Abstract
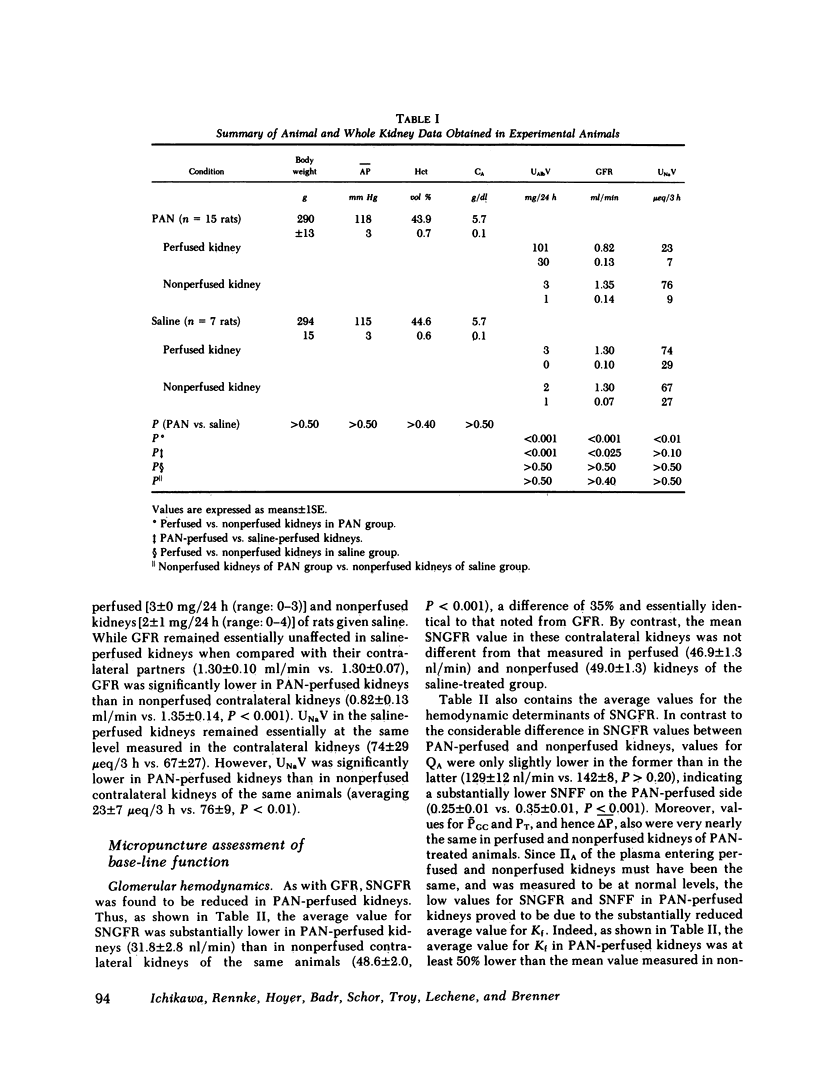
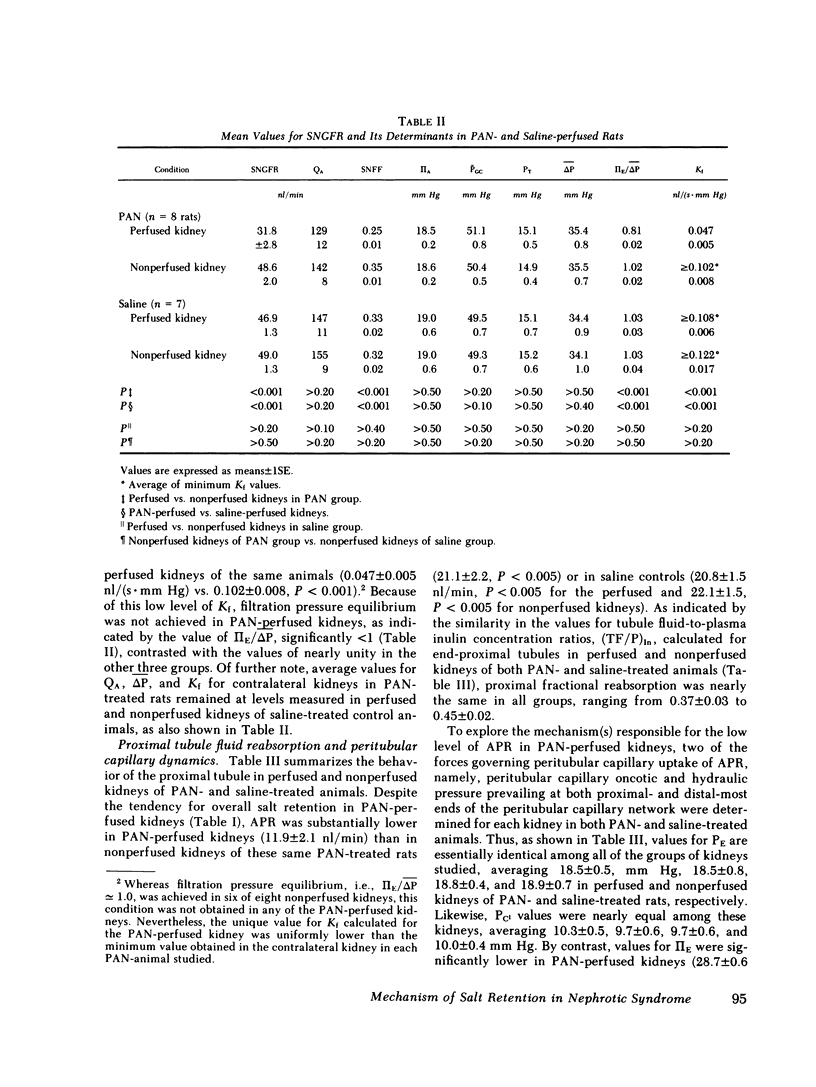
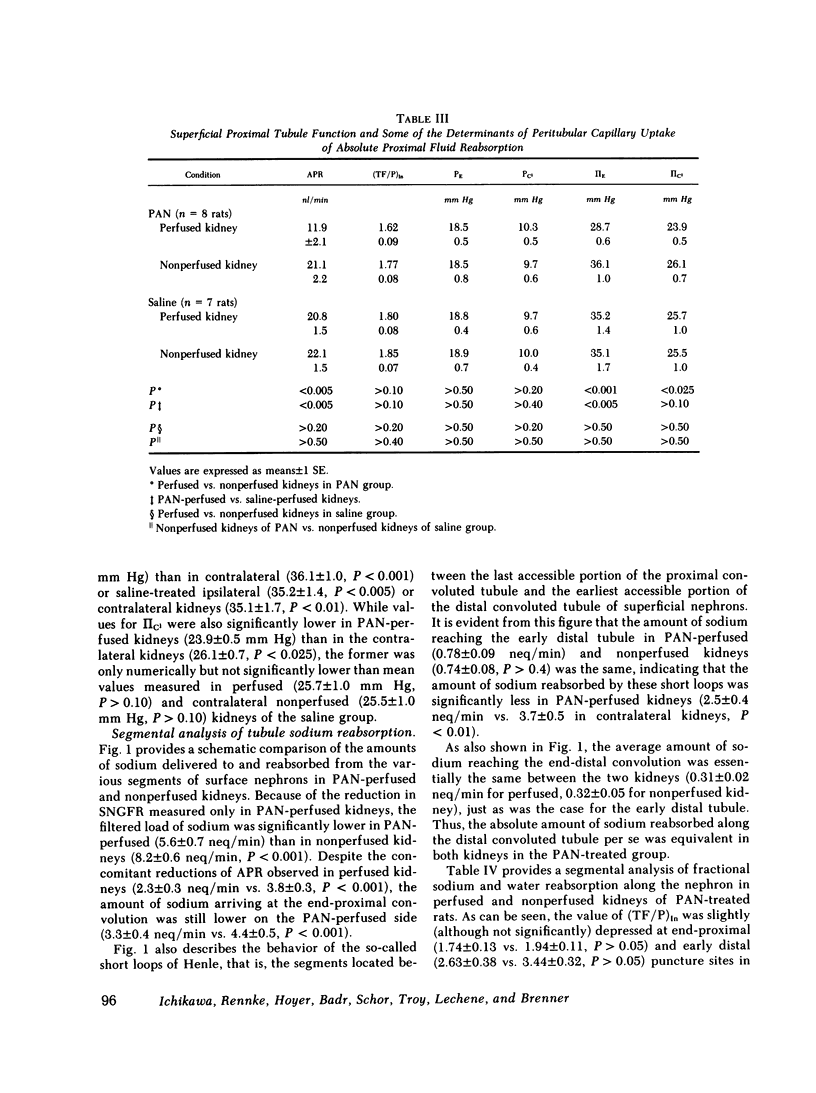
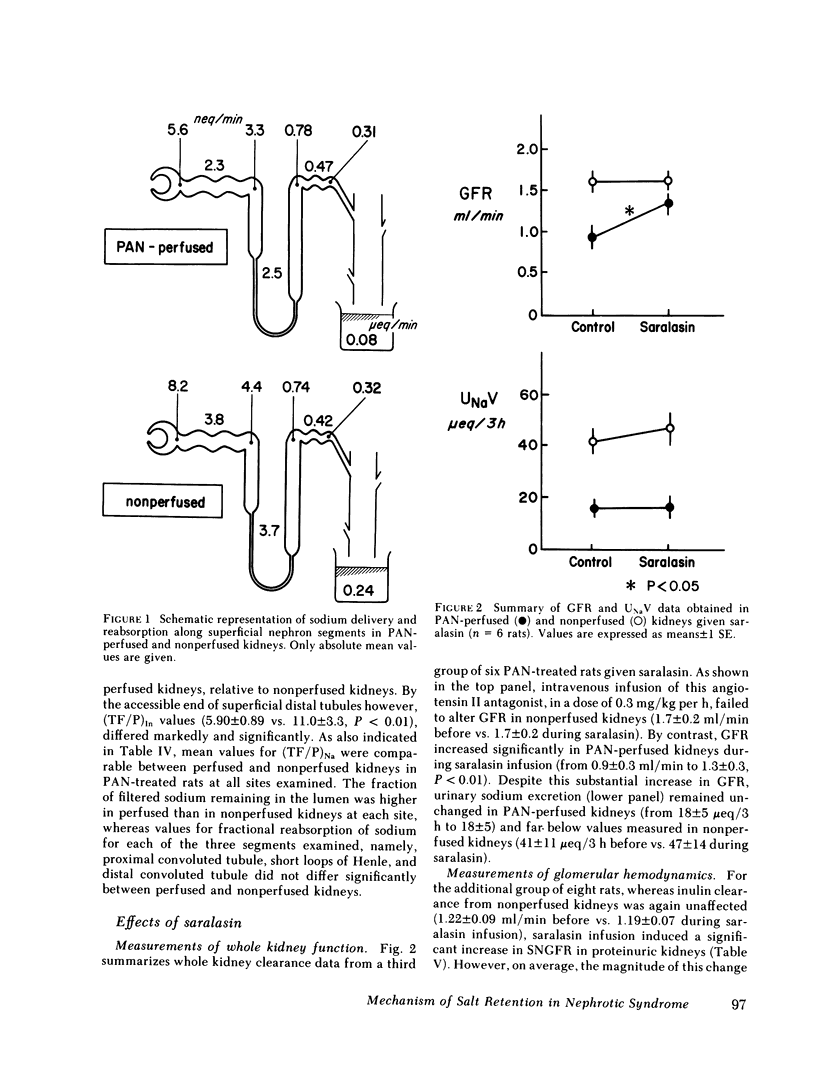
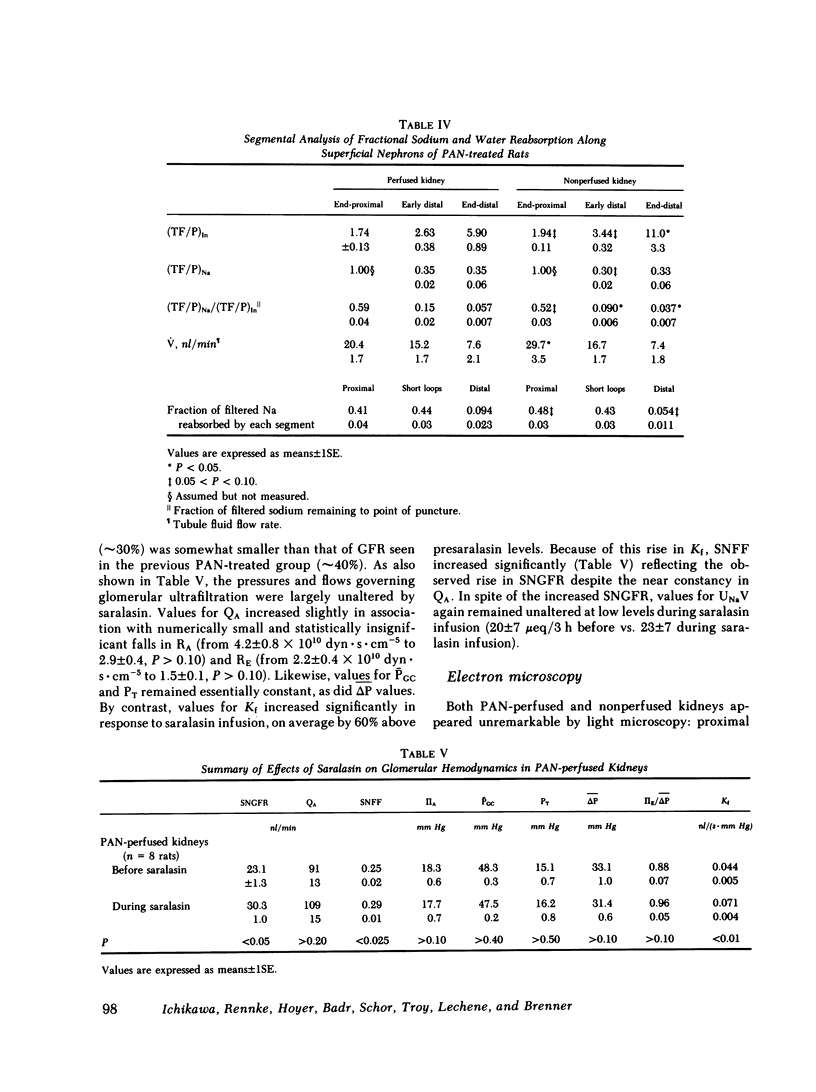
A unilateral model of puromycin aminonucleoside (PAN)-induced albuminuria was produced in Munich-Wistar rats to examine the mechanisms responsible for renal salt retention. 2 wk after selective perfusion of left kidneys with PAN (n = 8 rats) or isotonic saline (control, n = 7 rats), increases in albumin excretion and decreases in sodium excretion were demonstrated in PAN-perfused but not in nonperfused kidneys of PAN-treated rats although systemic plasma protein concentration remained at control level. Total kidney glomerular filtration rate (GFR) and superficial single nephron (SN) GFR were also reduced selectively in PAN-perfused kidneys, on average by approximately 30%, due primarily to a marked decline in the glomerular capillary ultrafiltration coefficient (Kf), which was also confined to PAN-perfused kidneys. Values for absolute proximal reabsorption (APR) were also selectively depressed in PAN-perfused kidneys, in keeping with a similarly selective decline in peritubular capillary oncotic pressure measured in these kidneys, the latter also a consequence of the fall in Kf. In a separate group of seven PAN-treated rats, however, no differences were detected between PAN-perfused and nonperfused kidneys in the absolute amount of sodium reaching the early (0.77 +/- 0.09 neq/min vs. 0.74 +/- 0.08, P greater than 0.40) and late portions of superficial distal tubules (0.31 +/- 0.02) neq/min vs. 0.32 +/- 0.05, P greater than 0.50), despite the lesser filtered load of sodium in PAN-perfused kidneys. Suppressed sodium reabsorption in both proximal convoluted tubules and short loops of Henle of PAN-perfused kidneys contributed to this equalization of sodium delivery rates to the late distal tubule, as did comparable reabsorption along distal convolutions. In two additional groups of PAN-treated rats, infusion of saralasin (0.3 mg/kg per h, i.v.) led to substantial increases in total kidney GFR and SNGFR in PAN-perfused but not in nonperfused kidneys. Despite these increases in total and SNGFR, urinary sodium excretion by PAN-perfused kidneys remained at a level far below that for nonperfused kidneys, again indicating that the antinatriuresis characterizing the PAN-perfused kidney is due to alterations in sodium handling by the tubules rather than changes in GFR. These results therefore indicate (a) that reductions in Kf and depressed sodium reabsorption by proximal tubules and Henle's loop segments in this model are brought about by intrarenal rather than circulating or systemic factors, and (b) assuming that superficial nephrons are representative of the entire nephron population, renal salt retention in this model is due primarily to intrarenal factor(s) acting beyond the distal convolution.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa M. A scanning electron microscopy of the glomerulus of normal and nephrotic rats. Lab Invest. 1970 Nov;23(5):489–496. [PubMed] [Google Scholar]
- BRICKER N. S., STOKES J. M., LUBOWITZ H., DEWEY R. R., BERNARD H. R., HARTROFT P. M. Experimentally induced permanent unilateral renal disease in dogs. J Lab Clin Med. 1958 Oct;52(4):571–579. [PubMed] [Google Scholar]
- Baylis C., Ichikawa I., Willis W. T., Wilson C. B., Brenner B. M. Dynamics of glomerular ultrafiltration. IX. Effects of plasma protein concentration. Am J Physiol. 1977 Jan;232(1):F58–F71. doi: 10.1152/ajprenal.1977.232.1.F58. [DOI] [PubMed] [Google Scholar]
- Bernard D. B., Alexander E. A., Couser W. G., Levinsky N. G. Renal sodium retention during volume expansion in experimental nephrotic syndrome. Kidney Int. 1978 Nov;14(5):478–485. doi: 10.1038/ki.1978.152. [DOI] [PubMed] [Google Scholar]
- Blantz R. C., Wilson C. B. Acute effects of antiglomerular basement membrane antibody on the process of glomerular filtration in the rat. J Clin Invest. 1976 Oct;58(4):899–911. doi: 10.1172/JCI108543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrer M. P., Baylis C., Robertson C. R., Brenner B. M., Troy J. L., Willis W. T. Mechanisms of the puromycin-induced defects in the transglomerular passage of water and macromolecules. J Clin Invest. 1977 Jul;60(1):152–161. doi: 10.1172/JCI108751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Reid J. J., Farquhar M. G. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest. 1976 Jan;34(1):43–59. [PubMed] [Google Scholar]
- Chandra M., Hoyer J. R., Lewy J. E. Renal function in rats with unilateral proteinuria produced by renal perfusion with aminonucleoside. Pediatr Res. 1981 Apr;15(4 Pt 1):340–344. doi: 10.1203/00006450-198104000-00010. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Troy J. L., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest. 1973 Jun;52(6):1500–1508. doi: 10.1172/JCI107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorhout E. J., Roos J. C., Boer P., Yoe O. H., Simatupang T. A. Observations on edema formation in the nephrotic syndrome in adults with minimal lesions. Am J Med. 1979 Sep;67(3):378–384. doi: 10.1016/0002-9343(79)90782-4. [DOI] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J Exp Med. 1961 Nov 1;114:699–716. doi: 10.1084/jem.114.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENK S., ANTONOWICZ I., CRAIG J. M., METCOFF J. Experimental nephrotic syndrome induced in rats by aminonucleoside; renal lesions and body electrolyte composition. Proc Soc Exp Biol Med. 1955 Jul;89(3):424–427. doi: 10.3181/00379727-89-21833. [DOI] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Glassock R. J. Sodium homeostasis in acute glomerulonephritis and the nephrotic syndrome. Contrib Nephrol. 1980;23:181–203. doi: 10.1159/000390007. [DOI] [PubMed] [Google Scholar]
- Grausz H., Lieberman R., Earley L. E. Effect of plasma albumin on sodium reabsorption in patients with nephrotic syndrome. Kidney Int. 1972;1(1):47–54. doi: 10.1038/ki.1972.7. [DOI] [PubMed] [Google Scholar]
- Green R., Moriarty R. J., Giebisch G. Ionic requirements of proximal tubular fluid reabsorption flow dependence of fluid transport. Kidney Int. 1981 Nov;20(5):580–587. doi: 10.1038/ki.1981.180. [DOI] [PubMed] [Google Scholar]
- Hoyer J. R., Mauer S. M., Michael A. F. Unilateral renal disease in the rat. I. Clinical, morphologic, and glomerular mesangial functional features of the experimental model produced by renal perfusion with aminonucleoside. J Lab Clin Med. 1975 May;85(5):756–768. [PubMed] [Google Scholar]
- Ichikawa I., Hoyer J. R., Seiler M. W., Brenner B. M. Mechanism of glomerulotubular balance in the setting of heterogeneous glomerular injury. Preservation of a close functional linkage between individual nephrons and surrounding microvasculature. J Clin Invest. 1982 Jan;69(1):185–198. doi: 10.1172/JCI110430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M., Seldin D. W., Kokko J. P. Effect of perfusion rate on the fluxes of water, sodium, chloride and urea across the proximal convoluted tubule. Kidney Int. 1977 Jan;11(1):18–27. doi: 10.1038/ki.1977.3. [DOI] [PubMed] [Google Scholar]
- Maddox D. A., Bennett C. M., Deen W. M., Glassock R. J., Knutson D., Brenner B. M. Control of proximal tubule fluid reabsorption in experimental glomerulonephritis. J Clin Invest. 1975 Jun;55(6):1315–1325. doi: 10.1172/JCI108051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mauer S. M., Fish A. J., Blau E. B., Michael A. F. The glomerular mesangium. I. Kinetic studies of macromolecular uptake in normal and nephrotic rats. J Clin Invest. 1972 May;51(5):1092–1101. doi: 10.1172/JCI106901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan T., Berliner R. W. A study by continuous microperfusion of water and electrolyte movements in the loop of Henle and distal tubule of the rat. Nephron. 1969;6(3):388–405. doi: 10.1159/000179741. [DOI] [PubMed] [Google Scholar]
- Oken D. E., Flamenbaum W. Micropuncture studies of proximal tubule albumin concentrations in normal and nephrotic rats. J Clin Invest. 1971 Jul;50(7):1498–1505. doi: 10.1172/JCI106635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pricam C., Humbert F., Perrelet A., Amherdt M., Orci L. Intercellular junctions in podocytes of the nephrotic glomerulus as seen with freeze-fracture. Lab Invest. 1975 Sep;33(3):209–218. [PubMed] [Google Scholar]
- Rocha A., Marcondes M., Malnic G. Micropuncture study in rats with experimental glomerulonephritis. Kidney Int. 1973 Jan;3(1):14–23. doi: 10.1038/ki.1973.3. [DOI] [PubMed] [Google Scholar]
- Ryan G. B., Karnovsky M. J. An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int. 1975 Oct;8(4):219–232. doi: 10.1038/ki.1975.105. [DOI] [PubMed] [Google Scholar]
- Schor N., Ichikawa I., Rennke H. G., Troy J. L., Brenner B. M. Pathophysiology of altered glomerular function in aminoglycoside-treated rats. Kidney Int. 1981 Feb;19(2):288–296. doi: 10.1038/ki.1981.19. [DOI] [PubMed] [Google Scholar]
- Tucker B. J., Blantz R. C. Effects of glomerular filtration dynamics on the glomerular permeability coefficient. Am J Physiol. 1981 Mar;240(3):F245–F254. doi: 10.1152/ajprenal.1981.240.3.F245. [DOI] [PubMed] [Google Scholar]
- VERNIER R. L., PAPERMASTER B. W., GOOD R. A. Aminonucleoside nephrosis. I. Electron microscopic study of the renal lesion in rats. J Exp Med. 1959 Jan 1;109(1):115–126. doi: 10.1084/jem.109.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam M. A., Cotran R. S., Karnovsky M. J. An ultrastructural study of glomerular permeability in aminonucleoside nephrosis using catalase as a tracer protein. J Exp Med. 1970 Dec 1;132(6):1168–1180. doi: 10.1084/jem.132.6.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viets J. W., Deen W. M., Troy J. L., Brenner B. M. Determination of serum protein concentration in nanoliter blood samples using fluorescamine or 9-phthalaldehyde. Anal Biochem. 1978 Aug 1;88(2):513–521. doi: 10.1016/0003-2697(78)90451-7. [DOI] [PubMed] [Google Scholar]
- Wright F. S. Increasing magnitude of electrical potential along the renal distal tubule. Am J Physiol. 1971 Mar;220(3):624–638. doi: 10.1152/ajplegacy.1971.220.3.624. [DOI] [PubMed] [Google Scholar]