Abstract
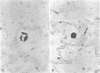
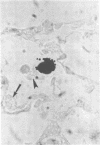
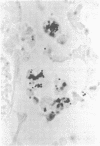
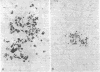
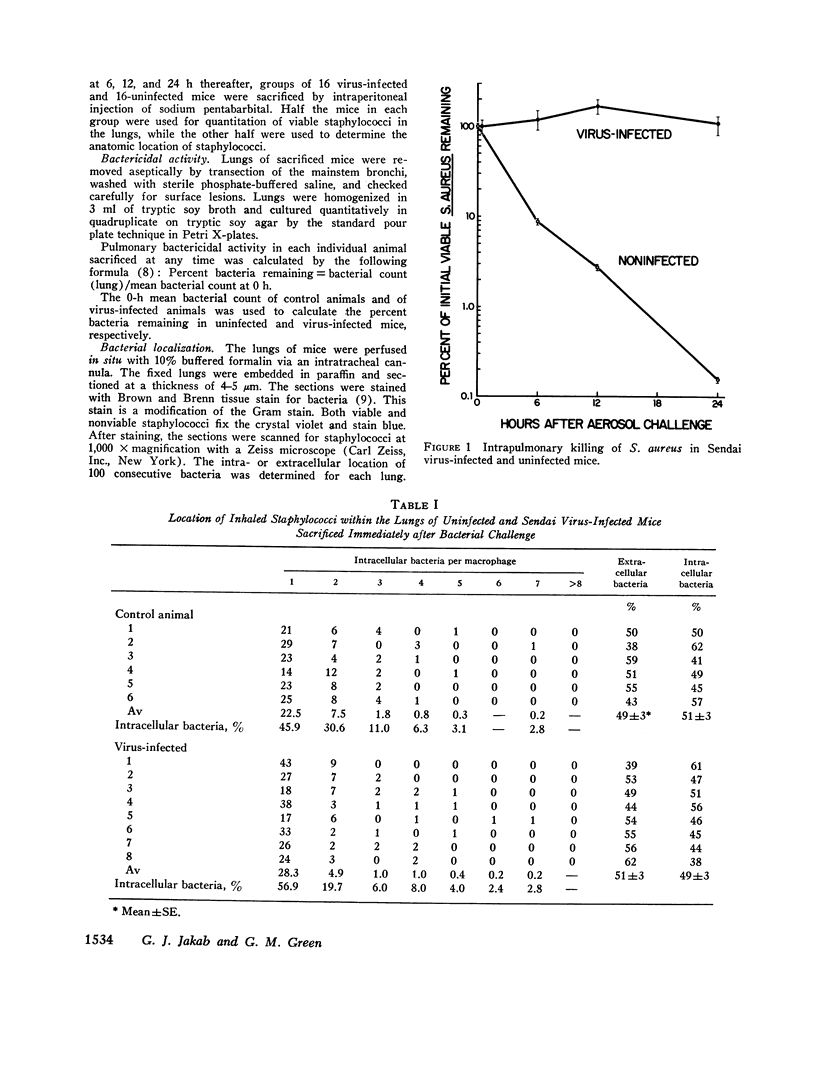
Bacterial multiplication associated with virus infections is related to defects in in situ bactericidal (phagocytic) mechanisms of the lung. To determine whether the phagocytic defect was in bacterial ingestion and/or intracellular digestion, mice were infected with a sublethal dose of aerosolized Sendai virus and challenged 7 days later with a finely dispersed aerosol of Staphylococcus aureus. Groups of uninfected and virus-infected mice were sacrificed at 0, 6, 12, and 24 h after challenge, the lungs were perfused with formalin in situ, and the intra- or extracellular location of the bacteria was determined histologically. At 0 h, 49% and 51% of the staphylococci had an intracellular location in virus and nonvirus-infected lungs, respectively. With time, decreasing numbers of staphylococci were observed within the phagocytic cells of nonvirus-infected lungs, mostly as single organisms or in small clusters of less than four. In contrast, in focal area of virus-infected lungs, increasing numbers of phagocytic cells showed clumps of more than 25 bacteria/cell. These data demonstrate that virus-infected suppression of pulmonary antibacterial activity against S. aureus is related primarily to defects in intracellular processing mechanisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHN Z. A. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963 Jan 1;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Pettis P., Beckerdite S., Franson R. Effects of phagocytosis by rabbit granulocytes on macromolecular synthesis and degradation in different species of bacteria. J Bacteriol. 1973 Aug;115(2):490–497. doi: 10.1128/jb.115.2.490-497.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILL F. A., COLE R. M. THE FATE OF A BACTERIAL ANTIGEN (STREPTOCOCCAL M PROTEIN) AFTER PHAGOCYTOSIS BY MACROPHAGES. J Immunol. 1965 Jun;94:898–915. [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERS J. F., MUDLER J., MASUREL N., vd KUIP L., TYRRELL D. A. Studies on the pathogenesis of influenza virus pneumonia in mice. J Pathol Bacteriol. 1962 Jan;83:207–217. [PubMed] [Google Scholar]
- Jakab G. J., Dick E. C. Synergistic effect in viral-bacterial infection: combined infection of the murine respiratory tract with Sendai virus and Pasteurella pneumotropica. Infect Immun. 1973 Nov;8(5):762–768. doi: 10.1128/iai.8.5.762-768.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. The effect of Sendai virus infection on bactericidal and transport mechanisms of the murine lung. J Clin Invest. 1972 Aug;51(8):1989–1998. doi: 10.1172/JCI107005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosli C. G. Synergism between respiratory viruses and bacteria. Yale J Biol Med. 1968 Apr-Jun;40(5-6):522–540. [PMC free article] [PubMed] [Google Scholar]
- Robinson T. W., Cureton R. J., Heath R. B. The pathogenesis of Sendai virus infection in the mouse lung. J Med Microbiol. 1968 Aug;1(1):89–95. doi: 10.1099/00222615-1-1-89. [DOI] [PubMed] [Google Scholar]
- Ruppert D., Jakab G. J., Sylwester D. L., Green G. M. Sources of variance in the measurement of intrapulmonary killing of bacteria. J Lab Clin Med. 1976 Mar;87(3):544–558. [PubMed] [Google Scholar]
- Sawyer W. D. Interaction of influenza virus with leukocytes and its effect on phagocytosis. J Infect Dis. 1969 Jun;119(6):541–556. doi: 10.1093/infdis/119.6.541. [DOI] [PubMed] [Google Scholar]
- Taylor R. N., Dietz T. M., Maxwell K. W., Marcus S. Effect of influenza virus infection on phagocytic and cytopeptic capacities of guinea pig macrophages. Immunol Commun. 1974;3(5):439–455. doi: 10.3109/08820137409061124. [DOI] [PubMed] [Google Scholar]