Abstract
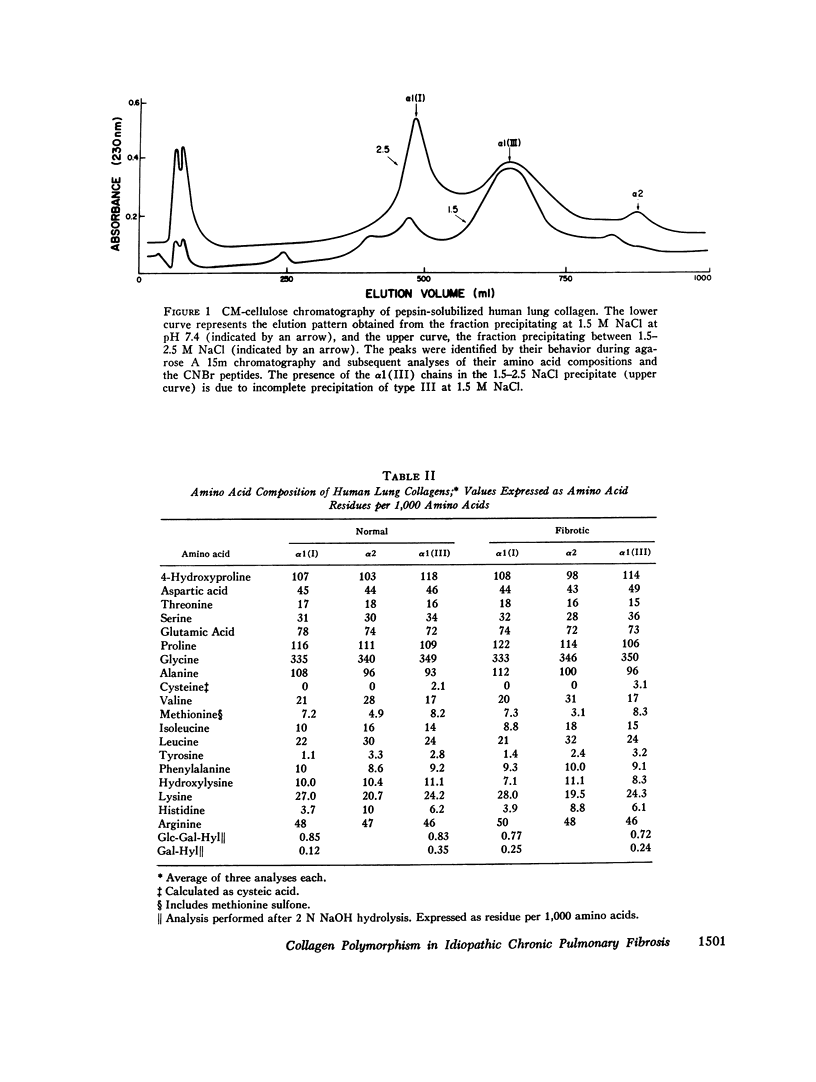
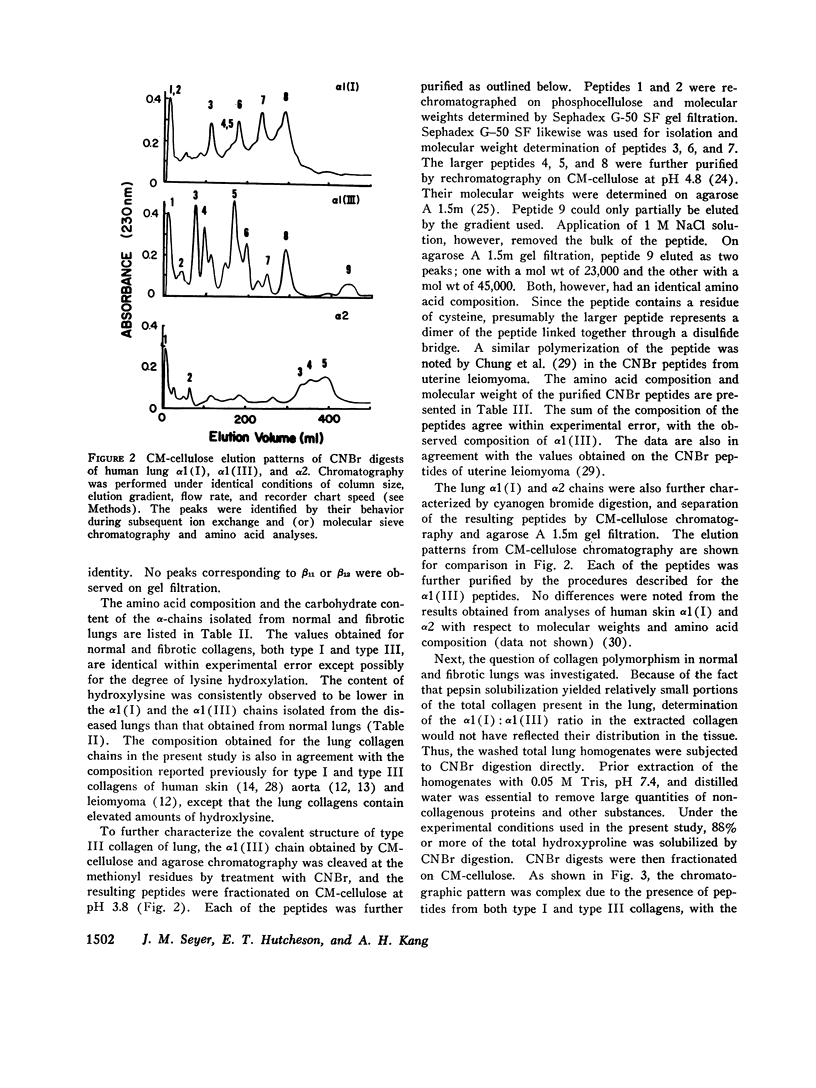
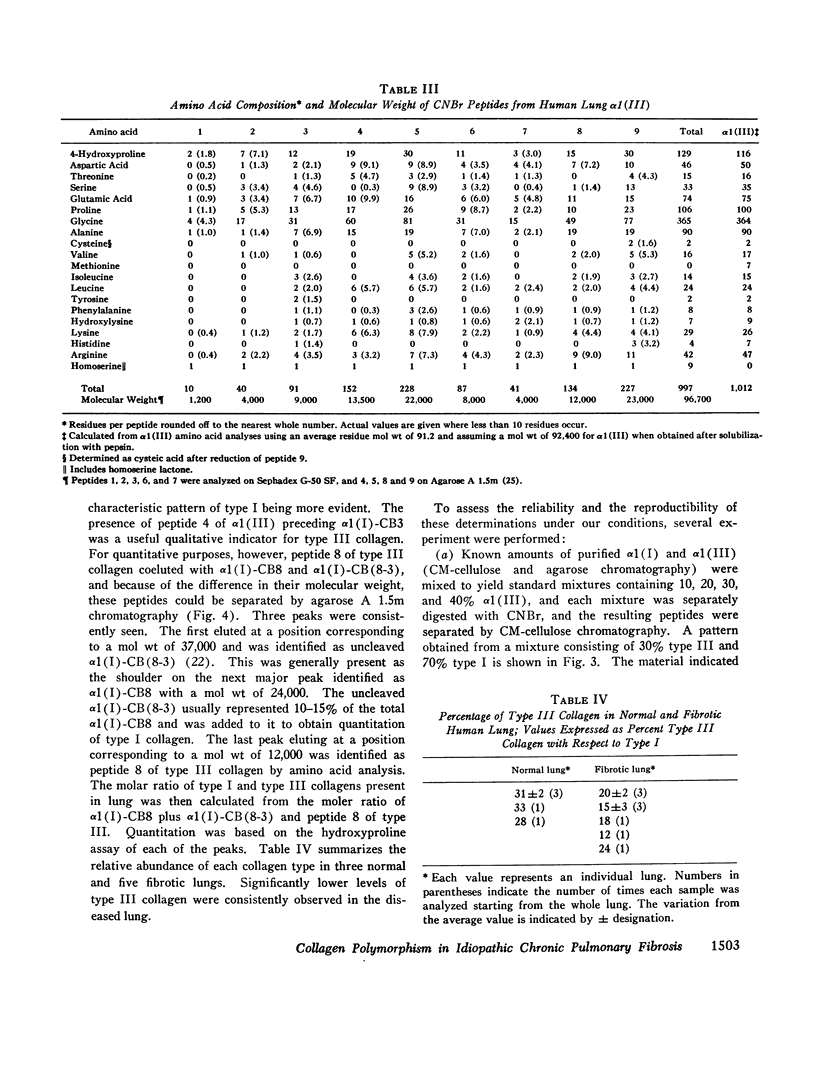
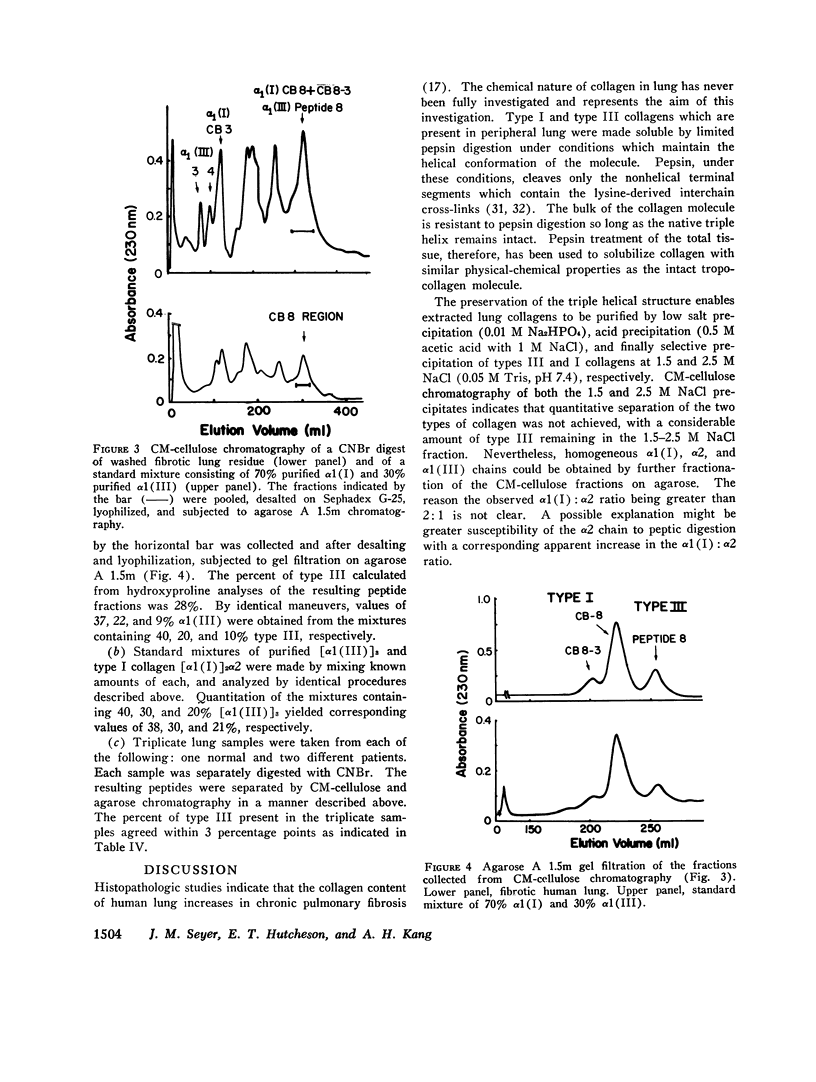
Collagens in normal human lung and in idiopathic chronic fibrosis were investigated in terms of their covalent structure and compared for possible alterations in the diseased state. Collagens were solubilized by limited digestion with pepsin under nondenaturing conditions, and after purification they, were fractionated into types I and III. Carboxymethylcellulose and agarose chromatography of both types I and III collagens, and amino acid and carbohydrate analyses of the resulting alpha-chains indicated that the alpha 1 (I), alpha 2, and alpha 1 (III) chains of normal human lung were identical with the human skin alpha-chains in all respects examined except that the normal lung chains contained higher levels of hydroxylysine. Examination of collagens obtained from the diseased lung revealed that the content of hydroxylysine of the alpha 1 (I) and the alpha 1 (III) chains appeared to be diminished as compared to the normal lung chains. The values, expressed as residues per 1,000 residues, are 7.1 and 8.3 for the alpha 1 (I) and the alpha 1 (III) chains, respectively, as compared to 10.0 and 11.1 for the alpha-chains from the normal tissue. The chromatographic properties and amino acid and carbohydrate composition of the alpha-chains from the diseased tissue were otherwise indistinguishable from those of normal lung. In addition, isolation and characterization of the CNBr peptides of alpha 1 (I), alpha 2 and alpha 1 (III) from the diseased lung revealed no significant differences from the CNBr peptides from other human tissues reported previously. Normal and diseased lungs were also digested with CNBr, and the resultant alpha 1 (I) and alpha 1 (III) peptides were separated chromatographically. The relative quantities of these peptides indicate that type III collagen constitutes 33% of the total collagen in normal human lung, with the remainder being type I, whereas in idiopathic chronic pulmonary fibrosis, the relative content of type III collagen is markedly diminished, ranging from 12 to 24% in different patients. These results indicate that an alteration in tissue collagen polymorphism as well as subtle variations in the collagen structure accompany the fibrotic process in the diseased state, and suggest that these alterations may have possible pathogenetic implications.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askenasi R. S., Kefalides N. A. Simple chromatographic method for determination of 14 C-labeled lysine, hydroxylysine, and hydroxylysine glycosides. Anal Biochem. 1972 May;47(1):67–72. doi: 10.1016/0003-2697(72)90279-5. [DOI] [PubMed] [Google Scholar]
- BORNSTEIN P., PIEZ K. A. A BIOCHEMICAL STUDY OF HUMAN SKIN COLLAGEN AND THE RELATION BETWEEN INTRA- AND INTERMOLECULAR CROSS-LINKING. J Clin Invest. 1964 Sep;43:1813–1823. doi: 10.1172/JCI105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORNSTEIN P., PIEZ K. A. COLLAGEN: STRUCTURAL STUDIES BASED ON THE CLEAVAGE OF METHIONYL BONDS. Science. 1965 Jun 4;148(3675):1353–1355. doi: 10.1126/science.148.3675.1353. [DOI] [PubMed] [Google Scholar]
- Bradley K. H., McConnell S. D., Crystal R. G. Lung collagen composition and synthesis. Characterization and changes with age. J Biol Chem. 1974 May 10;249(9):2674–2683. [PubMed] [Google Scholar]
- Bradley K., McConnell-Breul S., Crystal R. G. Collagen in the human lung. Quantitation of rates of synthesis and partial characterization of composition. J Clin Invest. 1975 Mar;55(3):543–550. doi: 10.1172/JCI107961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley K., McConnell-Breul S., Crystal R. G. Lung collagen heterogeneity. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2828–2832. doi: 10.1073/pnas.71.7.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. T., Piez K. A., Bornstein P. Isolation and characterization of the cyanogen bromide peptides from the alpha-1 chain of rat skin collagen. Biochemistry. 1967 Dec;6(12):3771–3780. doi: 10.1021/bi00864a022. [DOI] [PubMed] [Google Scholar]
- Chung E., Keele E. M., Miller E. J. Isolation and characterization of the cyanogen bromide peptides from the alpha 1(3) chain of human collagen. Biochemistry. 1974 Aug 13;13(17):3459–3464. doi: 10.1021/bi00714a006. [DOI] [PubMed] [Google Scholar]
- Cintron C. Hydroxylysine glycosides in the collagen of normal and scarred rabbit corneas. Biochem Biophys Res Commun. 1974 Sep 9;60(1):288–294. doi: 10.1016/0006-291x(74)90203-4. [DOI] [PubMed] [Google Scholar]
- Drake M. P., Davison P. F., Bump S., Schmitt F. O. Action of proteolytic enzymes on tropocollagen and insoluble collagen. Biochemistry. 1966 Jan;5(1):301–312. doi: 10.1021/bi00865a039. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Epstein E. H., Jr, Scott R. D., Miller E. J., Piez K. A. Isolation and characterization of the peptides derived from soluble human and baboon skin collagen after cyanogen bromide cleavage. J Biol Chem. 1971 Mar 25;246(6):1718–1724. [PubMed] [Google Scholar]
- Fietzek P. P., Rauterberg J. Cyanogen bromide peptides of type III collagen: first sequence analysis demonstrates homology with type I collagen. FEBS Lett. 1975 Jan 1;49(3):365–368. doi: 10.1016/0014-5793(75)80786-1. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick M., Hospelhorn V. D. Studies of human pulmonary connective tissue. II. Amino acid composition of residues following collagenase digestion of lung connective tissues. Am Rev Respir Dis. 1965 Nov;92(5):792–800. doi: 10.1164/arrd.1965.92.5.792. [DOI] [PubMed] [Google Scholar]
- Gallop P. M., Blumenfeld O. O., Seifter S. Structure and metabolism of connective 801 tissue proteins. Annu Rev Biochem. 1972;41:617–672. doi: 10.1146/annurev.bi.41.070172.003153. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Piez K. A., Gross J. Characterization of the alpha-chains of chick skin collagen and the nature of the NH2-terminal cross-link region. Biochemistry. 1969 Sep;8(9):3648–3655. doi: 10.1021/bi00837a023. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Piez K. A., Gross J. Characterization of the cyanogen bromide peptides from the alpha 1 chain of chick skin collagen. Biochemistry. 1969 Apr;8(4):1506–1514. doi: 10.1021/bi00832a029. [DOI] [PubMed] [Google Scholar]
- Kang A. H. Studies on the location of intermolecular cross-links in collagen. Isolation of a CNBr peptide containing -hydroxylysinonorleucine. Biochemistry. 1972 May 9;11(10):1828–1835. doi: 10.1021/bi00760a015. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Isolation and characterization of cyanogen bromide peptides from basement membrane collagen. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1151–1158. doi: 10.1016/0006-291x(72)90955-2. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A. Isolation of a collagen from basement membranes containing three identical - chains. Biochem Biophys Res Commun. 1971 Oct 1;45(1):226–234. doi: 10.1016/0006-291x(71)90073-8. [DOI] [PubMed] [Google Scholar]
- Low F. N. Extracellular components of the pulmonary alveolar wall. Arch Intern Med. 1971 May;127(5):847–852. [PubMed] [Google Scholar]
- Miller E. J., Lunde L. G. Isolation and characterization of the cyanogen bromide peptides from the alpha 1(II) chain of bovine and human cartilage collagen. Biochemistry. 1973 Aug 14;12(17):3153–3159. doi: 10.1021/bi00741a003. [DOI] [PubMed] [Google Scholar]
- Nold J. G., Kang A. H., Gross J. Collagen molecules: distribution of alpha chains. Science. 1970 Dec 4;170(3962):1096–1098. doi: 10.1126/science.170.3962.1096. [DOI] [PubMed] [Google Scholar]
- Piez K. A. Molecular weight determination of random coil polypeptides from collagen by molecular sieve chromatography. Anal Biochem. 1968 Nov;26(2):305–312. doi: 10.1016/0003-2697(68)90342-4. [DOI] [PubMed] [Google Scholar]
- Risteli J., Kivirikko K. I. Activities of prolyl hydroxylase, lysyl hydroxylase, collagen galactosyltransferase and collagen glucosyltransferase in the liver of rats with hepatic injury. Biochem J. 1974 Oct;144(1):115–122. doi: 10.1042/bj1440115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer H. Interstitial pneumonia. Annu Rev Med. 1967;18:423–442. doi: 10.1146/annurev.me.18.020167.002231. [DOI] [PubMed] [Google Scholar]
- Stanley N. N., Alper R., Cunningham E. L., Cherniack N. S., Kefalides N. A. Effects of a molecular change in collagen on lung structure and mechanical function. J Clin Invest. 1975 Jun;55(6):1195–1201. doi: 10.1172/JCI108037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B. P., Kang A. H., Trelstad R. L., Gross J. Collagen heterogeneity within different growth regions of long bones of rachitic and non-rachitic chicks. Biochem J. 1972 May;127(4):715–720. doi: 10.1042/bj1270715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelstad R. L. Human aorta collagens: evidence for three distinct species. Biochem Biophys Res Commun. 1974 Apr 8;57(3):717–725. doi: 10.1016/0006-291x(74)90605-6. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Kang A. H., Toole B. P., Gross J. Collagen heterogeneity. High resolution separation of native ( 1(I) 2 2 and ( 1(II) 3 and their component chains. J Biol Chem. 1972 Oct 25;247(20):6469–6473. [PubMed] [Google Scholar]


