Abstract
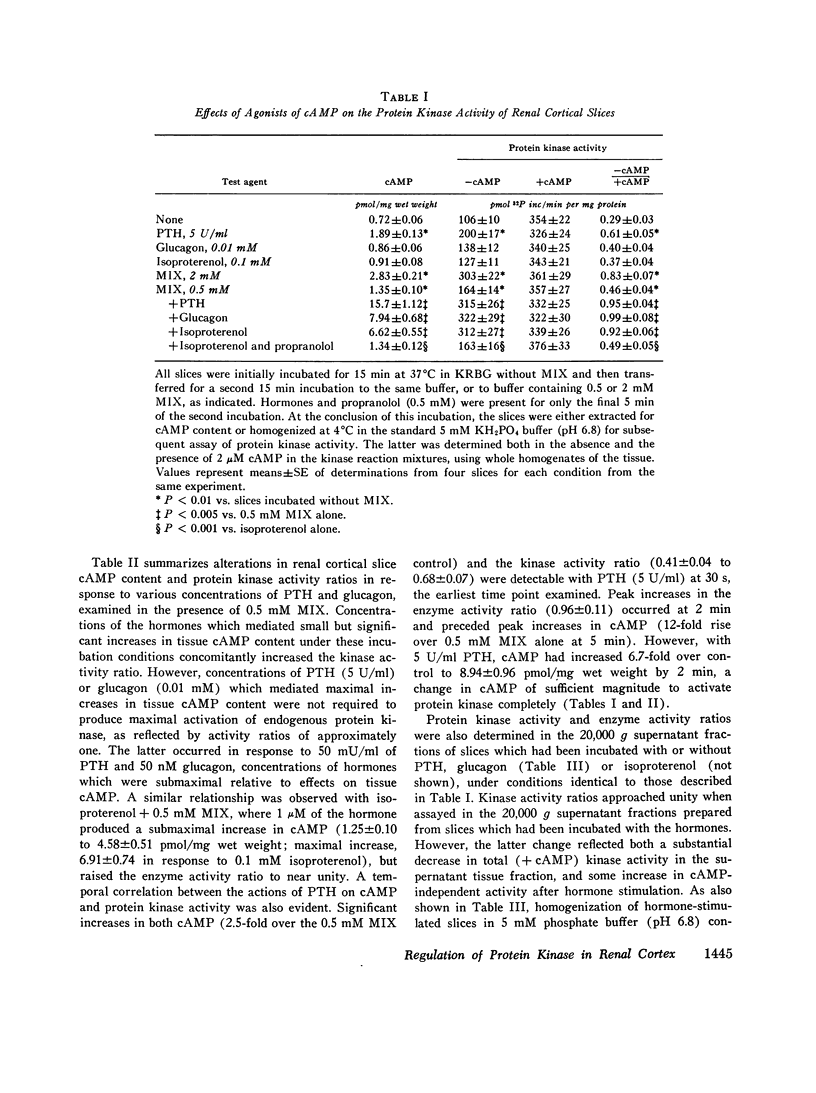
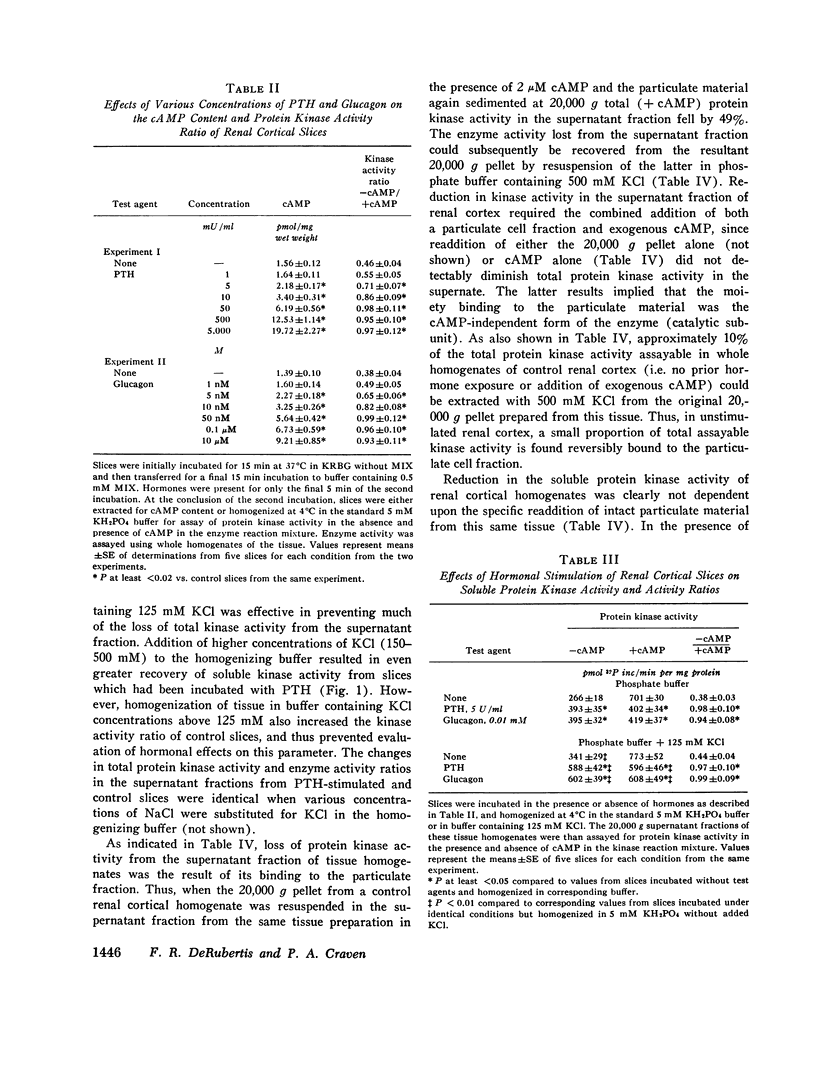
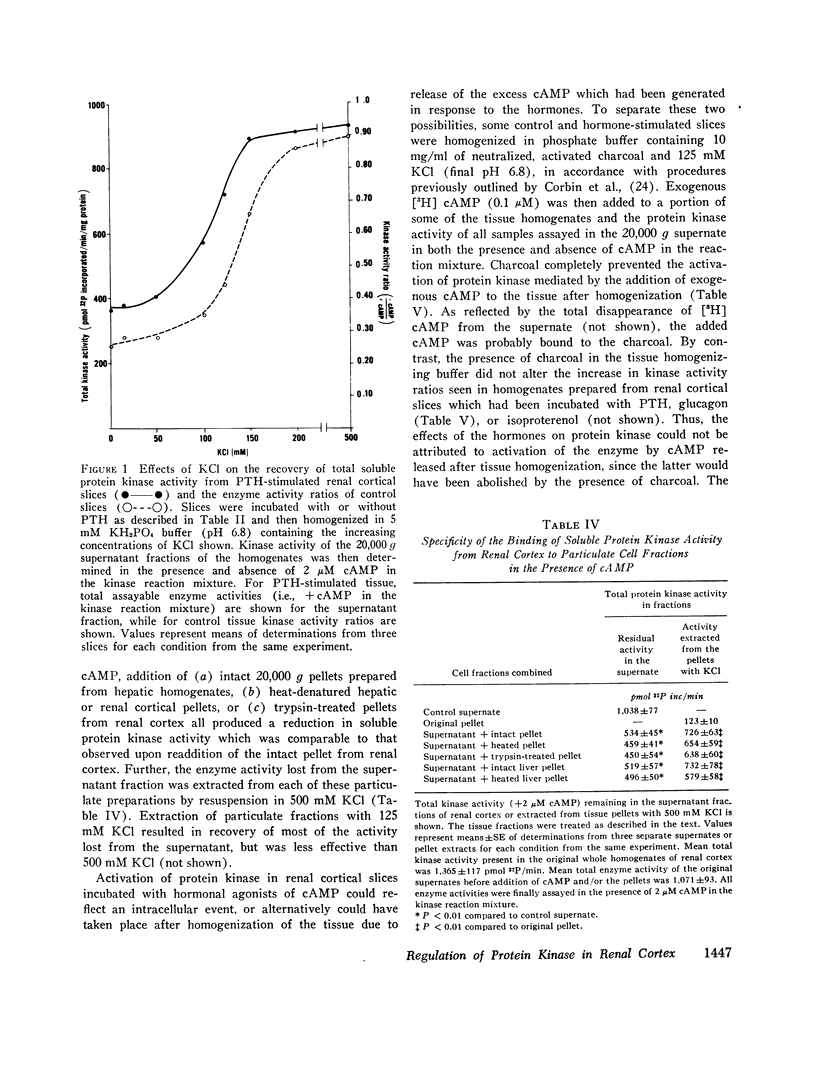
Many of the intracellular actions of cyclic adenosine 3',5'-monophosphate are expressed through phosphorylation reactions mediated by cAMP-dependent protein kinases, but little is known about hormonal control of endogenous protein kinase activity (PK) in kidney. In the present study, we examined the effects of parathyroid hormone, glucagon, and isoproterenol on cAMP and PK in slices of rat renal cortex. In the presence of 0.5 mM 1-methyl, 3-isobutyl xanthine, all three hormones activated PK in slices, as reflected by an increase in the ratio of enzyme activity assayable in homogenates of the slices without addition of cAMP to the kinase reaction mixture (cAMP-independent activity) over total enzyme activity (+2 uM cAMP in the reaction mixture). When enzyme activity was assayed in whole homogenates prepared from slices, the increase in the enzyme activity ratio (- cAMP/+cAMP) which followed hormonal stimulation was due entirely to an increase in cAMP-independent activity, with no change in total activity. In general, a good correlation existed between the alterations in tissue cAMP levels mediated by the hormones and/or 1-methyl, 3-isobutyl xanthine and concomitant alterations in PK. All three hormones increased PK activity ratios to near unity, suggesting complete enzyme activation. However, the concentrations of parathyroid hormone and glucagon which produced maximal activation of PK were much lower than those required for maximal cAMP responses. Studies with charcoal indicated that these hormonal actions on PK reflected intracellular events rather than representing activation of the enzyme during tissue homogenization, due to release of sequestered cAMP. Thus, homogenization of tissue in charcoal prevented activation of PK by subsequent addition of exogenous cAMP, but did not lower enzyme activity ratios in homogenates of hormone-stimulated cortical slices. When PK was determined in the 20,000 g supernatant fraction of renal cortical slices incubated with the hormones, enzyme activity ratios also increased, but total enzyme activity declined. Lost activity was recovered by extraction of particulate fractions with 500 mM KCl or NaCl, results which implied particulate binding of activated PK. Activated soluble PK from renal cortex was bound equally well by intact, heat- and trypsin-treated renal cortical pellets and by intact and heated hepatic pellets. Accordingly, the apparent translocation of enzyme in hormone stimulated cortex does not necessarily represent binding of the activated PK to specific acceptor sites in the particulate cell fractions or constitute a physiologic hormonal action. Activation of renal cortical PK by increasing concentrations of salts suggests that the enzyme in this tissue resembles the predominant type found in heart.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Issa H., Kratowich N., Mendicino J. Properties of soluble and bound protein kinases isolated from swine kidney. Eur J Biochem. 1974 Mar 1;42(2):461–473. doi: 10.1111/j.1432-1033.1974.tb03360.x. [DOI] [PubMed] [Google Scholar]
- Agus Z. S., Puschett J. B., Senesky D., Goldberg M. Mode of action of parathyroid hormone and cyclic adenosine 3',5'-monophosphate on renal tubular phosphate reabsorption in the dog. J Clin Invest. 1971 Mar;50(3):617–626. doi: 10.1172/JCI106532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell N. H. Evidence for a separate adenylate cyclase system responsive to beta-adrenergic stimulation in the renal cortex of the rat. Acta Endocrinol (Copenh) 1974 Nov;77(3):604–611. doi: 10.1530/acta.0.0770604. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Corbin J. D., King C. A., Krebs E. G. Interaction of the subunits of adenosine 3':5'-cyclic monophosphate-dependent protein kinase of muscle. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2444–2447. doi: 10.1073/pnas.68.10.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Soderling T. R., Park C. R. Hormonal regulation of adenosine 3',5'-monophosphate-dependent protein kinase. Adv Cyclic Nucleotide Res. 1975;5:265–279. [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M., Walsh D. A., Krebs E. G. Activation of adipose tissue lipase by skeletal muscle cyclic adenosine 3',5'- monophosphate-stimulated protein kinase. J Biol Chem. 1970 Sep 25;245(18):4849–4851. [PubMed] [Google Scholar]
- DeRubertis F. R., Chayoth R., Zor U., Field J. B. Evidence for persistent binding of biologically active thyrotropin to thyroid in vitro. Endocrinology. 1975 Jun;96(6):1579–1586. doi: 10.1210/endo-96-6-1579. [DOI] [PubMed] [Google Scholar]
- DeRubertis F. R., Craven P. Reduced sensitivity of the hepatic adenylate cyclase-cyclic AMP system to glucagon during sustained hormonal stimulation. J Clin Invest. 1976 Feb;57(2):435–443. doi: 10.1172/JCI108294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J. B., Bloom G., Kerins M. E., Chayoth R., Zor U. Activation of protein kinase in thyroid slices by thyroid-stimulating hormone. J Biol Chem. 1975 Jul 10;250(13):4903–4910. [PubMed] [Google Scholar]
- Forte L. R., Chao W. T., Walkenbach R. J., Byington K. H. Kidney membrane cyclic AMP receptor and cyclic AMP-dependent protein kinase activities: comparison of plasma membrane and cytoplasmic fractions. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1510–1517. doi: 10.1016/0006-291x(72)90511-6. [DOI] [PubMed] [Google Scholar]
- Gill G. N., Garren L. D. A cyclic-3',5'-adenosine monophosphate dependent protein kinase from the adrenal cortex: comparison with a cyclic AMP binding protein. Biochem Biophys Res Commun. 1970 May 11;39(3):335–343. doi: 10.1016/0006-291x(70)90581-4. [DOI] [PubMed] [Google Scholar]
- Gill J. R., Jr, Casper A. G. Depression of proximal tubular sodium reabsorption in the dog in response to renal beta adrenergic stimulation by isoproterenol. J Clin Invest. 1971 Jan;50(1):112–118. doi: 10.1172/JCI106464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann R. A., Lee S., DeAngelo A. B. Translocation of cytoplasmic protein kinase and cyclic adenosine monophosphate-binding protein to intracellular acceptor sites. Adv Cyclic Nucleotide Res. 1975;5:281–306. [PubMed] [Google Scholar]
- Keely S. L., Jr, Corbin J. D., Park C. R. On the question of translocation of heart cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1501–1504. doi: 10.1073/pnas.72.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchberger M. A., Tada M., Repke D. I., Katz A. M. Cyclic adenosine 3',5'-monophosphate-dependent protein kinase stimulation of calcium uptake by canine cardiac microsomes. J Mol Cell Cardiol. 1972 Dec;4(6):673–680. doi: 10.1016/0022-2828(72)90120-4. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- Kuo J. F., Krueger B. K., Sanes J. R., Greengard P. Cyclic nucleotide-dependent protein kinases. V. Preparation and properties of adenosine 3',5'-monophosphate-dependent protein kinase from various bovine tissues. Biochim Biophys Acta. 1970 Jul 15;212(1):79–91. doi: 10.1016/0005-2744(70)90180-4. [DOI] [PubMed] [Google Scholar]
- Kurokawa K., Massry S. G. Evidence for stimulation of renal gluconeogenesis by catecholamines. J Clin Invest. 1973 Apr;52(4):961–964. doi: 10.1172/JCI107261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K., Massry S. G. Evidence for two separate adenyl cyclase systems responding independently to parathyroid hormone and -adrenergic agents in the renal cortex of the rat. Proc Soc Exp Biol Med. 1973 May;143(1):123–126. doi: 10.3181/00379727-143-37266. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Means A. R., MacDougall E., Soderling T. R., Corbin J. D. Testicular adenosine 3':5'-monophosphate-dependent protein kinase. Regulation by follicle-stimulating hormone. J Biol Chem. 1974 Feb 25;249(4):1231–1238. [PubMed] [Google Scholar]
- Palmer W. K., Castagna M., Walsh D. A. Nuclear protein kinase activity in glucagon-stimulated perfused rat livers. Biochem J. 1974 Nov;143(2):469–471. doi: 10.1042/bj1430469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullman T. N., Lavender A. R., Aho I. Direct effects of glucagon on renal hemodynamics and excretion of inorganic ions. Metabolism. 1967 Apr;16(4):358–373. doi: 10.1016/0026-0495(67)90047-9. [DOI] [PubMed] [Google Scholar]
- Richardson M. C., Schulster D. The role of protein kinase activation in the control of steroidogenesis by adrenocorticotrophic hormone in the adrenal cortex. Biochem J. 1973 Dec;136(4):993–998. doi: 10.1042/bj1360993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roobol A., Alleyne G. A. Regulation of renal gluconeogenesis by calcium ions, hormones and adenosine 3':5'-cyclic monophosphate. Biochem J. 1973 May;134(1):157–165. doi: 10.1042/bj1340157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling T. R., Corbin J. D., Park C. R. Regulation of adenosine 3',5'-monophosphate-dependent protein kinase. II. Hormonal regulation of the adipose tissue enzyme. J Biol Chem. 1973 Mar 10;248(5):1822–1829. [PubMed] [Google Scholar]
- Soderling T. R., Hickenbottom J. P., Reimann E. M., Hunkeler F. L., Walsh D. A., Krebs E. G. Inactivation of glycogen synthetase and activation of phosphorylase kinase by muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1970 Dec 10;245(23):6317–6328. [PubMed] [Google Scholar]
- Sudilovsky O. In vivo regulation of hepatic protein kinase by adenosine 3',5'-monophosphate mediated glucagon stimulation. Biochem Biophys Res Commun. 1974 May 7;58(1):85–91. doi: 10.1016/0006-291x(74)90894-8. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]


