Abstract
T helper type 2 (Th2) cells, which produce interleukin-4 (IL-4), IL-5 and IL-13, control immunity to all forms of allergic inflammatory responses. Interleukin-21 (IL-21) reduces allergic symptoms in murine models and inhibits IL-4-induced IgE secretion by B cells. However, whether or not IL-21 directly affects Th2 cells, which leads to reduced allergic symptoms, is unclear. In this study, we investigated the effects of IL-21 on the differentiation and effector functions of Th2 cells. We found that IL-21 reduced the number of differentiated Th2 cells and these Th2 cells showed a diminished Th2 cytokine production. Interleukin-21 suppressed Th2 cytokine production of already polarized Th2 cells by down-regulation of transcription factor GATA-3. It also induced apoptosis of Th2 cells with decreased anti-apoptotic factor Bcl-2. Intranasal administration of IL-21 at the beginning of ovalbumin (OVA) sensitization or before OVA challenge decreased Th2 cytokines in the bronchoalveolar lavage fluid of OVA/alum-immunized allergic mice. In addition, the inhibitory effects of IL-21 on Th2 effector functions can also be found in allergic patients. Our results demonstrate that IL-21 suppresses the development of Th2 cells and functions of polarized Th2 cells. Hence, the administration of IL-21 may be considered for use as a preventive and therapeutic approach when dealing with Th2-mediated allergic diseases.
Keywords: apoptosis, cytokines, GATA3, IL-21, Th2
Introduction
CD4+ T cells are crucially involved in adaptive immune responses. T helper type 2 (Th2) cells are responsible for host immunity to extracellular parasites and the development of allergic asthma and other allergic inflammatory diseases.1 Th2 cells produce interleukin-4 (IL-4), IL-5 and IL-13, and have been shown to regulate B-cell class switching to IgE, recruit eosinophils leading to tissue eosinophilia, and induce mucus production, goblet cell metaplasia and airway hyper-responsiveness.1 At the molecular level, the expression of IL-4, IL-5 and IL-13 is controlled by the transcription factor GATA-3, a master regulator for Th2 cell differentiation.2–4
Interleukin-21 (IL-21) is produced by activated CD4+ T cells, Th2 cells, Th17 cells, follicular helper T cells and natural killer T cells.5–10 Interleukin-21 receptor (IL-21R) is composed of two subunits – a common γ-chain subunit, which is shared with IL-2, IL-4, IL-7, IL-9 and IL-15 receptors, and a unique receptor.11,12 The distribution of IL-21R on various immune cells indicates a role for IL-21 signalling across both the innate and the adaptive immune systems.8,11 IL-21 augments the activation, proliferation and differentiation of CD4+ and CD8+ T cells and regulates the survival and the profile of cytokines secreted by these cells,13–15 drives the differentiation of B cells into memory cells and terminally differentiated plasma cells,16 enhances cytotoxic activity of CD8+ T cells and natural killer cells,6,17 and contributes to the differentiation of follicular helper T cells and Th17 cells.5 Conversely, IL-21 exerts negative effects on other lymphoid cells, including B-cell apoptosis,18 inhibiting dendritic cell maturation and function,19 limiting the differentiation of inducible regulatory T cells, and suppressing Th1 cell differentiation.10,20,21 In particular, IL-21 also mediates suppressive effects by inducing IL-10.22
Interleukin-21 inhibits IL-4-induced IgE secretion by B cells.23–25 The increased levels of serum IgE in IL-21 or IL-21R-deficient mice most probably results from the ability of IL-21 to directly inhibit isotype switching to IgE by their B cells.23,24 Furthermore, in mouse models of allergic rhinitis and immediate hypersensitivity reaction in skin, administering IL-21 significantly reduces allergic symptoms by diminishing antigen-specific serum IgE or suppressing mast cell activation.26,27 In addition, systemic administration of IL-21 blocks antigen-induced anaphylaxis in a mouse model.28 In our previous study, we found that serum levels of IL-21 are decreased in patients with atopic dermatitis, which is a Th2-mediated allergic disease with elevated serum level of IgE, when compared with healthy controls.29 These results showed that IL-21 suppressed Th2-mediated allergic diseases. However, whether IL-21 directly affects Th2 cells, which could lead to a reduction in allergic symptoms, is unclear.
In this study, we investigated the effects of IL-21 on the differentiation of naive Th cells to Th2 cells and IL-21 on the regulation of Th2 effector functions in vitro and in vivo using ovalbumin (OVA) -specific Th2 models. We also examined the effects of IL-21 on T cells of patients with allergic rhinitis. Our results demonstrated that IL-21 could suppress the development and functions of Th2 cells.
Materials and methods
Mice
Female BALB/c mice aged 6–8 weeks were obtained from the National Laboratory Animal Centre, Taipei, Taiwan. DO11.10 transgenic mice in a BALB/c background carrying the MHC class II restricted rearranged T-cell receptor reacted to OVA antigen were purchased from The Jackson laboratory (Bar Harbor, ME). All mice were maintained in the Animal Centre of the College of Medicine, National Taiwan University. All experiments were performed following approval of The Institutional Animal Care and Use Committee (IACUC) of National Taiwan University College of Medicine and College of Public Health.
Th2 cell preparation and culture and IL-21 treatment
CD4+ T cells were isolated from the spleen of DO11.10 mice by positive selection with a microbead-conjugated monoclonal antibody to CD4 (clone GK1.5) (BD Biosciences, San Diego, CA). γ-Irradiated splenocytes from BALB/c mice were used as antigen-presenting cells. CD4+ T cells (5 × 105 cells/well) and antigen-presenting cells (5 × 106 cells/well) were placed in 48-well plates in 1 ml of complete RPMI medium supplemented with OVA peptide, recombinant mouse IL-4 (1000 U/ml) (BD Biosciences), recombinant mouse IL-2 (10 U/ml) (Peprotech Inc., Rocky Hill, NJ), and anti-mouse interferon-γ (IFN-γ; 10 μg/ml; Biolegend, San Diego, CA). After 2 weeks, cells were washed, counted, and re-stimulated under the same conditions used for the initial stimulation. According to the experimental design, pre-tested recombinant mouse IL-21 (100 ng/ml; PeproTech) or IL-21R:Fc (10 000 ng/ml; R&D Systems, Minneapolis, MN) was added before or after Th2 cell culture protocol.
Human peripheral blood mononuclear cell isolation and culture conditions
Heparinized peripheral blood samples from healthy individuals with no history of atopy and patients with mild allergic rhinitis were studied. Informed consents were obtained, and the study was approved by the Research Ethics Committee for The National Taiwan University Hospital. Peripheral blood mononuclear cells (PBMCs) (1 × 106 cells/ml) or CD4+ T cells were stimulated with 1 μg/ml anti-human CD3 and anti-human CD28 antibodies (Biolegend) and 10 U/ml IL-2 with or without pre-tested recombinant human IL-21 (100 ng/ml; Peprotech). Supernatant cytokines, transcription factors and apoptosis were assayed.
Intracellular cytokines staining
The harvested Th2 cells were labelled with FITC-conjugated anti-DO11.10 TCR antibody and Peridinin chlorophyll protein/Cy5.5-conjugated anti-mouse CD4 antibody (Biolegend) in 0·2% BSA in PBS and then fixed and permeabilized by fixation/permeabilization solution (BD Biosciences). Finally, they were stained with phycoerythrin-conjugated anti-mouse IL-4 antibody and allophycocyanin-conjugated anti-mouse IFN-γ antibody (BD Biosciences). Stained cells were analysed using a FACSCalibur (BD Biosciences) and the data obtained were analysed using flowjo software (Tree Star, Inc., Ashland, OR).
Apoptosis assay
The harvested CD4+ T cells were centrifuged and resuspended in 100 μl of 1× binding buffer [10 mm HEPES (pH 7·4), 140 mm NaCl, and 2·5 mm CaCl2] and stained with phycoerythrin-conjugated annexin V and 7-aminoactinomycin D (7-AAD; eBioscience, San Diego, CA) for 15 min at room temperature in the dark. Cells were diluted in 1× binding buffer and analysed on a FACSCalibur (BD Biosciences).
RNA isolation and quantitative RT-PCR
To assess the expression of T-bet, GATA-3 and RORγt, total RNA was isolated using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA) in accordance with the user's manual. For RT-PCR, total RNA was reverse transcribed into cDNA by oligonucleotide priming using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA). PCR was performed in triplicate on a 7500 Fast Real-Time PCR System (Applied Biosystems). SYBR Green DNA-binding dye was used in the amplification reactions. Fluorescence signals were analysed during each of 40 cycles (denaturation 15 seconds at 95°, annealing 30 seconds e at 60°, and extension 30 seconds at 72°). T-bet, GATA-3, and RORγt mRNA expression values were relative to individual internal controls, β-actin for mouse and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for human. Primer sequences used in PCR are in Table1.
Table 1.
Primer equences for quantitative PCR
| Gene name | Sequence | |
|---|---|---|
| Mouse T-bet | Forward | 5′- TCAACCAGCACCAGACAGAG -3′ |
| Reverse | 5′- AAACATCCTGTAATGGCTTGTG -3′ | |
| Mouse GATA-3 | Forward | 5′- TTATCAAGCCCAAGCGAAG -3′ |
| Reverse | 5′- TGGTGGTGGTCTGACAGTTC -3′ | |
| Mouse RORγt | Forward | 5′- GGAGCTCTGCCAGAATGAGC -3′ |
| Reverse | 5′- CAAGGCTCGAAACAGCTCCAC -3′ | |
| Mouse Bcl-2 | Forward | 5′- TGAGTACCTGAACCGGCATCT -3′ |
| Reverse | 5′- GGTCTTCAGAGACAGCCAGGA -3′ | |
| Mouse β-actin | Forward | 5′- TTCTTTGCAGCTCCTTCGTTGCCG -3′ |
| Reverse | 5′- TGGATGGCTACGTACATGGCTGGG -3′ | |
| Human T-bet | Forward | 5′- CCACCAGCCACTACAGGATG -3′ |
| Reverse | 5′- GGACGCCCCCTTGTTGTTT -3′ | |
| Human GATA-3 | Forward | 5′- TTAACATCGACGGTCAAGGC -3′ |
| Reverse | 5′- GGTAGGGATCCATGAAGCAG -3′ | |
| Human RORγt | Forward | 5′- TTAACATCGACGGTCAAGGC -3′ |
| Reverse | 5′- AGTTCTGCTGACGGGTGC -3′ | |
| Human GAPDH | Forward | 5′- TCATTTCCTGGTATGACAACG -3′ |
| Reverse | 5′- TTACTCCTTGGAGGCCATGT -3′ | |
Recombinant adenovirus constructs
Recombinant adenovirus was constructed using the Ad-Easy system with human phosphoglycerate kinase gene promoter (hPGK).30 Viruses were purified from infected cells 42–48 hr after infection by three freeze–thaw cycles followed by purification on Adeno-X™ Maxi Purification Kit (Clontech, San Diego, CA). Viral titres were measured by standard endpoint dilution assay using Ad293 cells. Ad-mock was identical, but contained no transgene in the expression cassette.
Th2-derived allergic model and IL-21 administration
BALB/c mice were immunized intaperitoneally with a total volume of 200 μl containing 50 μg OVA (grade V; Sigma) emulsified in 2 mg aluminium hydroxide (AlumImmject; Pierce Chemical, Rockford, IL) on day 0. On days 7, 14 and 21, mice were boosted with 25 μg OVA in 2 mg aluminium hydroxide. The mice were intranasally challenged with 100 μg OVA on day 29 or 31 (Figs4a and 5a). Two days after challenge, the mice were bled and then killed. Ad-IL-21 (1 × 108 plaque-forming units) were given before first sensitization (day 0) or before challenge (day 28).
Figure 4.
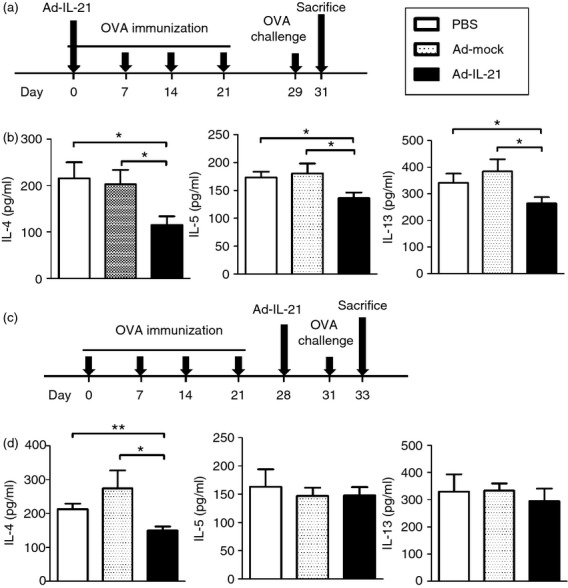
Interleukin-21 (IL-21) administration decreased bronchoalveolar lavage fluid (BALF) cytokines in ovalbumin (OVA)/alum immunized mice. (a) Experimental protocol. Mice were intranasally injected with Ad-IL-21 (1 × 108 plaque-forming units) and then immunized with OVA/alum on day 0. On days 7, 14 and 21, mice were boosted with OVA/alum. Mice were challenged with OVA on day 29 and killed on day 31. (b) BALF cytokines IL-4, IL-5 and IL-13 were detected by ELISA. (c) Experimental protocol. Mice were immunized with OVA on days 0, 7, 14 and 21, intranasally injected with Ad-IL-21 on day 28 and then challenged with OVA on day 31. Mice were killed on day 33. (d) BALF cytokines IL-4, IL-5 and IL-13 were detected by ELISA. Ad-IL-21 was IL-21-expressing adenovirus. Ad-mock was identical, but contained no transgene in the expression cassette. n = 7–10 mice per group. *P < 0·05; **P < 0·01.
Figure 5.
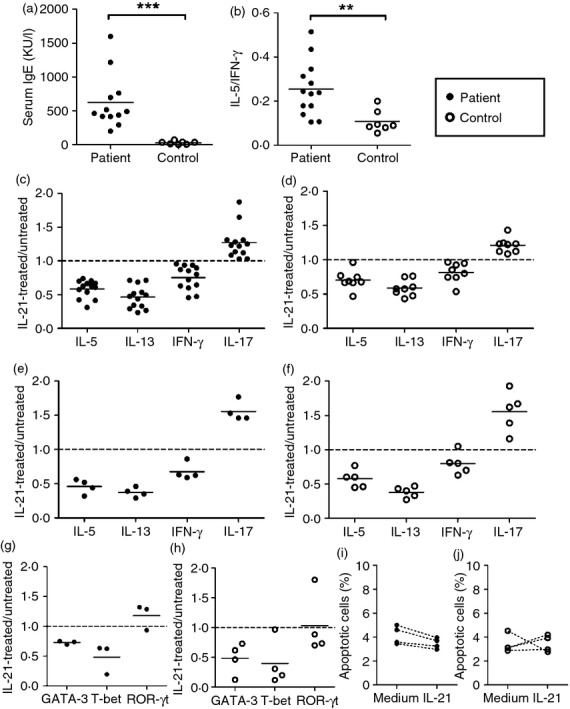
Decreased T helper type 2 (Th2) cytokine production was noted in interleukin-21 (IL-21) -treated human cells. (a) Serum levels of IgE in allergic subjects and controls were determined by ImmunoCAP. (b) Peripheral blood mononuclear cells (PBMCs) from allergic subjects and controls were stimulated with anti-CD3 and anti-CD28 antibodies and levels of IL-5 and interferon-γ (IFN-γ) in the culture supernatants were measured by ELISA. PBMCs (c, d) or CD4+ T cells (e–j) from patients (c, e, g, i) or controls (d, f, h, j) were stimulated with anti-CD3 and anti-CD28 antibodies in the presence of IL-21 or not. (c–f) Cytokines IL-5, IL-13, IFN-γ and IL-17 in the supernatants were detected by ELISA. (g, h) The expression of GATA-3, T-bet and RORγt was detected by quantitative RT-PCR. (i, j) Apoptosis was measured by staining with annexin V and 77-aminoactinomycin D (7-AAD) and analysing with a flow cytometer. Individual symbols represent an independent experiment and the horizontal lines represent the mean values. **P < 0·01; ***P < 0·001.
Analysis of cellular composition and cytokine production in the bronchoalveolar lavage fluid
Bronchoalveolar lavage fluid (BALF) was collected as previously described to analyse cellular components and cytokine production in the lung.31 Quantification of IL-4, IL-5 and IL-13 in the BALF was accomplished by using a commercially available ELISA kit (Duoset; R&D Systems).
Statistical analysis
Data from at least three independent experiments were used for statistical analysis. Results are expressed as the mean ± standard error of the mean (SEM). The Wilcoxon rank sum test and the Mann–Whitney U-test were used for statistical analysis. Significance levels were defined as P-values ≤ 0·05.
Results
Confirmation of Th2 cells
We established Th2 cells by culturing CD4+ T cells with Th2 skewing culture conditions including OVA peptide, IL-2, IL-4 and anti-IFN-γ antibodies. As shown in representative Fig.1(a), after re-stimulating with PMA and ionomycin, 29·21% of CD4+ T cells expressed IL-4 after two rounds of Th2 skewing culture, while only 1·74% of CD4+ T cells expressed IL-4 before Th2 polarizing culture (Th0). The percentage of IFN-γ-expressing CD4+ T cells in Th2-skewing cultured cells was decreased compared with unpolarized Th0 cells (1·26% and 10·39%, respectively; Fig.1a). Th0 cells stimulated with OVA secreted Th1 cytokine IFN-γ, but little or no Th2 cytokines IL-4, IL-5 and IL-13. In contrast, Th2 cells stimulated with OVA secreted huge amounts of Th2 cytokines IL-4, IL-5 and IL-13, but little or no Th1 cytokine IFN-γ. Interleukin-17 was noted as being very low in T cells under Th2-skewing culture conditions and Th0 cells (Fig.1b). In addition, cultured Th2 cells expressed high levels of Th2 transcription factor GATA-3, but did not express Th1 T-bet and Th17 RORγt (Fig.1c).
Figure 1.
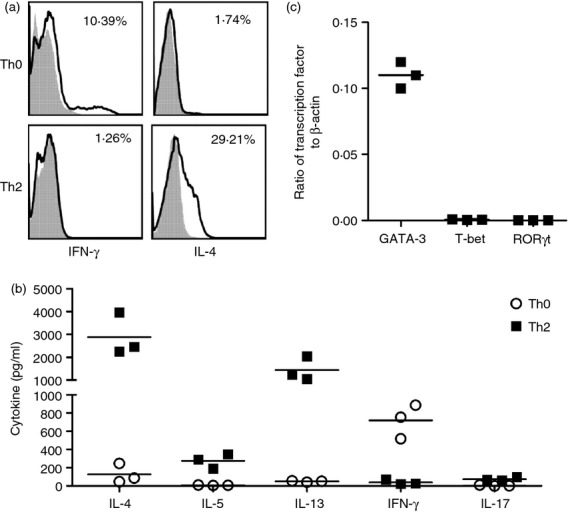
Confirmation of T helper type 2 (Th2) cells. Th2 cells were cultured as described in the Materials and methods section. (a) Th0 and Th2 cells were re-stimulated with PMA and ionomycin. Cytokines interferon-γ (IFN-γ) and interleukin-4 (IL-4) expression by Th0 and Th2 cells were measured by staining with surface anti-CD3, anti-CD4 and intracellular anti-cytokine antibodies. CD4+ T cells were gated and the cytokine expression in CD4+ T cells was analysed. The shadow area is an isotype control; the non-shadow area reflects cytokine expression. The frequencies of cytokine-producing CD4+ T cells were shown in each panel. (b) Th0 and Th2 cells were re-stimulated with OVA323–339 peptide-pulsed splenocytes. Cytokines IL-4, IL-5, IL-13, IFN-γ and IL-17 production in the culture supernatants were measured by ELISA. (c) Th2 cells were re-stimulated with PMA and ionomycin. Transcription factors GATA3, T-bet and RORγt in Th2 cells were measured by quantitative RT-PCR. Individual symbols represent an independent experiment and the horizontal lines represent the mean values of three independent experiments.
IL-21 suppressed Th2 cell differentiation
To study the effects of IL-21 on the development of Th2 cells in vitro, we cultured Th2 cells in Th2-skewing culture conditions with or without IL-21. After two rounds of culturing, the numbers of harvested cells were significantly lower in cells cultured with IL-21 (Fig.2a). Cells cultured in Th2-skewing culture conditions with IL-21 expressed the high levels of Th2 transcription factor GATA-3, but did not express Th1 T-bet and Th17 RORγt, which was the same with cells in Th2-skewing culture conditions without IL-21 (Fig.2b). For antigen-specific activation, Th2 cells harvested from Th2-skewing culture conditions with addition of IL-21 secreted decreased levels of IL-4, IL-5 and IL-13 compared with the same number of Th2 cells harvested from Th2-skewing culture conditions without IL-21. Treatment with IL-21 decreased IL-4 production by 22%, 20% and 23%; IL-5 production by 55%, 42% and 46%; and IL-13 production by 24%, 25% and 22% in three independent experiments. Importantly, the decreases of IL-4, IL-5 and IL-13 could be reversed by the addition of neutralizing IL-21R fusion protein (IL-21R:Fc) (Fig.2c). Taken together, IL-21 reduced the number of differentiated Th2 cells and these IL-21-cultured Th2 cells showed a diminished antigen-specific Th2 cytokine production. Hence, IL-21 suppressed not only the Th2 cell differentiation, but also the function of differentiated Th2 cells.
Figure 2.
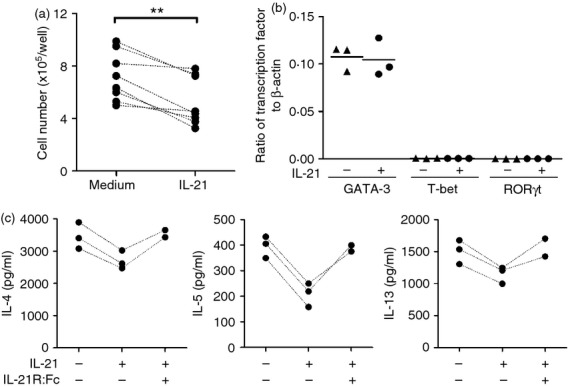
Interleukin-21 (IL-21) suppressed T helper type 2 (Th2) cell differentiation. T cells were cultured in Th2-skewing culture conditions with or without IL-21 or IL-21 plus IL-21R:Fc. (a) The harvested cells were counted and expressed as cell number per well. (b) GATA3, T-bet and RORγt expression in the harvested cells was measured by quantitative RT-PCR. (c) 5 × 105 harvested Th2 cells were re-stimulated with ovalbumin 323–339 (OVA323–339) peptide-pulsed splenocytes and IL-4, IL-5 and IL-13 in the supernatants were detected by ELISA. Individual symbols represent an independent experiment and the horizontal lines represent the mean values of three independent experiments. **P < 0·01.
IL-21 suppressed Th2 cytokine production of already polarized Th2 cells
We then investigated whether IL-21 suppressed the function of polarized Th2 cells. OVA-specific polarized Th2 cells were stimulated with OVA in the presence of IL-21 or not and the cytokine production was measured. As shown in Fig.3(a), decreased IL-4 (3–20%), IL-5 (6–20%) and IL-13 (8–33%) production was found in polarized Th2 cells treated with IL-21 compared with that without IL-21. Moreover, the decreases of IL-4, IL-5 and IL-13 could be reversed by the addition of antibodies to IL-21R (Fig.3a). Decreased GATA-3 was also observed in polarized Th2 cells treated with IL-21 (Fig.3b). Of note, the percentage of apoptotic cells, including early Annexin V+ 7-AAD− and late Annexin V+ 7-AAD− apoptotic cells, was increased in IL-21-treated Th2 cells compared with untreated Th2 cells (Fig.3c). In addition, anti-apoptotic factor Bcl-2 expression in IL-21-treated Th2 cells was decreased compared with untreated Th2 cells (Fig.3d).
Figure 3.
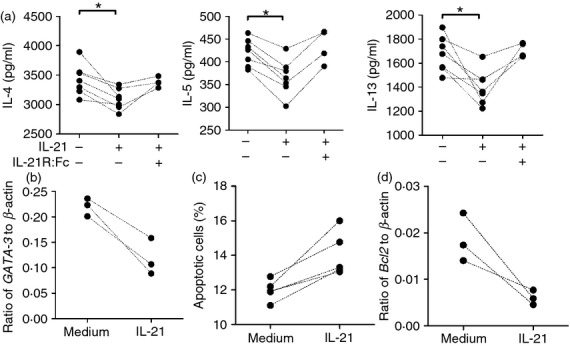
Interleukin-21 (IL-21) decreased cytokine production of polarized T helper type 2 (Th2) cells. Polarized Th2 cells (5 × 105 cells) were re-stimulated with ovalbumin 323–339 (OVA323–339) peptides pulsed splenocytes in the presence of IL-21 or IL-21 plus IL-21R:Fc. (a) Th2 cytokines IL-4, IL-5 and IL-13 in the supernatants were detected by ELISA. (b) GATA3 expression in Th2 cells was measured by quantitative RT-PCR. (c) Apoptosis of Th2 cells was measured by staining with annexin V and 7-aminoactinomycin D (7-AAD) and analysed with a flow cytometer. The apoptotic cells were annexin V+ 7-AAD− and annexin V+ 7-AAD+. (d) Bcl-2 expression of Th2 cells was measured by quantitative RT-PCR. Individual symbols represent an independent experiment. *P < 0·05.
IL-21 decreased Th2 cytokines in OVA-sensitized and challenged mice
We then investigated the effects of IL-21 on T cells in vivo. Intraperitoneal immunization followed by airway challenge with OVA is a standard method to induce Th2-driven allergic airway inflammation. We administered IL-21-expressing adenovirus (Ad-IL-21) at the beginning of OVA immunization and measured the Th2 cytokines after challenge with OVA (Fig.4a). We found that Th2 cytokines IL-4, IL-5 and IL-13 in the BALF were significantly decreased in mice receiving Ad-IL-21 (average 44% decrease in IL-4, 25% in IL-5 and 31% in IL-13 compared with Ad-mock group) (Fig.4b). We then administered Ad-IL-21 to OVA-immunized mice before OVA challenge to investigate the effects of IL-21 on the function of polarized Th2 cells (Fig.4c). We found that Th2 cytokine IL-4 in the BALF was significantly decreased (average 45% decrease compared to Ad-mock group) in mice receiving Ad-IL-21 whereas IL-5 and IL-13 remained unchanged (Fig.4d). Hence, IL-21 suppresses the development of Th2 cells and function of polarized Th2 cells in vitro and in vivo.
IL-21 decreased Th2 cytokine production of T cells from allergic patients and healthy subjects
We then investigated whether IL-21 affected the Th2 cells of humans. PBMCs or CD4+ T cells from patients with allergic rhinitis were stimulated with anti-CD3 and anti-CD28 antibodies in the presence of IL-21 or without IL-21, and Th2 cytokine secretion from activated T cells was measured. Patients with allergic rhinitis were characterized by their typical nasal symptoms and increased levels of serum IgE (Fig.5a). Increased IL-5/IFN-γ in activated T cells was noted in patients with allergic rhinitis, suggesting Th2 favouring in these patients (Fig.5b). Production of IL-5 and IL-13 was significantly decreased in PBMCs and CD4+ T cells treated with IL-21 (Fig.5c,e). However, IL-4 production was relatively low in activated human T cells (data not shown). In addition, decreased IFN-γ was also observed in activated PBMCs and CD4+ T cells treated with IL-21. In contrast, increased IL-17 was found in activated PBMCs and in CD4+ T cells treated with IL-21 (Fig.5c,e). The Th2 cytokine transcription factor GATA-3 was decreased in IL-21-treated activated CD4+ T cells. In addition, decreased Th1 cytokine transcription factor T-bet and increased Th17 cytokine transcription factor RORγt were found (Fig.5g). However, the percentage of apoptotic cells was not changed in IL-21-treated CD4+ T cells compared with untreated CD4+ T cells (Fig.5i). Moreover, decreased IL-5, IL-13 and IFN-γ and transcription factors GATA-3 and T-bet, increased IL-17, and unchanged apoptosis were also found in IL-21-treated cells from healthy subjects (Fig.5d,f,h,j).
Discussion
In this study, we investigated the regulatory effects of IL-21 on Th2 cells in vitro and in vivo. Our results demonstrate that IL-21 suppresses Th2 cell development and the cytokine production ability of differentiated Th2 cells. Interleukin-21 decreases cytokine production of polarized Th2 cells and induces Th2 cell apoptosis by down-regulating Bcl-2. In vivo, IL-21 decreases Th2 cytokines in Th2-driven OVA-sensitized and OVA-challenged mice. In the study of patients with allergic diseases, IL-21 suppresses the IL-5 and IL-13 production of activated T cells by down-regulation of GATA-3.
Previous studies have shown that IL-21 inhibits IL-4-induced IgE secretion by B cells and IL-21R-deficient mice make high levels of IgE.23–25,32 The administration of exogenous IL-21 during immunization and challenge of mice with OVA reduces IgE production and impairs the ensuing recruitment of eosinophils into the airway.24 Interleukin-21 significantly reduces immediate hypersensitivity reactions in mouse skin by diminishing serum IgE or suppressing mast cell activation.27 Moreover, intranasal IL-21 administration alleviates murine allergic rhinitis by inhibiting local expression of IL-4, IL-5 and IL-13, IgE production by B cells and eotaxin production by fibroblast.26,28 By administration of Ad-IL-21 in Th2-driven OVA-induced airway inflammation, we found that IL-21 decreased Th2 cytokines in the BALF of OVA-immunized mice (Fig.4b,d). Therefore, IL-21 suppresses allergic responses by influencing not only the IgE production of B cells but also the secretion of Th2-associated cytokines by T cells.
In our results, IL-21 suppressed IL-4, IL-5 and IL-13 production of polarized Th2 cells with a decreased expression of GATA-3. GATA-3 was preferentially expressed in Th2 cells compared with Th1 cells.2 The regulation of IL-4, IL-5 and IL-13 controlled by the transcription factor GATA-3 has been shown to induce changes in the chromatin structure at the Il-4 locus and subsequently transactivate the IL-5 and IL-13 promoters.33 GATA-3 is not only sufficient to direct the differentiation of Th cells into the Th2 pathway, but is also essential to the function of Th2 cells. Anti-sense GATA-3 RNA has been shown to suppress the expression of IL-4 and/or IL-5 in Th2 cells.34 In addition, nasal administration of anti-sense GATA-3 oligonucleotides or intratracheal administration of GATA-3 short hairpin RNAs suppressed the expression of GATA-3 and significantly attenuated allergic airway inflammation in animal models of asthma.35,36
In the study of allergic patients, we found that IL-21 suppressed both Th2 cells and Th1 cells (Fig.5). Studies in murine systems have shown that exposure of naive T cells to IL-21 results in decreased production of IFN-γ, but not other Th1 cell cytokines, upon secondary stimulation.10,20 The inhibitory effect of IL-21 on IFN-γ production relied on the ability of IL-21 to repress the expression of Eomesodermin, a T-box transcription factor.37 In contrast, IL-21 increased IL-17 production of activated T cells according to our results (Fig.5c–f). Previous study has shown that IL-21 is produced by Th17 cells and is a functionally important product of Th17 cells, suggesting that IL-21 serves as an autocrine regulator of IL-17 production and helps to promote/sustain Th17 lineage commitment.5,21 Hence, IL-21 could regulate Th1, Th2 and Th17. The different signals of IL-21 through different target molecules of different T helper cells need to be further investigated.
We found that IL-21 suppressed the activity of polarized Th2 cells to secrete IL-4, IL-5 and IL-13 in vitro, but IL-21 suppressed only IL-4 in OVA-immunized airway inflammation mice. Type 2 innate lymphoid cells (ILC2) are non-B, non-T cells and secrete IL-5 and IL-13 after IL-33 or IL-25 stimulation, which results in the induction of type 2 responses to helminths and allergens.38 ILC2 cells have been found to represent a large proportion of the total IL-5-producing and IL-13-producing cells in the lungs in various models of asthma.39 Notably, even in allergic asthma induced with OVA/alum, ILC2 produce almost the same amount of IL-5 and IL-13 that T cells do.39 Hence, we proposed that in our IL-21-treated OVA-immunized mice, Th2 cells were suppressed by IL-21 but ILC2 were not. Because ILC2 secrete IL-5 and IL-13, but not IL-4, the levels of IL-4 in the BALF of IL-21-treated OVA-immunized mice were decreased, but IL-5 and IL-13 remained unchanged.
Previous studies demonstrated that IL-21 induces apoptosis of B cells and antigen-specific CD8 T cells by down-regulation of anti-apoptotic factor Bcl-2 and Bcl-xL.18,25,40 Interleukin-21 induces apoptosis of conventional dendritic cells via signal transducer and activator of transcription 3 and Bim.41 Moreover, IL-21 induces Bε cell apoptosis and then suppresses IgE responses.25 These results suggested that IL-21 induces the expression of an upstream pro-apoptotic protein that results in the down-modulation of Bcl-2 and induced apoptosis of Th2 cells.
In conclusion, our results demonstrated that IL-21 suppresses Th2 cell development and functions and IL-21 induces Th2 cell apoptosis. Furthermore, the suppressive effects of IL-21 on Th2 cells have also been noted in humans. Hence, IL-21 should be considered as applicable for therapeutic use in human Th2-mediated allergic diseases.
Acknowledgments
This work was supported by the National Science Council, Taiwan (NSC 101-2320-B-002 -027 -MY3).
Glossary
Abbreviations:
- BALF
bronchoalveolar lavage fluid
- IFN-γ
interferon-γ
- IL-4
interleukin-4
- IL-21R
interleukin-21 receptor
- ILC2
type 2 innate lymphoid cells
- OVA
ovalbumin
- PBMCs
peripheral blood mononuclear cells
- Th2 cells
T helper type 2 cells
Disclosure
The authors declare no conflict of interest.
References
- Paul WE, Zhu J. How are TH2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–35. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Takemoto N, Kurata H, Kamogawa Y, Miyatake S, O'Garra A, Arai N. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. J Exp Med. 2000;192:105–15. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Vahedi G, Sun HW, et al. Discrete roles of STAT4 and STAT6 transcription factors in tuning epigenetic modifications and transcription during T helper cell differentiation. Immunity. 2010;32:840–51. doi: 10.1016/j.immuni.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–10. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–34. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PLoS ONE. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M, Grusby MJ. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon γ-producing Th1 cells. J Exp Med. 2002;196:969–77. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta DS, Wurster AL, Grusby MJ. Biology of IL-21 and the IL-21 receptor. Immunol Rev. 2004;202:84–95. doi: 10.1111/j.0105-2896.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- Parrish-Novak J, Foster DC, Holly RD, Clegg CH. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J Leukoc Biol. 2002;72:856–63. [PubMed] [Google Scholar]
- Strengell M, Sareneva T, Foster D, Julkunen I, Matikainen S. IL-21 up-regulates the expression of genes associated with innate immunity and Th1 response. J Immunol. 2002;169:3600–5. doi: 10.4049/jimmunol.169.7.3600. [DOI] [PubMed] [Google Scholar]
- Strengell M, Matikainen S, Siren J, Lehtonen A, Foster D, Julkunen I, Sareneva T. IL-21 in synergy with IL-15 or IL-18 enhances IFN-γ production in human NK and T cells. J Immunol. 2003;170:5464–9. doi: 10.4049/jimmunol.170.11.5464. [DOI] [PubMed] [Google Scholar]
- Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT, Pallone F, Monteleone G. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178:732–9. doi: 10.4049/jimmunol.178.2.732. [DOI] [PubMed] [Google Scholar]
- Konforte D, Simard N, Paige CJ. IL-21: an executor of B cell fate. J Immunol. 2009;182:1781–7. doi: 10.4049/jimmunol.0803009. [DOI] [PubMed] [Google Scholar]
- Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–48. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta DS, Wurster AL, Whitters MJ, Young DA, Collins M, Grusby MJ. IL-21 induces the apoptosis of resting and activated primary B cells. J Immunol. 2003;170:4111–8. doi: 10.4049/jimmunol.170.8.4111. [DOI] [PubMed] [Google Scholar]
- Brandt K, Bulfone-Paus S, Foster DC, Ruckert R. Interleukin-21 inhibits dendritic cell activation and maturation. Blood. 2003;102:4090–8. doi: 10.1182/blood-2003-03-0669. [DOI] [PubMed] [Google Scholar]
- Mehta DS, Wurster AL, Weinmann AS, Grusby MJ. NFATc2 and T-bet contribute to T-helper-cell-subset-specific regulation of IL-21 expression. Proc Natl Acad Sci USA. 2005;102:2016–21. doi: 10.1073/pnas.0409512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolski R, Kim HP, Zhu W, Levy DE, Leonard WJ. IL-21 mediates suppressive effects via its induction of IL-10. J Immunol. 2009;182:2859–67. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki K, Spolski R, Feng CG, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–4. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- Suto A, Nakajima H, Hirose K, et al. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line Cε transcription of IL-4-stimulated B cells. Blood. 2002;100:4565–73. doi: 10.1182/blood-2002-04-1115. [DOI] [PubMed] [Google Scholar]
- Harada M, Magara-Koyanagi K, Watarai H, et al. IL-21-induced Bε cell apoptosis mediated by natural killer T cells suppresses IgE responses. J Exp Med. 2006;203:2929–37. doi: 10.1084/jem.20062206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromura Y, Kishida T, Nakano H, Hama T, Imanishi J, Hisa Y, Mazda O. IL-21 administration into the nostril alleviates murine allergic rhinitis. J Immunol. 2007;179:7157–65. doi: 10.4049/jimmunol.179.10.7157. [DOI] [PubMed] [Google Scholar]
- Tamagawa-Mineoka R, Kishida T, Mazda O, Katoh N. IL-21 reduces immediate hypersensitivity reactions in mouse skin by suppressing mast cell activation or IgE production. J Invest Dermatol. 2011;131:1513–20. doi: 10.1038/jid.2011.73. [DOI] [PubMed] [Google Scholar]
- Kishida T, Hiromura Y, Shin-Ya M, et al. IL-21 induces inhibitor of differentiation 2 and leads to complete abrogation of anaphylaxis in mice. J Immunol. 2007;179:8554–61. doi: 10.4049/jimmunol.179.12.8554. [DOI] [PubMed] [Google Scholar]
- Lin SC, Chuang YH, Yang YH, Chiang BL. Decrease in interleukin-21 in children suffering with severe atopic dermatitis. Pediatr Allergy Immunol. 2011;22:869–75. doi: 10.1111/j.1399-3038.2011.01209.x. [DOI] [PubMed] [Google Scholar]
- Juan SH, Lee TS, Tseng KW, Liou JY, Shyue SK, Wu KK, Chau LY. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;104:1519–25. doi: 10.1161/hc3801.095663. [DOI] [PubMed] [Google Scholar]
- Chuang YH, Wang TC, Jen HY, Yu AL, Chiang BL. α-Galactosylceramide-induced airway eosinophilia is mediated through the activation of NKT cells. J Immunol. 2011;186:4687–92. doi: 10.4049/jimmunol.1003659. [DOI] [PubMed] [Google Scholar]
- Shang XZ, Ma KY, Radewonuk J, Li J, Song XY, Griswold DE, Emmell E, Li L. IgE isotype switch and IgE production are enhanced in IL-21-deficient but not IFN-γ-deficient mice in a Th2-biased response. Cell Immunol. 2006;241:66–74. doi: 10.1016/j.cellimm.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Ho IC, Tai TS, Pai SY. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat Rev Immunol. 2009;9:125–35. doi: 10.1038/nri2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DH, Yang L, Ray A. Differential responsiveness of the IL-5 and IL-4 genes to transcription factor GATA-3. J Immunol. 1998;161:3817–21. [PubMed] [Google Scholar]
- Finotto S, De Sanctis GT, Lehr HA, et al. Treatment of allergic airway inflammation and hyperresponsiveness by antisense-induced local blockade of GATA-3 expression. J Exp Med. 2001;193:1247–60. doi: 10.1084/jem.193.11.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Huang HY, Chiang BL. Lentiviral-mediated GATA-3 RNAi decreases allergic airway inflammation and hyperresponsiveness. Mol Ther. 2008;16:60–5. doi: 10.1038/sj.mt.6300309. [DOI] [PubMed] [Google Scholar]
- Suto A, Wurster AL, Reiner SL, Grusby MJ. IL-21 inhibits IFN-γ production in developing Th1 cells through the repression of Eomesodermin expression. J Immunol. 2006;177:3721–7. doi: 10.4049/jimmunol.177.6.3721. [DOI] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–94. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–16. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- Barker BR, Parvani JG, Meyer D, Hey AS, Skak K, Letvin NL. IL-21 induces apoptosis of antigen-specific CD8+ T lymphocytes. J Immunol. 2007;179:3596–603. doi: 10.4049/jimmunol.179.6.3596. [DOI] [PubMed] [Google Scholar]
- Wan CK, Oh J, Li P, et al. The cytokines IL-21 and GM-CSF have opposing regulatory roles in the apoptosis of conventional dendritic cells. Immunity. 2013;38:514–27. doi: 10.1016/j.immuni.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


