Abstract
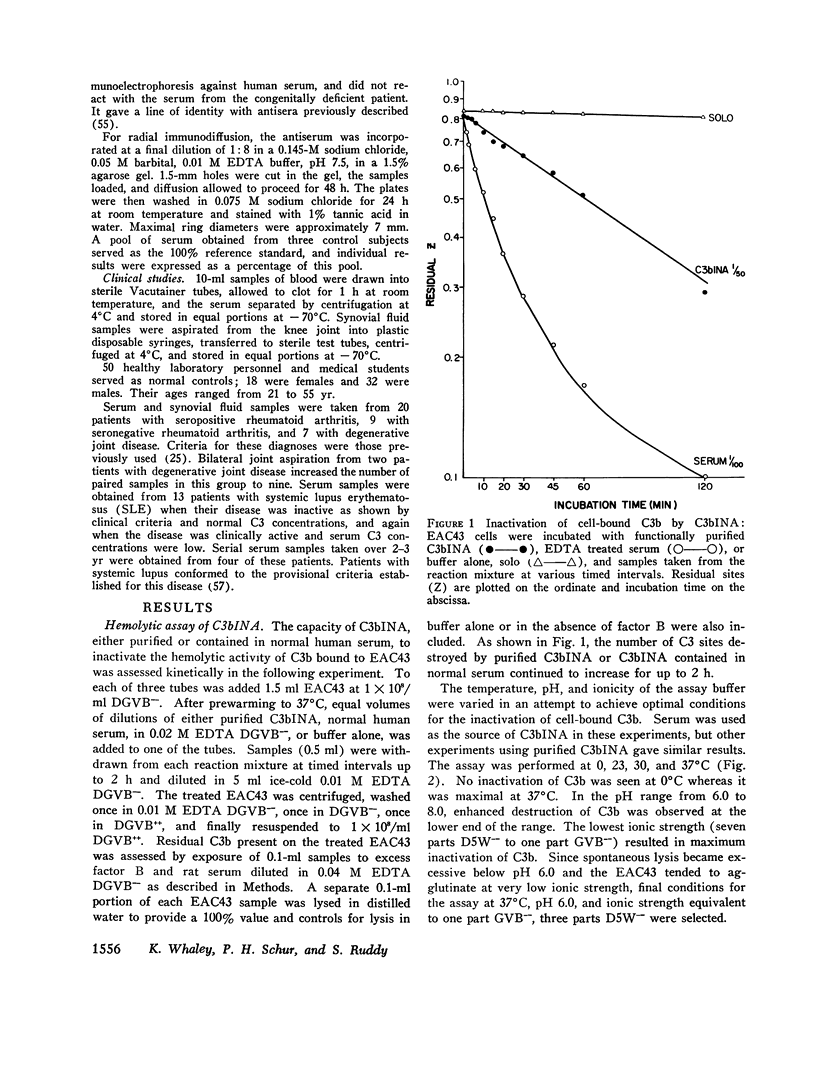
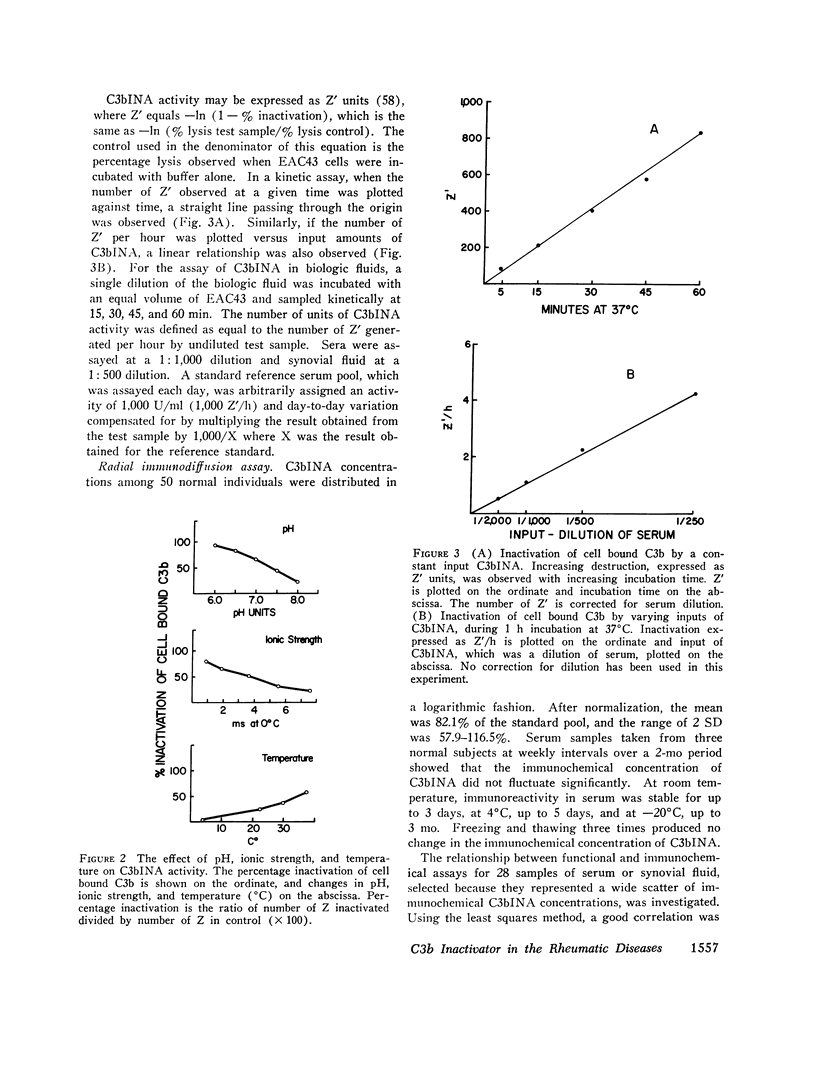
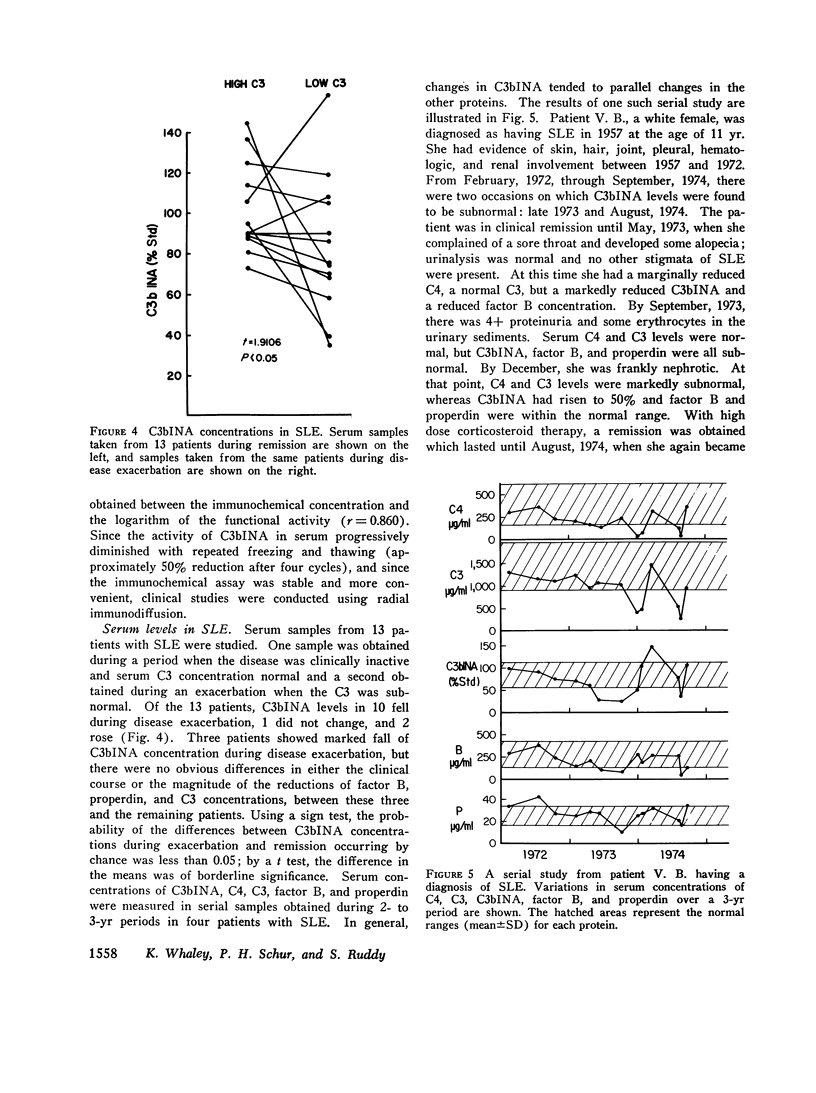
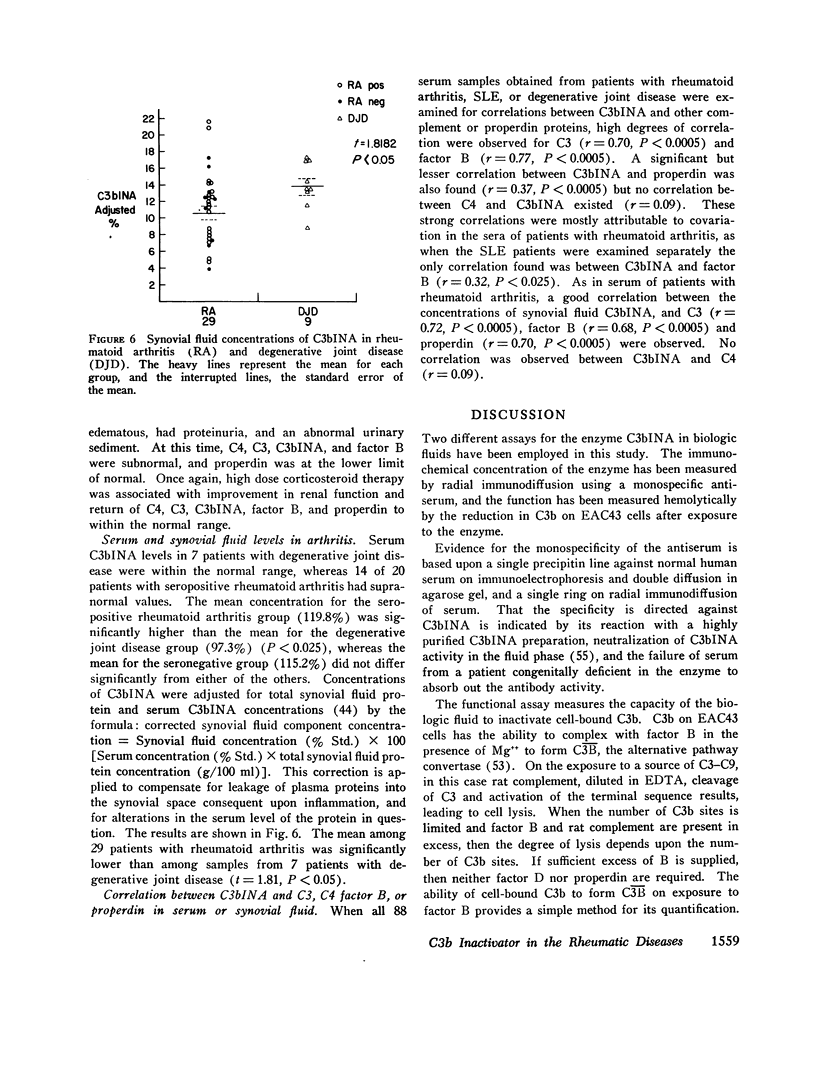
C3b inactivator (C3bINA) has been measured in biologic fluids by radial immunodiffusion using a monospecific antiserum prepared in rabbits, and by a hemolytic assay which measures the reduction in the capacity of EAC43 cells bearing limited C3b sites to form C3B, the alternative pathway C3 convertase. The radial immunodiffusion and hemolytic assays show a good correlation (r = 0.86 P less than 0.001). Measurement of C3bINA concentrations in the sera of patients with systemic lupus erythematosus showed that during exacerbations of disease activity C3bINA concentrations tended to be lower, usually in association with reductions in C4, C3, factor B, and properdin, and sometimes with reductions of the alternative pathway proteins, factor B, and properdin alone. Supranormal values for C3bINA were found in the sera of 14 of 20 patients with seropositive rheumatoid arthritis and 3 of 9 seronegative patients, but none of 7 patients with degenerative joint disease. Synovial fluid concentrations of C3bINA, after correction for total synovial fluid protein and serum concentration of the enzyme, were significantly reduced in patients with rheumatoid arthritis compared to patients with degenerative joint disease (P less than 0.05). In both serum and synovial fluid from patients with rheumatoid arthritis, there was a good correlation between the concentrations of C3bINA and those of C3, factor B, and properdin, but not that of C4, suggesting that levels of C3bINA may serve to modulate recruitment of the properdin amplification loop in this disease.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson N., Alper C. A., Lachmann P. J., Rosen F. S., Jandl J. H. Deficiency of C3 inactivator in man. J Immunol. 1971 Jul;107(1):19–27. [PubMed] [Google Scholar]
- Alper C. A., Rosen F. S. Alper CA, Rosen FS: Studies of the in vivo behavior of human C'3 in normal subjects and patients. J Clin Invest. 1967 Dec;46(12):2021–2034. doi: 10.1172/JCI105691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton M. C., Schur P. H. The complement system in rheumatoid synovitis. II. Intracytoplasmic inclusions of immunoglobulins and complement. Arthritis Rheum. 1971 Jan-Feb;14(1):87–95. doi: 10.1002/art.1780140111. [DOI] [PubMed] [Google Scholar]
- Charlesworth J. A., Williams D. G., Sherington E., Lachmann P. J., Peters D. K. Metabolic studies of the third component of complement and the glycine-rich beta glycoprotein in patients with hypocomplementemia. J Clin Invest. 1974 Jun;53(6):1578–1587. doi: 10.1172/JCI107708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bracco M. M., Manni J. A. Serum levels of C1q, C1r and C1s in normal and pathologic sera. Arthritis Rheum. 1974 Mar-Apr;17(2):121–128. doi: 10.1002/art.1780170204. [DOI] [PubMed] [Google Scholar]
- FOSTIROPOULOS G., AUSTEN K. F., BLOCH K. J. TOTAL HEMOLYTIC COMPLEMENT (CH50) AND SECOND COMPONENT OF COMPLEMENT (C2 HU) ACTIVITY IN SERUM AND SYNOVIAL FLUID. Arthritis Rheum. 1965 Apr;8:219–232. doi: 10.1002/art.1780080206. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Properdin: binding to C3b and stabilization of the C3b-dependent C3 convertase. J Exp Med. 1975 Oct 1;142(4):856–863. doi: 10.1084/jem.142.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F., Ruddy S. Formation of a hemolytically active cellular intermediate by the interaction between properdin factors B and D and the activated third component of complement. J Exp Med. 1973 Dec 1;138(6):1305–1313. doi: 10.1084/jem.138.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewurz H., Pickering R. J., Mergenhagen S. E., Good R. A. The complement profile in acute glomerulonephritis systemic lupus erythematosus and hypocomplementemic chronic glomerulonephritis. Contrasts and experimental correlations. Int Arch Allergy Appl Immunol. 1968;34(6):556–570. doi: 10.1159/000230149. [DOI] [PubMed] [Google Scholar]
- Gewurz H., Pickering R. J., Naff G., Snyderman R., Mergenhagen S. E., Good R. A. Decreased properdin activity in acute glomerulonephritis. Int Arch Allergy Appl Immunol. 1969;36(6):592–598. doi: 10.1159/000230780. [DOI] [PubMed] [Google Scholar]
- Gigli I., Nelson R. A., Jr Complement dependent immune phagocytosis. I. Requirements for C'1, C'4, C'2, C'3. Exp Cell Res. 1968 Jul;51(1):45–67. doi: 10.1016/0014-4827(68)90158-4. [DOI] [PubMed] [Google Scholar]
- Gigli I., Ruddy S., Austen K. F. The stoichiometric measurement of the serum inhibition of the first component of complement by the inhibition of immune hemolysis. J Immunol. 1968 Jun;100(6):1154–1164. [PubMed] [Google Scholar]
- Goetzl E. J., Metzger H. Affinity labeling of a mouse myeloma protein which binds nitrophenyl ligands. Kinetics of labeling and isolation of a labeled peptide. Biochemistry. 1970 Mar 3;9(5):1267–1278. doi: 10.1021/bi00807a031. [DOI] [PubMed] [Google Scholar]
- Hedberg H. Studies on synovial fluid in arthritis. I. The total complement activity. II. The occurrence of mononuclear cells with in vitro cytotoxic effect. Acta Med Scand Suppl. 1967;479:1–137. [PubMed] [Google Scholar]
- Hunsicker L. G., Ruddy S., Austen K. F. Alternate complement pathway: factors involved in cobra venom factor (CoVF) activation of the third component of complement (C3). J Immunol. 1973 Jan;110(1):128–138. [PubMed] [Google Scholar]
- Hunsicker L. G., Ruddy S., Carpenter C. B., Schur P. H., Merrill J. P., Müller-Eberhard H. J., Austen K. F. Metabolism of third complement component (C3) in nephritis. Involvement of the classic and alternate (properdin) pathways for complement activation. N Engl J Med. 1972 Oct 26;287(17):835–840. doi: 10.1056/NEJM197210262871701. [DOI] [PubMed] [Google Scholar]
- Hurd E. R., Kinsella D., Ziff M. Immunohistologic studies of synoviocytes and synovial exudate cells. J Exp Med. 1971 Sep 1;134(3 Pt 2):296s–205s. [PubMed] [Google Scholar]
- Kinsella T. D., Baum J., Ziff M. Immunofluorescent demonstration of an IgG-B1C complex in synovial lining cells of rheumatoid synovial membrane. Clin Exp Immunol. 1969 Feb;4(2):265–271. [PMC free article] [PubMed] [Google Scholar]
- Koffler D., Agnello V., Carr R. I., Kunkel H. G. Variable patterns of immunoglobulin and complement deposition in the kidneys of patients with systemic lupus erythematosus. Am J Pathol. 1969 Sep;56(3):305–316. [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Liske R. The preparation and properties of alexinated intermediates that react with conglutinin. I. Guinea-pig complement. Immunology. 1966 Sep;11(3):243–254. [PMC free article] [PubMed] [Google Scholar]
- Lachmann P. J., Müller-Eberhard H. J. The demonstration in human serum of "conglutinogen-activating factor" and its effect on the third component of complement. J Immunol. 1968 Apr;100(4):691–698. [PubMed] [Google Scholar]
- McLean R. H., Michael A. F. Properdin anc C3 proactivator: alternate pathway components in human glomerulonephritis. J Clin Invest. 1973 Mar;52(3):634–644. doi: 10.1172/JCI107225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Götze O. C3 proactivator convertase and its mode of action. J Exp Med. 1972 Apr 1;135(4):1003–1008. doi: 10.1084/jem.135.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Polley M. J., Calcott M. A. Formation and functional significance of a molecular complex derived from the second and the fourth component of human complement. J Exp Med. 1967 Feb 1;125(2):359–380. doi: 10.1084/jem.125.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON R. A., Jr The immune-adherence phenomenon; an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science. 1953 Dec 18;118(3077):733–737. doi: 10.1126/science.118.3077.733. [DOI] [PubMed] [Google Scholar]
- NILSSON U. R., MUELLER-EBERHARD H. J. ISOLATION OF BETA IF-GLOBULIN FROM HUMAN SERUM AND ITS CHARACTERIZATION AS THE FIFTH COMPONENT OF COMPLEMENT. J Exp Med. 1965 Aug 1;122:277–298. doi: 10.1084/jem.122.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Nicol P. A., Lachmann P. J. The alternate pathway of complement activation. The role of C3 and its inactivator (KAF). Immunology. 1973 Feb;24(2):259–275. [PMC free article] [PubMed] [Google Scholar]
- PEKIN T. J., Jr, ZVAIFLER N. J. HEMOLYTIC COMPLEMENT IN SYNOVIAL FLUID. J Clin Invest. 1964 Jul;43:1372–1382. doi: 10.1172/JCI105013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin L. H., Lambert P. H., Miescher P. A. Complement breakdown products in plasma from patients with systemic lupus erythematosus and patients with membranoproliferative or other glomerulonephritis. J Clin Invest. 1975 Jul;56(1):165–176. doi: 10.1172/JCI108065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Atkinson J. P., Newball H. H., Frank M. M. Receptors for immunoglobulin and complement on human alveolar macrophages. J Immunol. 1975 Jun;114(6):1813–1819. [PubMed] [Google Scholar]
- Ross G. D., Polley M. J., Rabellino E. M., Grey H. M. Two different complement receptors on human lymphocytes. One specific for C3b and one specific for C3b inactivator-cleaved C3b. J Exp Med. 1973 Oct 1;138(4):798–811. doi: 10.1084/jem.138.4.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Polley M. J. Specificity of human lymphocyte complement receptors. J Exp Med. 1975 May 1;141(5):1163–1180. doi: 10.1084/jem.141.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. A stoichiometric assay for the fourth component of complement in whole human serum using EAC'la-gp and functionally pure human second component. J Immunol. 1967 Dec;99(6):1162–1172. [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. Activation of the complement system in rheumatoid synovitis. Fed Proc. 1973 Feb;32(2):134–137. [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. C3 inactivator of man. I. Hemolytic measurement by the inactivation of cell-bound C3. J Immunol. 1969 Mar;102(3):533–543. [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. C3b inactivator of man. II. Fragments produced by C3b inactivator cleavage of cell-bound or fluid phase C3b. J Immunol. 1971 Sep;107(3):742–750. [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. The complement system in rheumatoid synovitis. I. An analysis of complement component activities in rheumatoid synovial fluids. Arthritis Rheum. 1970 Nov-Dec;13(6):713–723. doi: 10.1002/art.1780130601. [DOI] [PubMed] [Google Scholar]
- Ruddy S., Britton M. C., Schur P. H., Austen K. F. Complement components in synovial fluid: activation and fixation in seropositive rheumatoid arthritis. Ann N Y Acad Sci. 1969 Dec 10;168(1):161–172. doi: 10.1111/j.1749-6632.1969.tb43105.x. [DOI] [PubMed] [Google Scholar]
- Ruddy S., Colten H. R. Rheumatoid arthritis. Biosynthesis of complement proteins by synovial tissues. N Engl J Med. 1974 Jun 6;290(23):1284–1288. doi: 10.1056/NEJM197406062902304. [DOI] [PubMed] [Google Scholar]
- Ruddy S., Everson L. K., Schur P. H., Austen K. F. Hemolytic assay of the ninth complement complement component: elevation and depletion in rheumatic diseases. J Exp Med. 1971 Sep 1;134(3 Pt 2):259s–275s. [PubMed] [Google Scholar]
- Ruddy S., Fearon D. T., Austen K. F. Depressed synovial fluid levels of properdin and properdin factor B in patients with rheumatoid arthritis. Arthritis Rheum. 1975 Jul-Aug;18(4):289–295. doi: 10.1002/art.1780180401. [DOI] [PubMed] [Google Scholar]
- Ruddy S., Hunsicker L. G., Austen K. F. C3b inactivator of man. 3. Further purification and production of antibody to C3b INA. J Immunol. 1972 Mar;108(3):657–664. [PubMed] [Google Scholar]
- Schur P. H., Sandson J. Immunologic factors and clinical activity in systemic lupus erythematosus. N Engl J Med. 1968 Mar 7;278(10):533–538. doi: 10.1056/NEJM196803072781004. [DOI] [PubMed] [Google Scholar]
- Schutte M., DiCamelli R., Murphy P., Sadove M., Gewurz H. C3 proactivator (C3PA) as an acute phase reactant. Clin Exp Immunol. 1974 Oct;18(2):251–256. [PMC free article] [PubMed] [Google Scholar]
- Shin H. S., Pickering R. J., Mayer M. M. The fifth component of the guinea pig complement system. II. Mechanism of SAC1,4,2,3,5b formation and C5 consumption by EAC1,4,2,3. J Immunol. 1971 Feb;106(2):473–479. [PubMed] [Google Scholar]
- Sliwinski A. J., Zvaifler N. J. Decreased synthesis of the third component of complement (C3) in hypocomplementemic systemic lupus erythematosus. Clin Exp Immunol. 1972 May;11(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Tamura N., Nelson R. A., Jr Three naturally-occurring inhibitors of components of complement in guinea pig and rabbit serum. J Immunol. 1967 Sep;99(3):582–589. [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J., Bokisch V. A. Binding of soluble immune complexes to human lymphoblastoid cells. I. Characterization of receptors for IgG Fc and complement and description of the binding mechanism. J Exp Med. 1974 Oct 1;140(4):877–894. doi: 10.1084/jem.140.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verroust P. J., Wilson C. B., Cooper N. R., Edgington T. S., Dixon F. J. Glomerular complement components in human glomerulonephritis. J Clin Invest. 1974 Jan;53(1):77–84. doi: 10.1172/JCI107562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Complement-derived leukotactic factors in inflammatory synovial fluids of humans. J Clin Invest. 1971 Mar;50(3):606–616. doi: 10.1172/JCI106531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein A., Peters K., Brown D., Bluestone R. Metabolism of the third component of complement (C3) in patients with rheumatoid arthritis. Arthritis Rheum. 1972 Jan-Feb;15(1):49–56. doi: 10.1002/art.1780150108. [DOI] [PubMed] [Google Scholar]
- Wellek B., Hahn H. H., Opferkuch W. Evidence for macrophage C3d-receptor active in phagocytosis. J Immunol. 1975 May;114(5):1643–1645. [PubMed] [Google Scholar]
- Westberg N. G., Naff G. B., Boyer J. T., Michael A. F. Glomerular deposition of properdin in acute and chronic glomerulonephritis with hypocomplementemia. J Clin Invest. 1971 Mar;50(3):642–649. doi: 10.1172/JCI106534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. G., Peters D. K., Fallows J., Petrie A., Kourilsky O., Morel-Maroger L., Cameron J. S. Studies of serum complement in the hypocomplementaemic nephritides. Clin Exp Immunol. 1974 Nov;18(3):391–405. [PMC free article] [PubMed] [Google Scholar]
- Ziegler J. B., Rosen F. S., Alper C. A., Grupe W., Lepow I. H. Metabolism of properdin in normal subjects and patients with renal disease. J Clin Invest. 1975 Sep;56(3):761–767. doi: 10.1172/JCI108147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvaifler N. J. Breakdown products of C 3 in human synovial fluids. J Clin Invest. 1969 Aug;48(8):1532–1542. doi: 10.1172/JCI106119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvaifler N. J. Rheumatoid synovitis. An extravascular immune complex disease. Arthritis Rheum. 1974 May-Jun;17(3):297–305. doi: 10.1002/art.1780170315. [DOI] [PubMed] [Google Scholar]


