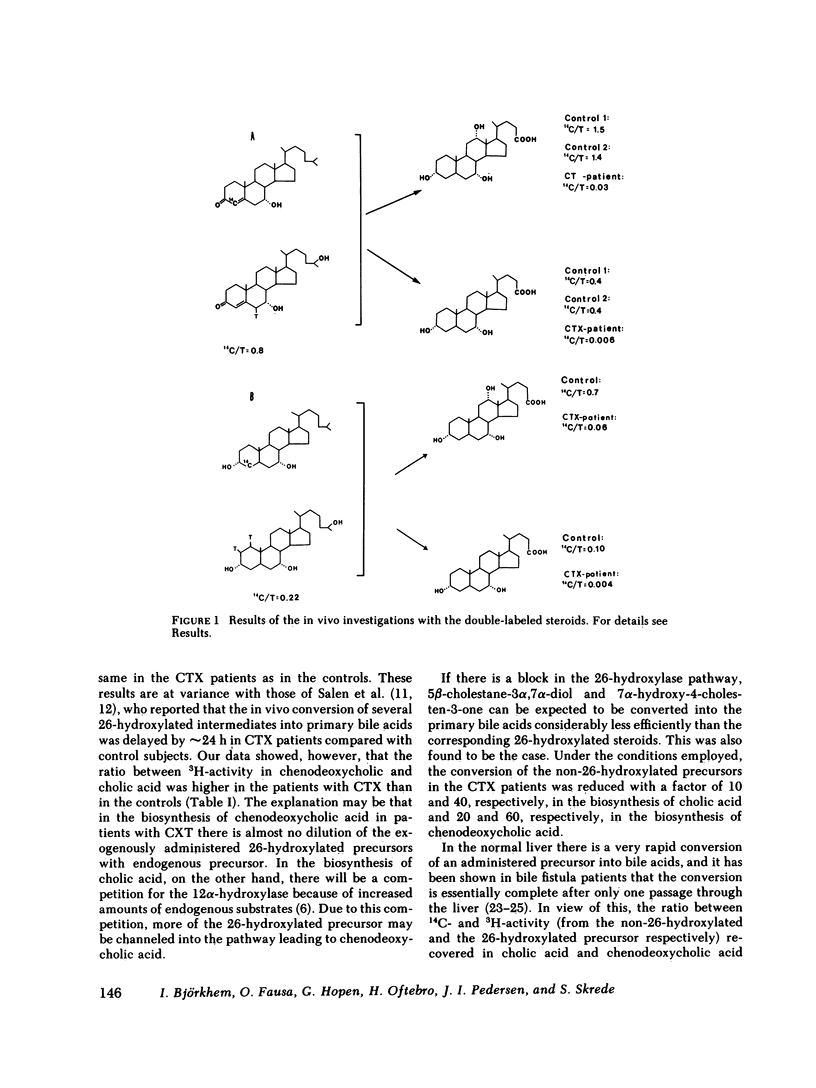
Abstract
On the basis of different in vitro studies, we have previously suggested that the basic metabolic defect in the rare inherited disease cerebrotendinous xanthomatosis (CTX) is a lack of a hepatic mitochondrial C27-steroid 26-hydroxylase, involved in the normal biosynthesis of bile acids (1980. J. Clin. Invest. 65: 1418-1430; 1981. J. Lipid Res. 22: 191-200; 22: 632-640). In the present work, this hypothesis was tested in vivo. One patient with CTX and two control subjects received intravenously a mixture of [4-14C]7 alpha-hydroxy-4-cholesten-3-one and [6 beta-3H]7 alpha,26-dihydroxy-4-cholesten-3-one, steroids believed to be important precursors of chenodeoxycholic acid. The ratio between 14C and 3H in cholic acid and chenodeoxycholic acid isolated from bile of the CTX-patient was approximately 1/40 and 1/60 of those of the control subjects, respectively. Another patient with CTX and one control subject received a mixture of [4-14C]5 beta-cholestane-3 alpha,7 alpha-diol and [1,2-3H]5 beta-cholestane-3 alpha,7 alpha,26-triol, both possible precursors to chenodeoxycholic acid. In this case the 14C/3H ratio in cholic acid and chenodeoxycholic acid from the patient with CTX was 1/10 and 1/15, respectively, compared with that of the control subject. The most likely explanation for these findings is that very little of the 14C-precursors, i.e. without a 26-hydroxyl group, can be converted into cholic acid and chenodeoxycholic acid because of a defect of the 26-hydroxylase step. The results obtained are in accord with our previous findings in vitro. The results further underline the importance of the 26-hydroxylase pathway in the normal biosynthesis of cholic acid and chenodeoxycholic acid in man.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkhem I., Danielsson H., Einarsson K. On the conversion of cholesterol to 5-beta-cholestane-3-alpha, 7-alpha-diol in guinea pig liver homogenates. Eur J Biochem. 1967 Oct;2(3):294–302. doi: 10.1111/j.1432-1033.1967.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Gustafsson J., Johansson G., Persson B. Biosynthesis of bile acids in man. Hydroxylation of the C27-steroid side chain. J Clin Invest. 1975 Mar;55(3):478–486. doi: 10.1172/JCI107954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Gustafsson J. Omega-hydroxylation of steriod side-chain in biosynthesis of bile acids. Eur J Biochem. 1973 Jul 2;36(1):201–212. doi: 10.1111/j.1432-1033.1973.tb02902.x. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Oftebro H., Skrede S., Pedersen J. I. Assay of intermediates in bile acid biosynthesis using isotope dilution--mass spectrometry: hepatic levels in the normal state and in cerebrotendinous xanthomatosis. J Lipid Res. 1981 Feb;22(2):191–200. [PubMed] [Google Scholar]
- Björkhem I. On the mechanism of the enzymatic conversn of cholest-5-ene-3-beta, 7-alpha-diol into 7-alpha-hydroxycholest-4-en-3-one. Eur J Biochem. 1969 Apr;8(3):337–344. doi: 10.1111/j.1432-1033.1969.tb00533.x. [DOI] [PubMed] [Google Scholar]
- Björkhem I. Stereochemistry of the enzymatic conversion of a delta 4-3-oxosteroid into a 3-oxo-5 beta-steroid. Bile acids and steroids 208. Eur J Biochem. 1969 Jan;7(3):413–417. doi: 10.1111/j.1432-1033.1969.tb19625.x. [DOI] [PubMed] [Google Scholar]
- Danielsson H., Sjövall J. Bile acid metabolism. Annu Rev Biochem. 1975;44:233–253. doi: 10.1146/annurev.bi.44.070175.001313. [DOI] [PubMed] [Google Scholar]
- Hanson R. F., Klein P. D., Williams G. C. Bile acid formation in man: metabolism of 7 -hydroxy-4-cholesten-3-one in bile fistula patients. J Lipid Res. 1973 Jan;14(1):50–53. [PubMed] [Google Scholar]
- Hanson R. F. The formation and metabolism of 3 ,7 -dihydroxy-5 -cholestan-26-oic acid in man. J Clin Invest. 1971 Oct;50(10):2051–2055. doi: 10.1172/JCI106698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAKITA M., WELLS W. W. Quantitative analysis of fecal bile acids by gas-liquid chromatography. Anal Biochem. 1963 Jun;5:523–530. doi: 10.1016/0003-2697(63)90072-1. [DOI] [PubMed] [Google Scholar]
- Oftebro H., Björkhem I., Skrede S., Schreiner A., Pederson J. I. Cerebrotendinous xanthomatosis: a defect in mitochondrial 26-hydroxylation required for normal biosynthesis of cholic acid. J Clin Invest. 1980 Jun;65(6):1418–1430. doi: 10.1172/JCI109806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oftebro H., Björkhem I., Størmer F. C., Pedersen J. I. Cerebrotendinous xanthomatosis: defective liver mitochondrial hydroxylation of chenodeoxycholic acid precursors. J Lipid Res. 1981 May;22(4):632–640. [PubMed] [Google Scholar]
- Oftebro H., Saarem K., Björkhem I., Pedersen J. I. Side chain hydroxylation of C27-steroids and vitamin D3 by a cytochrome P-450 enzyme system isolated from human liver mitochondria. J Lipid Res. 1981 Nov;22(8):1254–1264. [PubMed] [Google Scholar]
- Orme-Johnson W. H., Beinert H. Reductive titrations of iron-sulfur proteins containing two to four iron atoms. J Biol Chem. 1969 Nov 25;244(22):6143–6148. [PubMed] [Google Scholar]
- Pedersen J. I., Godager H. K. Purification of NADPH-ferredoxin reductase from rat liver mitochondria. Biochim Biophys Acta. 1978 Jul 7;525(1):28–36. doi: 10.1016/0005-2744(78)90196-1. [DOI] [PubMed] [Google Scholar]
- Salen G., Shefer S., Cheng F. W., Dayal B., Batta A. K., Tint G. S. Cholic acid biosynthesis: the enzymatic defect in cerebrotendinous xanthomatosis. J Clin Invest. 1979 Jan;63(1):38–44. doi: 10.1172/JCI109275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salen G., Shefer S., Mosbach E. H., Hauser S., Cohen B. I., Nicolau G. Metabolism of potential precursors of chenodeoxycholic acid in cerebrotendinous xanthomatosis (CTX). J Lipid Res. 1979 Jan;20(1):22–30. [PubMed] [Google Scholar]
- Salen G., Shefer S., Setoguchi T., Mosbach E. H. Bile alcohol metabolism in man. Conversion of 5beta-cholestane-3alpha, 7alpha,12alpha, 25-tetrol to cholic acid. J Clin Invest. 1975 Jul;56(1):226–231. doi: 10.1172/JCI108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi T., Salen G., Tint G. S., Mosbach E. H. A biochemical abnormality in cerebrotendinous xanthomatosis. Impairment of bile acid biosynthesis associated with incomplete degradation of the cholesterol side chain. J Clin Invest. 1974 May;53(5):1393–1401. doi: 10.1172/JCI107688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer S., Cheng F. W., Dayal B., Hauser S., Tint G. S., Salen G., Mosbach E. H. A 25-hydroxylation pathway of cholic acid biosynthesis in man and rat. J Clin Invest. 1976 Apr;57(4):897–903. doi: 10.1172/JCI108366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swell L., Gustafsson J., Schwartz C. C., Halloran L. G., Danielsson H., Vlahcevic Z. R. An in vivo evaluation of the quantitative significance of several potential pathways to cholic and chenodeoxycholic acids from cholesterol in man. J Lipid Res. 1980 May;21(4):455–466. [PubMed] [Google Scholar]
- Taniguchi S., Hoshita N., Okuda K. Enzymatic characteristics of CO-sensitive 26-hydroxylase system for 5beta-cholestane-3 alpha, 7 alpha, 12 alpha-triol in rat-liver mitochondria and its intramitochondrial localization. Eur J Biochem. 1973 Dec 17;40(2):607–617. doi: 10.1111/j.1432-1033.1973.tb03233.x. [DOI] [PubMed] [Google Scholar]
- Vlahcevic Z. R., Schwartz C. C., Gustafsson J., Halloran L. G., Danielsson H., Swell L. Biosynthesis of bile acids in man. Multiple pathways to cholic acid and chenodeoxycholic acid. J Biol Chem. 1980 Apr 10;255(7):2925–2933. [PubMed] [Google Scholar]


