of acanthocytosis. Trans Assoc Am Physicians. 1965;78:334–342. [PubMed] [Google Scholar]
- LaCelle P. L. Alteration of membrane deformability in hemolytic anemias. Semin Hematol. 1970 Oct;7(4):355–371. [PubMed] [Google Scholar]
- MENGEL C. E., KANN H. E., Jr, HEYMAN A., METZ E. EFFECTS OF IN VIVO HYPEROXIA ON ERYTHROCYTES. II. HEMOLYSIS IN A HUMAN AFTER EXPOSURE TO OXYGEN UNDER HIGH PRESSURE. Blood. 1965 May;25:822–829. [PubMed] [Google Scholar]
- Melhorn D. K., Gross S. Vitamin E-dependent anemia in the premature infant. I. Effects of large doses of medicinal iron. J Pediatr. 1971 Oct;79(4):569–580. doi: 10.1016/s0022-3476(71)80302-5. [DOI] [PubMed] [Google Scholar]
- Mengel C. E., Kann H. E., Jr Effects of in vivo hyperoxia on erythrocytes. 3. In vivo peroxidation of erythrocyte lipid. J Clin Invest. 1966 Jul;45(7):1150–1158. doi: 10.1172/JCI105421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel C. E., Kann H. E., Jr, Meriwether W. D. Studies of paroxysmal nocturnal hemoglobinuria erythrocytes: increased lysis and lipid peroxide formation by hydrogen peroxide. J Clin Invest. 1967 Nov;46(11):1715–1723. doi: 10.1172/JCI105662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R. Influence of temperature and method of centrifugation on the separation of erythrocytes. J Lab Clin Med. 1973 Aug;82(2):334–341. [PubMed] [Google Scholar]
- O'Malley B. W., Mengel C. E., Meriwether W. D., Zirkle L. G., Jr Inhibition of erythrocyte acetylcholinesterase by peroxides. Biochemistry. 1966 Jan;5(1):40–44. doi: 10.1021/bi00865a006. [DOI] [PubMed] [Google Scholar]
- Oski F. A., Barness L. A. Vitamin E deficiency: a previously unrecognized cause of hemolytic anemia in the premature infant. J Pediatr. 1967 Feb;70(2):211–220. doi: 10.1016/s0022-3476(67)80416-5. [DOI] [PubMed] [Google Scholar]
- RAMANUJAM K., SMITH M. Haemolytic anaemia during treatment of leprosy with diaminodiphenylsulphone by mouth. Lancet. 1951 Jan 6;1(6645):21–22. doi: 10.1016/s0140-6736(51)93495-2. [DOI] [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Rasbridge M. R., Scott G. L. The haemolytic action of dapsone: changes in the red-cell membrane. Br J Haematol. 1973 Feb;24(2):183–193. doi: 10.1111/j.1365-2141.1973.tb05738.x. [DOI] [PubMed] [Google Scholar]
- Rasbridge M. R., Scott G. L. The haemolytic action of dapsone: the effect on red-cell glycolysis. Br J Haematol. 1973 Feb;24(2):169–181. doi: 10.1111/j.1365-2141.1973.tb05737.x. [DOI] [PubMed] [Google Scholar]
- Ritchie J. H., Fish M. B., McMasters V., Grossman M. Edema and hemolytic anemia in premature infants. A vitamin E deficiency syndrome. N Engl J Med. 1968 Nov 28;279(22):1185–1190. doi: 10.1056/NEJM196811282792202. [DOI] [PubMed] [Google Scholar]
- Stocks J., Kemp M., Dormandy T. L. Increased susceptibility of red-blood-cell lipids to autooxidation in haemolytic states. Lancet. 1971 Feb 6;1(7693):266–269. doi: 10.1016/s0140-6736(71)91004-x. [DOI] [PubMed] [Google Scholar]
- Tappel A., Fletcher B., Deamer D. Effect of antioxidants and nutrients on lipid peroxidation fluorescent products and aging parameters in the mouse. J Gerontol. 1973 Oct;28(4):415–424. doi: 10.1093/geronj/28.4.415. [DOI] [PubMed] [Google Scholar]
- Tudhope G. R., Hopkins J. Plasma tocopherol levels and the susceptibility of erythrocytes to Heinz body formation. Clin Sci Mol Med. 1974 May;46(5):635–645. doi: 10.1042/cs0460635. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Williams M. L., Shoot R. J., O'Neal P. L., Oski F. A. Role of dietary iron and fat on vitamin E deficiency anemia of infancy. N Engl J Med. 1975 Apr 24;292(17):887–890. doi: 10.1056/NEJM197504242921704. [DOI] [PubMed] [Google Scholar]


