Abstract
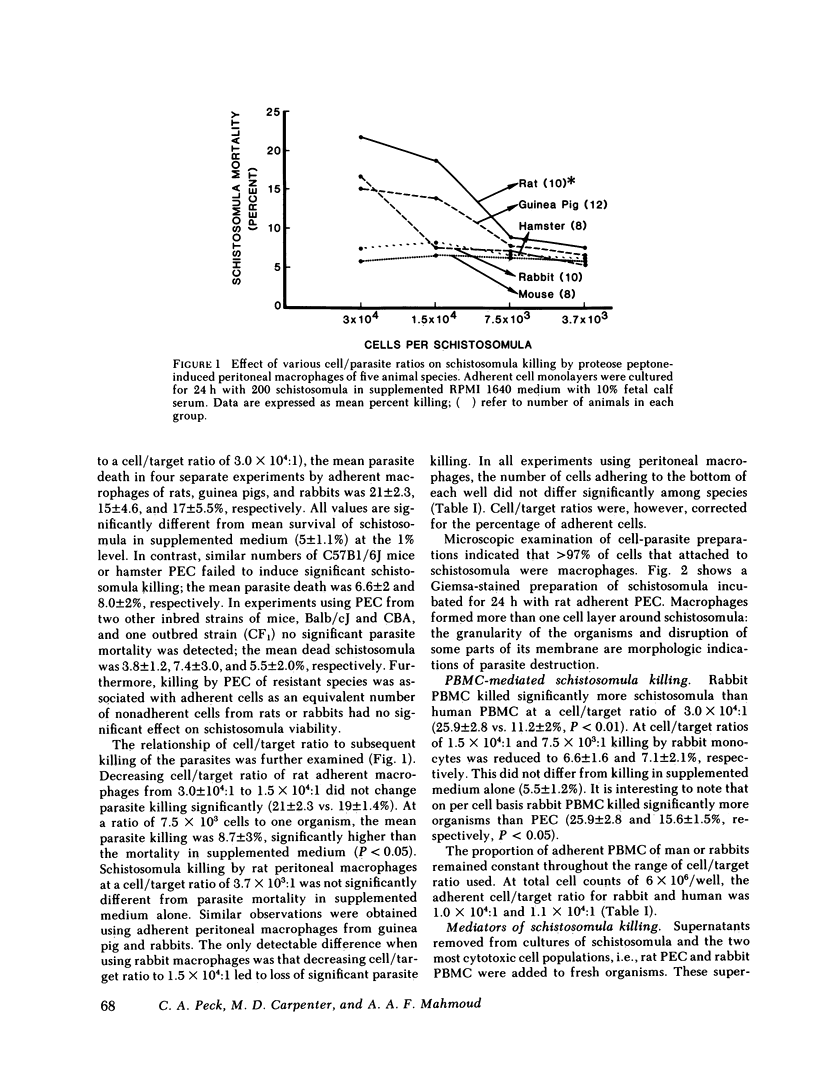
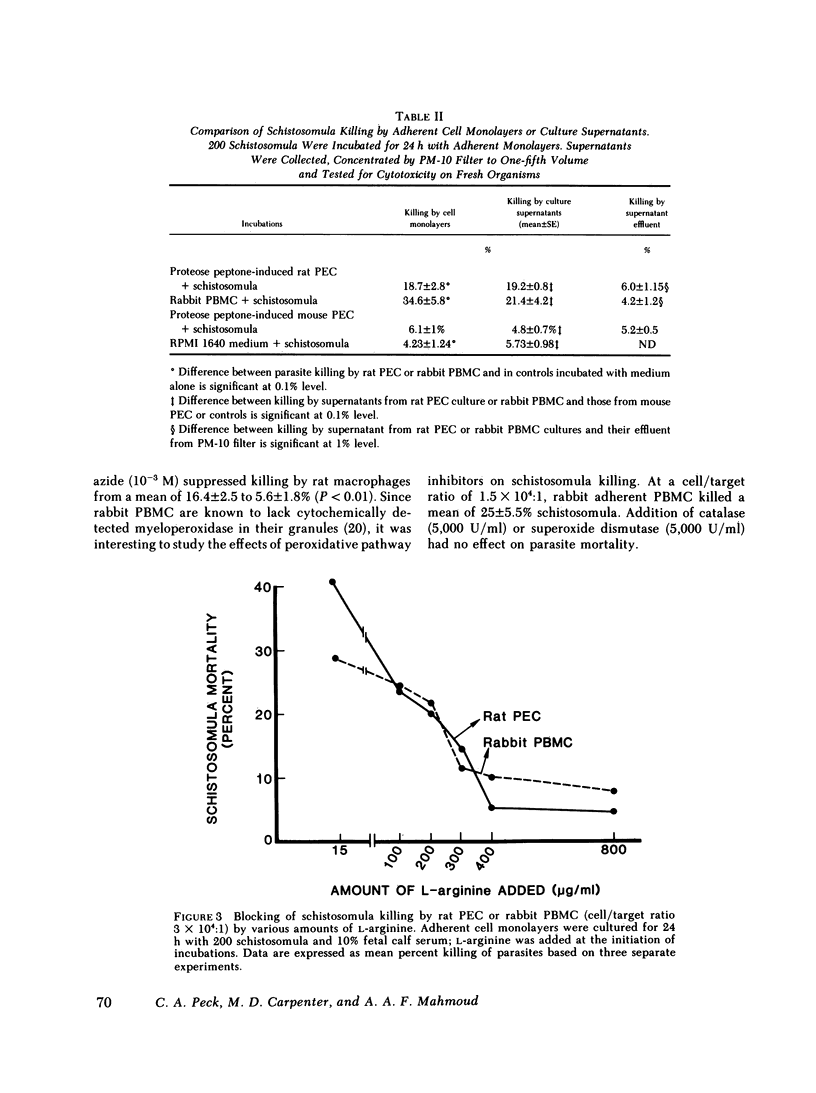
Resistance to infection with the multicellular parasite Schistosoma mansoni has been previously demonstrated to vary among several host species. The current investigation was designed to examine the basis for this species-related resistance in vitro. Adherent peritoneal macrophages or peripheral blood mononuclear cells from several species of host animals were incubated with S. mansoni schistosomula for 18-24 h; parasite viability was then assayed by methylene blue exclusion. Peritoneal exudate macrophages from susceptible species, such as mice (C57Bl/6) and hamsters killed, respectively, 6.6 +/- 2 and 8.0 +/- 2% of incubated schistosomula. In contrast, cells from resistant species: rats, guinea pigs, and rabbits, killed 21 +/- 2.3, 15 +/- 4.6, and 17 +/- 5.5%, respectively. Furthermore, blood monocytes from rabbits resulted in a mean of 25.9 +/- 2.8% dead organisms. Schistosomula killing by mononuclear phagocytes obtained from resistant species (rats or rabbits) was dependent on the cell/parasite ratio. Significant schistosomula mortality resulted from culture supernatants of rat macrophages or rabbit monocytes. Killing by cells from both species was significantly reduced upon addition of L-arginine, while catalase reduced killing only by rat macrophages. We conclude that mononuclear phagocytes may play a key role in species-related innate resistance to schistosomiasis; their in vitro schistosomulicidal activity parallels the known in vivo susceptibility of the donor species. Killing is mediated by lysosomal enzymes (arginase) and by products of oxidative metabolism, the predominant mechanism depends on the specific animal species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUCE J. I., LLEWELLYN L. M., SADUN E. H. Susceptibility of wild mammals to infection by Schistosoma mansoni. J Parasitol. 1961 Oct;47:752–756. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- CHEEVER A. W. A COMPARATIVE STUDY OF SCHISTOSOMA MANSONI INFECTIONS IN MICE, GERBILS, MULTIMAMMATE RATS AND HAMSTERS. I. THE RELATION OF PORTAL HYPERTENSION TO SIZE OF HEPATIC GRANULOMAS. Am J Trop Med Hyg. 1965 Mar;14:211–226. doi: 10.4269/ajtmh.1965.14.211. [DOI] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Capron M., Bazin H. Specific IgE antibodies in immune adherence of normal macrophages to Schistosoma mansoni schistosomules. Nature. 1975 Feb 6;253(5491):474–475. doi: 10.1038/253474a0. [DOI] [PubMed] [Google Scholar]
- Clegg J. A., Smithers S. R. Death of schistosome cercariae during penetration of the skin. II. Penetration of mammalian skin by Schistosoma mansoni. Parasitology. 1968 Feb;58(1):111–128. doi: 10.1017/s0031182000073479. [DOI] [PubMed] [Google Scholar]
- Colley D. G., Savage A. M., Lewis F. A. Host responses induced and elicited by cercariae, schistosomula, and cercarial antigenic preparations. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):88–95. doi: 10.4269/ajtmh.1977.26.88. [DOI] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Mahmoud A. A. Killing of schistosomula of Schistosoma mansoni by normal human monocytes. J Immunol. 1979 Aug;123(2):949–951. [PubMed] [Google Scholar]
- James S. L., Lazdins J. K., Meltzer M. S., Sher A. Macrophages as effector cells of protective immunity in murine schistosomiasis. Cell Immunol. 1982 Mar 1;67(2):255–266. doi: 10.1016/0008-8749(82)90218-0. [DOI] [PubMed] [Google Scholar]
- James S. L., Sher A., Lazdins J. K., Meltzer M. S. Macrophages as effector cells of protective immunity in murine schistosomiasis. II. Killing of newly transformed schistosomula in vitro by macrophages activated as a consequence of Schistosoma mansoni infection. J Immunol. 1982 Apr;128(4):1535–1540. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis A. I., Aikawa M., Mahmoud A. F. Mouse antibody-dependent eosinophil and macrophage adherence and damage to schistosomula of Schistosoma mansoni. J Immunol. 1979 Feb;122(2):398–405. [PubMed] [Google Scholar]
- Kassis A. I., Warren K. S., Mahmoud A. A. Antibody-dependent complement-mediated killing of schistosomula in intraperitoneal diffusion chambers in mice. J Immunol. 1979 Oct;123(4):1659–1662. [PubMed] [Google Scholar]
- Kazura J. W., Fanning M. M., Blumer J. L., Mahmoud A. A. Role of cell-generated hydrogen peroxide in granulocyte-mediated killing of schistosomula of Schistosoma mansoni in vitro. J Clin Invest. 1981 Jan;67(1):93–102. doi: 10.1172/JCI110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- Kung J. T., Brooks S. B., Jakway J. P., Leonard L. L., Talmage D. W. Suppression of in vitro cytotoxic response by macrophages due to induced arginase. J Exp Med. 1977 Sep 1;146(3):665–672. doi: 10.1084/jem.146.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Peters P. A., Civil R. H., Remington J. S. In vitro killing of schistosomula of Schistosoma mansoni by BCG and C. parvum-activated macrophages. J Immunol. 1979 May;122(5):1655–1657. doi: 10.2196/41502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S., Peters P. A. A role for the eosinophil in acquired resistance to Schistosoma mansoni infection as determined by antieosinophil serum. J Exp Med. 1975 Oct 1;142(4):805–813. doi: 10.1084/jem.142.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds G. R., Ellner J. J., Kearse L. A., Jr, Kazura J. W., Mahmoud A. A. Role of arginase in killing of schistosomula of Schistosoma mansoni. J Exp Med. 1980 Jun 1;151(6):1557–1562. doi: 10.1084/jem.151.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds G. R., Ellner J. J., el-Kholy A., Mahmoud A. A. Monocyte-mediated killing of schistosomula of Schistosoma mansoni: alterations in human Schistosomiasis mansoni and tuberculosis. J Immunol. 1981 Oct;127(4):1538–1542. [PubMed] [Google Scholar]
- Senft A. W. Studies in arginine metabolism by schistosomes. II. Arginine depletion in mammals and snails infected with S. mansoni or S. hematobium. Comp Biochem Physiol. 1967 May;21(2):299–306. doi: 10.1016/0010-406x(67)90790-6. [DOI] [PubMed] [Google Scholar]
- Sher A., Butterworth A. E., Colley D. G., Cook J. A., Freeman G. L., Jr, Jordan P. Immune responses during human schistosomiasis mansoni. II. Occurrence of eosinophil-dependent cytotoxic antibodies in relation to intensity and duration of infection. Am J Trop Med Hyg. 1977 Sep;26(5 Pt 1):909–916. [PubMed] [Google Scholar]
- VON LICHTENBERG F., SADUN E. H., BRUCE J. I. Tissue responses and mechanisms of resistance in schistosomiasis mansoni in abnormal hosts. Am J Trop Med Hyg. 1962 May;11:347–356. doi: 10.4269/ajtmh.1962.11.347. [DOI] [PubMed] [Google Scholar]



