Abstract
Among the mechanisms that control chromosome segregation in bacteria are highly-conserved partitioning systems comprising three components: ParA protein (a deviant Walker-type ATPase), ParB protein (a DNA-binding element) and multiple cis-acting palindromic centromere-like sequences, designated parS. Ten putative parS sites have been identified in the P. aeruginosa PAO1 genome, four localized in close proximity of oriC and six, diverged by more than one nucleotide from a perfect palindromic sequence, dispersed along the chromosome. Here, we constructed and analyzed P. aeruginosa mutants deprived of each single parS sequence and their different combinations. The analysis included evaluation of a set of phenotypic features, chromosome segregation, and ParB localization in the cells. It was found that ParB binds specifically to all ten parS sites, although with different affinities. The P. aeruginosa parS mutant with all ten parS sites modified (parS null) is viable however it demonstrates the phenotype characteristic for parA null or parB null mutants: slightly slower growth rate, high frequency of anucleate cells, and defects in motility. The genomic position and sequence of parS determine its role in P. aeruginosa biology. It transpired that any one of the four parS sites proximal to oriC (parS1 to parS4), which are bound by ParB with the highest affinity, is necessary and sufficient for the parABS role in chromosome partitioning. When all these four sites are mutated simultaneously, the strain shows the parS null phenotype, which indicates that none of the remaining six parS sites can substitute for these four oriC-proximal sites in this function. A single ectopic parS2 (inserted opposite oriC in the parS null mutant) facilitates ParB organization into regularly spaced condensed foci and reverses some of the mutant phenotypes but is not sufficient for accurate chromosome segregation.
Introduction
Accurate DNA segregation to progeny cells is fundamental to the survival of organisms and continuity of life. In Prokaryotes, pioneering studies on the segregation of low-copy-number plasmids have revealed the existence of partitioning systems (par) ensuring active distribution of DNA molecules to daughter cells and thus their stable inheritance in bacterial populations [1–3]. The great majority of plasmidic par systems comprise three components: an NTPase (component A) that forms a dynamic scaffold for plasmid movement, specific DNA-binding protein (component B), and a cis-acting centromere-like sequence(s) recognized and bound by B-component, all together forming a ‘minimalist’ DNA segregation machine [4].
Bacterial genomics has revealed the presence of an operon encoding homologs of type IA plasmidic Par proteins (usually designated ParA and ParB) in close proximity of the origin of replication, oriC [5–7], in the vast majority of bacteria with the exception of two families of γ-proteobacteria, Enterobacteriaceae (e.g., E. coli) and Pasteurellaceae (e.g., Haemophilus influenzae) and one family of Mollicutes, Mycoplasmataceae (e.g. Mycoplasma sp). The highly-conserved multiple copies of parS, the cis-acting centromere-like sequence, are mainly localized in the ori domain comprising 20% of the genome around oriC [7], although in some species, e.g., Bacillus subtilis and Pseudomonas aeruginosa, additional parS sequences are dispersed outside the ori domain [8, 9]. The hydrolytic activity of ParA, P-loop ATPase with a deviant Walker A motif [10], provides energy and orchestrates the movement of the nucleoprotein complex of ParB bound to its cognate parS site(s) [2, 11–14]. The chromosomal partitioning systems participate in the chromosome segregation by orienting the ori domain spatially [15–17], directing the newly replicated origins to the cell poles [18–29], compacting the chromosome by creating a platform for SMC loading [30–32], and holding the ori domains at the poles until completion of cell division [12, 29, 33–35].
Numerous studies on various bacterial species (with singular or multipartite genomes, with a simple or complex cell cycle) have revealed on one hand the highly conserved nature of the partitioning components, and on the other the participation of parABS systems not only in chromosome segregation but also in other vital cell processes in a species-specific manner [36]. The parABS systems may be involved in the regulation of replication [15, 27, 37–41], initiation of sporulation [42, 43], septation and DNA translocation [16, 21, 23, 30, 44–46] as well as growth control and cytokinesis [12, 34, 35, 47–53] or motility [54, 55]. Transcriptomic analyses of par mutants have demonstrated the role of Par proteins as global transcriptional regulators in P. aeruginosa [56] and Vibrio cholerae [57].
The interactions of ParA and ParB homologues with one another and with other proteins have been studied thoroughly [9, 12, 13, 16, 29–35, 37–39, 49–55, 58, 59]. The interactions of chromosomal ParBs with the centromere-like sequences have been also analyzed, demonstrating their ability to specifically bind parS, spread on DNA, form nucleoprotein complexes and transcriptionally silence genes adjacent to parS [8–9, 57, 60–62].
Less is known about why there are multiple parS sites on the chromosome and the roles they play. The binding site for chromosomal ParB, first identified for Spo0J (ParB) in B. subtilis [8, 63] as the 16-nucleotide sequence tGTTtCAcGTGAAAAa/g, seems to be highly conserved in the primary chromosomes throughout the bacterial kingdom [7]. The secondary chromosomes of multipartite bacterial genomes possess their own parABS systems [7] demonstrating intra- as well as inter-species structural and functional diversity [25, 64, 65]. Whereas the postulated role of ParB interactions with parS sequences in the ori domain is to form nucleoprotein complexes that facilitate origin separation and their directional movements [25, 27, 33, 44, 45], the significance of ParB binding to the parS sites outside the ori domain has not been fully evaluated [8, 9, 62].
Our studies on bacterial chromosome segregation have been conducted on the clinically important opportunistic pathogen P. aeruginosa representing bacteria with a simple cell cycle. It was shown that P. aeruginosa PAO1161 parA and parB mutants were non-lethal but demonstrated wide range of pleiotropic defects, such as slower growth rate, higher frequency of anucleate cells (more than 400-fold), impaired motility (swimming and swarming) and abnormal colony morphology [54, 55]. Similar phenotypes were observed in populations of cells overproducing one of the partitioning proteins, ParA or ParB, demonstrating importance of their proper stoichiometry [54, 55]. Likewise plasmidic members of the family [66], ParB of P. aeruginosa can polymerize and spread along DNA after binding to the centromere-like sequence, causing transcriptional silencing of neighboring genes in a test plasmid [9]. In P. aeruginosa cells ParB forms large, compact nucleoprotein complexes co-localizing with ori domains, visualized by use of immunofluorescence as 1 to 4 foci, depending on the stage of growth [55]. In parA null mutant ParB foci are much weaker and irregularly distributed whereas in various parB point mutants, defective in dimerization/ polymerization and interactions with ParA, multiple small foci are dispersed [54, 55, 67, 68]. A transcriptomic analysis of P. aeruginosa par mutants [56] has revealed changes in the expression of multiple operons indicating an important role of Par proteins (especially ParB) in coordinating different cell processes either directly through interactions with DNA or indirectly through interactions with putative partner proteins (Glabski K., unpublished). Molecular analysis of ParB derivatives has led to the identification of a dimerization domain [9, 55, 67], domains interacting with parS [55, 68], and a polymerization domain responsible for spreading around parS sequence to form the nucleoprotein complex [68]. Also for ParA of P. aeruginosa a dimerization domain and a domain of interactions with its partner ParB have been mapped in the central part of the protein [58].
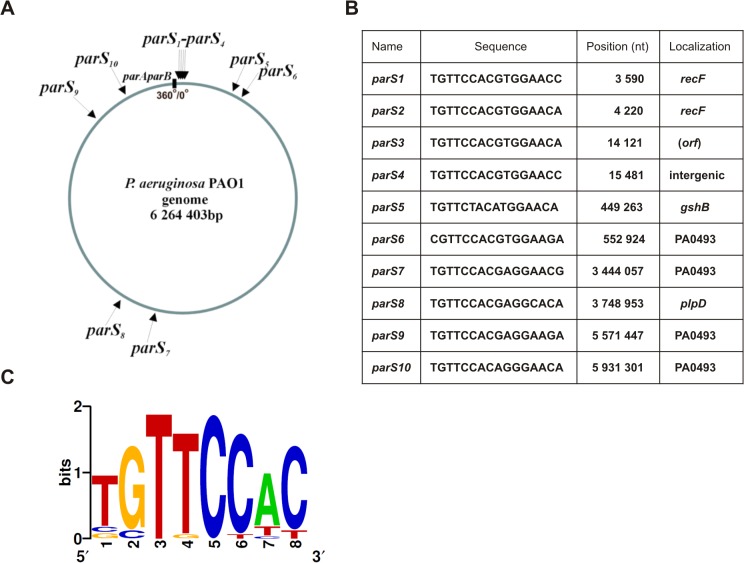
The parAB genes are localized ∼ 7 kb counterclockwise from oriC (Fig. 1A) in the reference PAO1 genome [69] and ten putative parS sites (numbered clockwise starting from oriC) are distributed along the chromosome with eight of them residing in the ori domain [9]. Among the parS sites, two designated parS2 and parS3 are perfect palindromes TGTTCCAC/GTGGAACA, parS1 and parS4 have one mismatch TGTTCCAC/GTGGAAC C, and the remaining six have two different mismatches (Table 1). The parAB operon and a single perfect palindromic parS2 sequence have been shown to stabilize otherwise unstable plasmid [9]. In vitro tests have demonstrated specific ParB binding to ds oligonucleotides corresponding to parS1, parS2 or parS7, with the highest affinity of ParB towards the perfectly palindromic parS2 oligonucleotide [9]. It has been hypothesized that the varied ParB affinity and localization in/outside the ori domain could be related to the different roles of individual parS sequences in P. aeruginosa biology, e.g., chromosome segregation versus regulation of gene expression.
Fig 1. The parS sites and their localization in the Pseudomonas aeruginosa genome.
(A) Circular map of the P. aeruginosa genome with locations of putative ParB binding sequences [9]. Position of the parAparB operon is shown as black rectangle, grey arrow marks oriC, black arrows indicate predicted parS sites. (B) Nucleotide sequences, genomic coordinates and gene locations of the parS sites. The sequences are presented in a clockwise configuration. The coordinates are given according to the genomic sequence of the PAO1-UW strain [69]. (C) Sequence logo for all twenty 8-bp half-sites in the P. aeruginosa PAO1-UW genome Error! Bookmark not defined.). Nucleotides at positions 2 and 5 are invariant in all half-sites.
Table 1. Nucleotide substitutions introduced into parS sequences.
| Name | Wild-type sequence | Mutated sequence | Restriction enzyme |
|---|---|---|---|
| parS1 | TGTTCCACGTGGAACC | TaTTtCAtGTaGAgCC | Eco72I (−) |
| parS2 | TGTTCCACGTGGAACA | TGTTtCAtGTaGAgCA | Eco72I (−) |
| parS3 | TGTTCCACGTGGAACA | cGTgCCcCGaGGgACg | Eco72I (−) |
| parS4 | TGTTCCACGTGGAACC | DELETION | NA |
| parS5 | TGTTCTACATGGAACA | TaTTgTAtATGGAgCA | SacII (−) |
| parS6 | CGTTCCACGTGGAAGA | CGTcCCtCGcGGcAGg | Eco72I (−) |
| parS7 | TGTTCCACGAGGAACG | TaTTtCAtGAaGAgCG | BshTI (−) |
| parS8 | TGTTCCACGAGGCACA | TaTTtCAtGAaGCgCA | NruI (+) |
| parS9 | TGTTCCACGAGGAAGA | TaTTtCAtGAaGAgGA | BglII (+) |
| parS10 | TGTTCCACAGGGAACA | TaTTtCAtAGaGAgCA | BshTI (+) |
Deviation from the perfect palindrome (parS2/parS3) are indicated in bold italics, and introduced mutations are in lower case. The mutations destroy (−) or create (+) a restriction site that was used to distinguish between the wt and mutated sequence. NA—not applicable.
Here, we constructed and characterized a set of P. aeruginosa PAO1161 parS mutants with each single parS altered and their various combinations including a parS null mutant (all ten parS sites modified) to shed light on their roles in the cell cycle. P. aeruginosa parS null mutant is viable although impaired in growth, chromosome segregation and motility. Analysis of mutant strains demonstrated that a single, high affinity ParB binding site in the proximity of oriC is necessary and sufficient for accurate chromosome segregation.
Materials and Methods
Bacterial strains and growth conditions
The E. coli and P. aeruginosa bacterial strains used and constructed in this study, are listed in S1 Table. Bacteria were grown at 30°C or 37°C in L-broth or L-agar (L-broth with 1.5% agar [w/v]). If needed, the media were supplemented with antibiotics: for E. coli strains benzyl penicillin (Pn) at final concentration 150 μg ml-1 in liquid medium and 300 μg ml-1 in agar plates, 30 μg ml-1 streptomycin (Sm), 50 μg ml-1 kanamycin (Km) or 10 μg ml-1chloramphenicol were applied; for P. aeruginosa strains 100 μg ml-1 chloramphenicol (Cm), 300 μg ml-1 carbenicillin (Cb) and 300 μg ml-1 rifampicin (Rif) were used. The L-agar used for blue/white screening contained 0.1 mM IPTG (isopropyl-β-D-thiogalactopyranoside) and 40 μg ml-1 X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside). Bacterial growth was monitored by measurements of optical density at 600 nm (OD600) and c.f.u. ml-1 after plating on L-agar.
Plasmids, oligonucleotides and DNA manipulations
The plasmids used and constructed during this study are listed in S2 Table, applied oligonucleotides are presented in S3 Table. Plasmid DNA was isolated by alkaline method and standard genetic procedures were used as recommended [70]. Chromosomal DNA templates for PCRs were prepared from 1 ml overnight cultures. Pelleted cells were washed with sterile water, re-suspended in 100 μl of sterile ddH20 and boiled. Standard PCR [71] was performed using 5 μl of boiled cell suspension and 5 pmoles of each primer. Fidelity of the amplified DNA sequence was verified by DNA sequencing in the internal sequencing facility (DNA Sequencing and Oligonucleotides Synthesis Laboratory, IBB PAS, Warsaw, Poland).
PCR site-directed mutagenesis
The QuickChange site-directed mutagenesis method was applied (Stratagene) with the pAKE600 plasmid [72] derivatives containing parS sequences as the templates. Only a few nucleotide substitutions were introduced in the primers for the PCR site-directed mutagenesis of intragenic parS sites not to modify the amino acid sequences of the orfs. To facilitate the screening of clones with mutagenized plasmid DNA, an appropriate restriction site was disabled or introduced as indicated in Table 1. The PCR-amplified plasmid DNA was treated with DpnI to remove the template DNA and then used for transformation of E. coli DH5α strain. After the initial screening, the presence of mutations was verified by DNA sequencing of PCR-amplified fragments.
Bacterial transformation
Competent E. coli cells were prepared by the standard CaCl2 method [70]. Competent P. aeruginosa cells were prepared as described previously [73].
Introduction of mutated parS alleles into P. aeruginosa PAO1161 chromosome by homologous recombination
The competent E. coli S17–1 cells (S1 Table) were transformed with pAKE600 suicide vector derivatives [72] to construct donor strains for conjugation. Bacterial conjugation was done on L-agar by mixing 100 μl of overnight cultures of E. coli S-17 (pAKE600 derivatives) donors and P. aeruginosa PAO1161 RifR recipient strain and incubation for 24 h at 37°C. The bacterial mixtures were washed off the plates with 2 ml of L-broth, and diluted cell suspensions were plated on L-agar with rifampicin and carbenicillin to select for transconjugants/ integrants.
The integrants of PAO1161 RifR (pAKE600 derivatives) were treated as described previously [54]. The allele integration and then allele exchange was verified by PCR using chromosomal DNA as a template and the adequate pairs of primers. The PCR-amplified fragments with putative mutated parS sequences were digested with appropriate restriction enzymes, and also sequenced to confirm the presence of the mutations.
Purification of His6-tagged ParB protein
Exponentially growing E. coli BL21(DE3) strain with pKLB28 (pET28 derivative, encoding His6-ParB) was induced with 0.5 mM IPTG at a cell density of 108 cells ml-1 and grown with shaking at 37°C for additional 2 h. The cells were harvested by centrifugation and sonicated in 50 mM phosphate buffer pH 8.0 containing 300 mM NaCl. Overproduced His6-tagged ParB protein was purified on Ni-agarose columns (Protino Ni-TED 1000, Macherey-Nagel) with an imidazole gradient in the same buffer. The quality of the purified protein was verified by SDS-PAGE using a Pharmacia PHAST gel system.
DNA-binding affinity assay
The electrophoretic mobility shift assay (EMSA) was performed according to Ringgaard et al. [74]. 6 pmoles of the double-stranded oligonucleotide labeled with the fluorescent dye (Cy3 or Cy5) were incubated with increasing quantities of His6-ParB protein in the presence of 18 pmoles of non-specific ds oligonucleotides as a competitor DNA in binding buffer (10 mM Tris-HCl pH 7.5, 0.5 mM dithiothreitol, 50 mM KCl, 1 mM MgCl2) [75] in a total volume of 20 μl. In dissociation experiments the constant amount of 240 pmoles of His6-ParB was added to 6 pmoles of fluorescently labeled ds oligonucleotides and increasing amounts of unlabeled ds parS2 oligonucleotide (18, 60, 90, 120, 180 pmoles, respectively). After 15 min incubation at 37°C the samples were separated on 5% polyacrylamide gels in 0.5 x Tris-borate-EDTA buffer (TBE) [70]. The DNA was visualized using FluorChemQ MultiImageIII ChemiImager and the images were captured using Alpha View software (Alpha Innotech).
ParB silencing test
E. coli DH5α strain was transformed with pGB2 [76] and its derivatives (with wt or mutated parS sequences inserted) selecting for SmR clones. The transformants cells were made competent and then transformed with an estimated 1 μg of either the pGBT30 (lacI q tacp) expression vector [77] or its derivative pKLB2 (lacI q tacp-parB). 100 μl of undiluted and serially diluted transformation mixtures were plated in repetitions on different selection plates. The selection was either for an incoming plasmid only (L-agar with Pn), for both resident and incoming plasmids (L-agar with Pn and Sm) or for both resident and incoming plasmids on plates supplemented with 0.5 mM IPTG to induce ParB production. The ratio of number of colonies on dual selection plates with IPTG versus the number of colonies on L-agar with Pn reflected the strength of ParB-parS binding and spreading on DNA (silencing ability).
Motility assay
For motility assays, P. aeruginosa PAO1161 derivatives strains were taken from a deep-frozen stock, spread on L-agar plates and grown overnight at 37°C. Then bacteria from single colonies were used to inoculate test plates with sterile toothpicks and such plates were incubated for 24 h at 37°C. For the swimming assay, tryptone plates (1% tryptone, 0.5% NaCl, 0.3% agar) were used; for the swarming test, plates containing 0.5% Bacto agar and supplemented with 5 g l-1 of dextrose and 8 g l-1 of nutrient broth were inoculated [78]. All sets of plates were standardized by using the same volume of medium. Independent assays were repeated at least three times each strain with wt PAO1161, PAO1161 parA null and PAO1161 parB null mutants as the control strains.
Colony morphology
Colonies of P. aeruginosa strains were observed after 24 h incubation on L-agar plates at 37°C using stereomicroscope Nikon SMZ1500, and images were captured with NIS-Elements 2.10 software.
Preparation of anti-ParB antiserum
For immunofluorescence microscopy, rabbit anti-ParB antibodies [9] were affinity purified as described previously [79]. Affi-Gel 10 (Bio-Rad) was used as the support for the purified ParB in 20 μl columns made of protein gel loading tips.
Fluorescence microscopy (DAPI staining and immunofluorescence)
Wild-type P. aeruginosa PAO1161, parA null, parB null, and parS mutants were grown in L-broth. At an OD600 of 0.4 the cells were collected and used to prepare microscopic slides. Fixing and permeabilization of cells and subsequent 4,6-diamidino-2-phenylindole (DAPI) staining were carried out as described previously [55]. A coverslip was placed on a slide with a 1:4 (v/v) solution of DAPI (1 μg ml-1) and Vectashield (mounting medium, Vector Laboratories). Cells were studied with Carl Zeiss Axio Imager.M2 utilizing lens EC Plan-Neofluar 100x/ 1.30 Oil ph 3 M27 and camera AxioCamMR5. The pictures were captured and analyzed with the AxioVision Rel.4.8.2 program (Carl Zeiss). Affinity-purified anti-ParB antibodies (40 μl) were used as the primary antibodies (1:100 dilution in 2% [w/v] bovine serum albumin–PBS), followed by 40 μl of anti-rabbit immunoglobulin G (IgG) conjugated to fluorescein isothiocyanate (FITC) (6.9 μg ml-1 in 2% [wt/vol] bovine serum albumin–PBS) (Sigma). The images were analyzed as described above.
Results
Cloning of mutated parS alleles
Bioinformatic analysis of PAO1 genome [69] has predicted that parS4 is located in an intergenic region (Fig. 1B) whereas nine other parSs are most likely situated within coding sequences. To mutagenize PAO1161, the laboratory strain originated from PAO1, we decided to use deletion to disable parS4 while for the other sites as many point mutations as possible were introduced without altering the coding sequence. For parS4 deletion, two fragments of approximately 250 nt each corresponding to the parS4 flanking sequences were linked in the multi-copy narrow-host-range plasmid pAKE600 [72] to obtain pPJB14 (S2 Table). DNA fragments of approximately 500 nt encompassing each of the other nine parS sites were PCR-amplified on PAO1161 DNA using appropriate pairs of primers (S3 Table), cloned into pAKE600 and obtained plasmids (S2 Table) were subjected to PCR-based site-directed mutagenesis. Each pair of mutagenic primers introduced several nucleotide substitutions into a given parS and simultaneously destroyed or created a new restriction site to facilitate screening (Table 1). The introduced changes in the parS inserts were confirmed by DNA sequencing.
In vivo ParB binding to the modified parS sequences
Before introducing the modified parS alleles into P. aeruginosa genome, we verified whether the introduced substitutions affected the ParB binding affinity towards those sites.
Upon binding to parS, P. aeruginosa ParB can (as can also plasmidic ParB representatives of group IA) spread along the DNA, thereby silencing the adjacent promoters [9, 66, 68, 80]. It has been demonstrated [9] that when parS is cloned upstream of the repA gene in the test plasmid pGB2 [76] and ParB excess is supplied from a compatible vector, the plasmid is lost when not selected for. Thus, native parSs and their mutated versions were cloned into pGB2 and obtained plasmids were introduced to E. coli DH5α strain. The SmR transformants of DH5α carrying the pGB2 derivatives were then transformed with expression vectors: pGBT30 (lacI q tacp) [77] or its derivative pKLB2 (lacI q tacp-parB) [9]. Double transformants were selected either for the incoming plasmid (plates with Pn), for both the incoming and resident plasmids (plates with Sm and Pn), or for both plasmids under conditions of ParB overproduction (plates with Sm, Pn and 0.5 mM IPTG). When DH5α (pGB2) with no parS cloned was transformed with either pGBT30 or pKLB2, the numbers of colonies were similar regardless of the type of selection plates applied. No incompatibility was observed between pGB2 derivatives containing diverse parS sequences and the empty expression vector pGBT30 either (data not shown). However, when DH5α (pGB2-parS2/par3) or DH5α (pGB2-parS1/parS4) strains (plasmids pABB812 and pABB822, respectively, S2 Table) were transformed with pKLB2, the number of transformants selected on the double selection plates with IPTG was approximately 104-fold lower than the number of transformants growing on plates with Pn only (Fig. 2). This confirmed the previously described [9] inability of pGB2 carrying a perfect palindrome (parS2/parS3) or a palindrome with one mismatch (parS1/parS4) to replicate in the presence of ParB excess. When DH5α (pGB2-parS1*), DH5α (pGB2-parS2*) or DH5α (pGB2-parS3*) strains (with plasmids pPJB27, pPJB28 or pPJB29, respectively, S2 Table) were transformed with pKLB2 the number of transformants growing under double selection in the presence or absence of ParB excess was the same (Fig. 2). This indicated that those three mutated versions of parS sites were not bound by ParB or were bound with too low affinity to block ParB binding/spreading, hence no plasmid loss in the “silencing test” was observed.
Fig 2. ParB silencing test for wild type and mutated versions of parS.
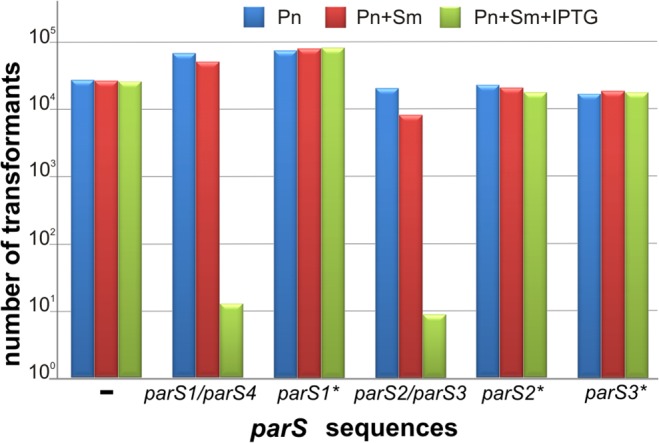
E. coli DH5α transformants carrying pGB2 derivatives with individual wt parS sequences or their mutated versions were transformed with pKLB2 (tacp-parB P.a.). Three independent transformation experiments were conducted and representative results are demonstrated. Undiluted transformation mixtures and their 10- and 100-fold dilutions were plated on three types of selection plates. Numbers of colonies growing on L-agar plates with Pn (blue bar), Pn and Sm (red bar), and double selection plates with 0.5 mM IPTG (green bar) are shown for the undiluted samples.
Similar transformation experiments (“silencing tests”) were carried out for pGB2 derivatives with native parS5 to parS10 insertions (plasmids pPJB22 to pJB26, S2 Table). The number of colonies of DH5α (pGB2-parS derivative) (pLKB2) strains when plated on L agar with Pn, Sm and 0.5 mM IPTG was slightly lower (2- to 5-fold) than number of colonies selected on L-agar with Pn, for the incoming plasmid only (data not shown). Such weak “silencing” effect of ParB binding to the wild-type parS sites containing two mismatches (parS5 to parS10) indicated that in vivo assay would not be sufficiently sensitive to analyze putative differences in ParB affinity between this group of wt parSs and their mutated versions.
In vitro ParB binding to wild-type and modified parS sequences and the hierarchy of wt parS sites
To analyze the effect of the mutations introduced in parS sequences on ParB binding, an in vitro EMSA test with fluorescently labeled ds oligonucleotides and His6-tagged ParB was performed.
Pairs of differently labeled ds oligonucleotides representing each wt parS and its mutated version were used in the EMSA. An unrelated ds oligonucleotide was used as a control to test for unspecific DNA binding by ParB. His6-ParB protein bound specifically to parS2/parS3 oligonucleotide whereas at the same range of concentrations it did not form specific complexes with the control ds oligonucleotide (S1 Fig.). At ParB concentrations >5 μM, non-specific interactions with the control fragment produced smearing. To minimize non-specific binding, a competitor DNA was added in further experiments.
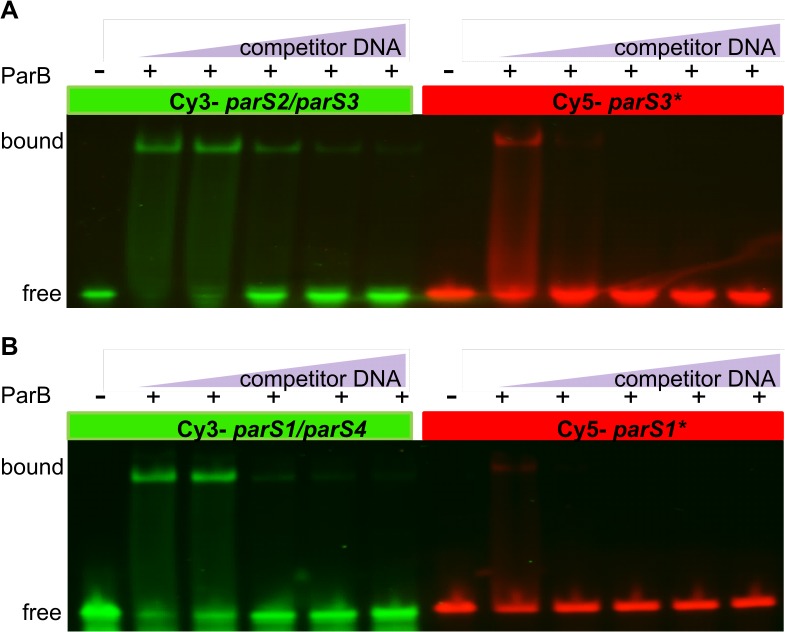
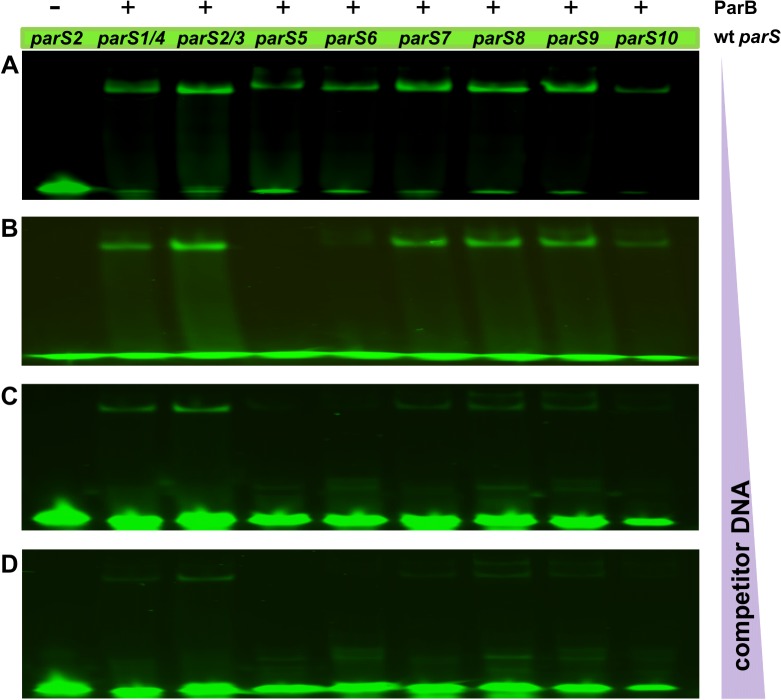
Specific ParB binding was detected for each of the wt parS sequences tested (data not shown). To compare affinity of ParB towards a wt parS and its mutated version, ParB was outcompeted from the respective complex by an excess of unlabelled wt parS2/parS3 oligonucleotide. The results for parS2/parS3 vs. parS3* and parS1 vs. parS1* are shown in Fig. 3, and for parS5—parS10 and their mutated versions in S2 Fig. This assay showed that the modified variants of almost all parS sequences tested were bound by ParB with an affinity 2- to 10-fold lower than were their wt counterparts. Only for the parS5, parS5* pair no significant difference in the ParB affinity was observed. A combination of assays for the ParB binding affinity towards different wt parS sequences and out-competing ParB from the complexes formed by wt parS2/parS3 (Fig. 4) allowed us to establish the hierarchy of in vitro ParB binding to various wt parS sequences as follows: parS2/parS3 > parS1/parS4> parS(7–8–9)> parS10> parS6> parS5.
Fig 3. ParB binds modified parS sites with lower affinity than the wild-type counterparts.
Differently labelled wt and their mutated versions were used in EMSA (A) parS2/3 vs. parS3* and (B) parS1 vs. parS1*. Six pmoles of labelled nucleotides were incubated with 240 pmoles of His6-ParB and increasing amounts (18, 60, 90, 120, 180 pmoles) of unlabelled ds parS2 oligonucleotide used as competitor DNA. After incubation at 37°C for 15 min, the complexes were separated on a native 5% polyacrylamide gel in TBE buffer, the DNA was visualized with FluorChemQ MultiImageIII ChemiImager and the images were captured using AlphaView software (Alpha Innotech).
Fig 4. The hierarchy of ParB binding to parS sequences.
Fluorescently labelled ds parS oligonucleotides (6 pmoles) were incubated with 240 pmoles of His6-ParB and increasing amounts of unlabelled ds parS2 as competitor: (A) 18 pmoles (B) 60 pmoles (C) 90 pmoles and (D) 180 pmoles. Complexes were visualized as described in Fig. 3.
Construction of a library of PAO1161 parS mutants
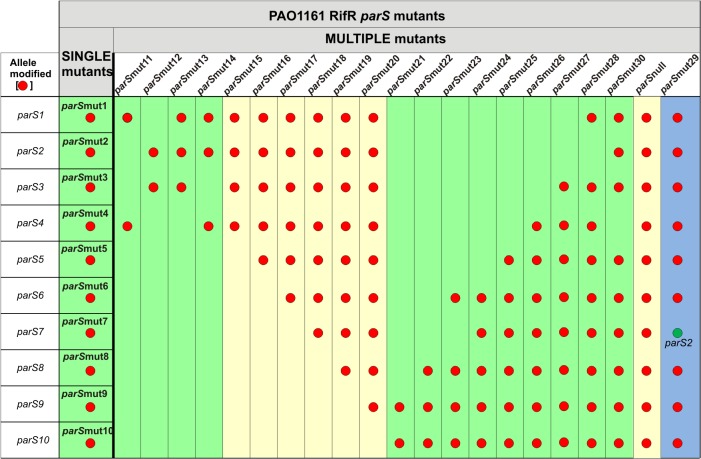
The in vitro tests indicated that, with the exception of parS5, the introduced nucleotide substitutions (although limited in number) weakened the ParB binding. All ten pAKE600 derivatives bearing mutated parS sequences were introduced separately into E. coli S17–1 and the transformants were used as donors in conjugation with PAO1161 RifR [54]. Since pAKE600 derivatives are incapable of replication in Pseudomonas, selection for transconjugants could only pick up cells with the plasmid integrated into the chromosome by homologous recombination using the provided regions of homology (parS flanks). The replacement of the chromosomal wild-type parS sequences in the P. aeruginosa chromosome by their modified counterparts was verified by sequencing. With this approach a set of ten mutants with each single parS site modified was obtained (PAO1161 parSmut1- parSmut10). To construct multiple parS mutants we used PAO1161 parSmut1 or parSmut10 as starting strains in which all other parS sequences were mutated sequentially using the same allele exchange approach, until the parS null strain with all ten sites modified was obtained (Fig. 5, S1 Table). Additionally, mutant strains PAO1161 parSmut12, parSmut13 and parSmut30 were constructed to address the requirement for the perfect (parS2 and parS3) or nearly perfect (parS1 and parS4) palindromes (Fig. 5). Finally, to study the effect of the genomic location of the perfectly palindromic parS sequence parS2/parS3 was inserted into the mutated parS7 site (opposite oriC) in the parS null mutant to obtain PAO1161 parSmut29 (parS null parSmut7::parS2). The thirty one PAO1161 derivatives, 10 single and 21 multiple parS mutants (Fig. 5, S1 Table), together with the parental strain, PAO1161 parB null and PAO1161 parA null mutant strains as controls were analyzed.
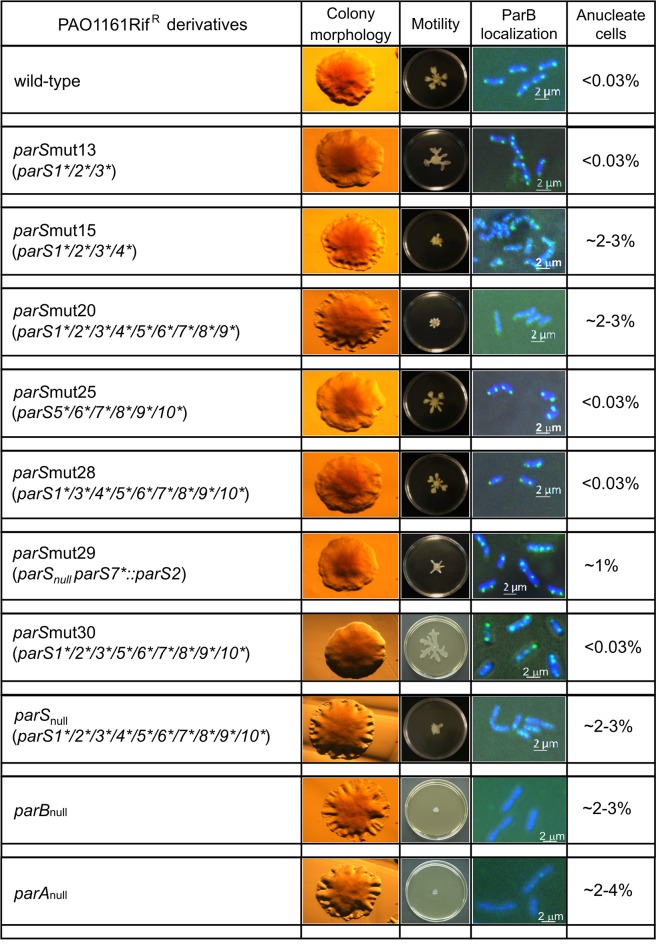
Fig 5. Summary of phenotypes of various parS mutants.
The collection comprises 10 mutants with each individual parS modified (single) and 21 multiple mutants with between two and ten (parS null) parS sequences modified (red dots). Mutants in columns highlighted in green demonstrate wild-type phenotypes (wt-like), those highlighted in yellow show phenotypes similar to those observed for the parA null and parB null mutants (parAB-like). The PAO1161 RifR parSmut29 mutant has ectopic parS2 (green dot) replacing parS7* in parS null mutant (highlighted in blue).
Phenotypic analysis of parS mutants
The phenotypic characterization of the PAO1161 derivatives constructed was based on the previous analysis of the parA and parB mutants. Both parA null and parB null mutants of PAO1161 demonstrated a slower growth rate (10% increase in the division time), produced slightly elongated cells with up to 7% anucleate cells (during growth on rich medium), and many more cells with aberrant chromosome separation, formed wrinkled colonies and were defective in two types of motility, swarming and swimming [54, 55]. Hence, all thirty one parS mutants were tested for these traits and additionally, using immunofluorescence, for the ability of ParB to form foci and their subcellular localization. Since the mutants’ profiles fell into two main categories, the results are presented only for chosen parS mutants in Fig. 6. The first group of mutants (class wt-like, highlighted in green in Fig. 5) did not differ phenotypically from the parental strain PAO1161. The second group of parS mutants (class parAB-like, highlighted in yellow in Fig. 5) were similar in many aspects to the parA and parB mutants.
Fig 6. Phenotypes of selected PAO1161 parS mutants.
Colony morphology, swarming motility, intracellular ParB localization with the use of FITC-conjugated anti-ParB antibodies, and proportion of anucleate cells after DAPI staining are shown for representative mutant strains. As the controls wt PAO1161 RifR and parA null and parB null mutants were tested in each set of experiments. The percentage of anucleate cells is the mean from at least three independent experiments, with approximately 1000 cells counted in each experiment.
All single parS mutants belonged to the first group, as did multiple mutants derived from PAO1161 parSmut10 (parSmut21 to parSmut28), represented by PAO1161 parSmut25 in Fig. 6. The PAO1161 parSmut28 with all parS sites modified except parS2 and PAO1161 parSmut30 with all parS sites modified except parS4 had the wild-type phenotypes (Fig. 5 and Fig. 6). Notably, mutation of parS2 in PAO1161 parSmut28 leading to PAO1161 parS null strain impaired chromosome segregation, slowed the growth rate, affected motility and colony formation, and caused diffusion of the immunofluorescence signal of ParB (Figs. 5 and 6). In the second set of multiple parS mutants constructed in the background of PAO1161 parSmut1 (or parSmut2), neither double mutants in the sites closest to oriC (PAO1161 parSmut11 and PAO1161 parSmut12) nor triple mutants (PAO1161 parSmut13 and PAO1161 parSmut14) differed from the parental strain in the tests conducted (Fig. 5). The mutant phenotype appeared when all four parS1 to parS4 sites were modified, as in the quadruple mutant PAO1161 parSmut15 and all its derivatives, PAO1161 parSmut16 to parSmut20 (Figs. 5 and 6).
A comparison of two mutants with nine parS sequences mutated and a single parS2 site, either present in the genome at its natural location (PAO1161 parSmut28) or moved to the oriC-distal position of parS7 (PAO1161 parSmut29), revealed different phenotypes as PAO1161 parSmut29 strain with parS2 at the ectopic position, only partially reversed defects of the parS null strain. Restored swimming ability (data not shown), slightly improved swarming and wild type colony morphology, and compaction of ParB on the genome into one to four foci regularly distributed in the cell (Fig. 6), indicated the restoration of some ParB functions. However, the percentage of anucleate cells was still higher and the growth rate lower (data not shown) than for wt strain or PAO1161 parSmut28, indicating imperfect chromosome segregation. One should note here that introduction of parS2/parS3 into the parS7 site affected the coding sequence of a gene of unknown function, pa3071, changing two amino acid residues (E85V and R87H) in its product, but it seems unlikely that the observed defects, typical for mutants with a disturbed par system, resulted from those changes.
Discussion
Chromosomal parABS systems participate not only in chromosome segregation but also in other fundamental processes: DNA replication, nucleoid condensation, cell division, coordination of chromosome segregation and cytokinesis, cell-to-cell communication, and motility [36]. Numerous studies have been conducted to elucidate the involvement of both Par proteins in these diverse cell functions. Here we aimed to characterize the third element of the system, the ParB-binding centromere-like parS sequence, in particular the roles of multiple parS motifs in the bacterial chromosome.
We analyzed the ParB affinity for all ten putative parS sequences previously identified [9] in the genome of P. aerugionosa PAO1 [69], the parental strain of the laboratory derivative PAO1161 (Fig. 1A). Eight of these sites are located within the ori domain (20% of the chromosome around oriC), with four clustered <16 kb from the origin of replication (parS1-parS4). The remaining two parS sequences, parS7 and parS8, are located opposite oriC on the circular chromosome of PAO1 (∼3 Mb away).
The in vitro test of ParB binding to ds oligonucleotides corresponding to the wt parS sequences confirmed that i/ ParB binds specifically to all ten predicted parS sequences and ii/ there is a hierarchy of ParB binding related to the degree of divergence of the palindromic parS structure and the position of the mismatched nucleotides. ParB preferentially binds the perfect palindromes parS2 and parS3, then with a slightly lower affinity parS1 and parS4 with a single mismatched pair of nucleotides. The remaining six parS sequences containing various double mismatches are clearly ordered. The parS7, parS8 and parS9 sequences that have one arm of the palindrome identical with the parS2/parS3 sequence and two diverged nucleotides in the other arm are bound with a higher affinity than are parS6 and parS5, in which both arms differ from parS2/parS3 (Table 1). These data also suggest that an intact core of the palindrome (CACGTG) is important for the interactions with ParB since i/ parS10 with two mutations in one arm within the central core demonstrates a lower binding affinity than parS7, parS8 and parS9 and ii/ parS5 diverged in the core part of both palindromic arms is bound by ParB with the lowest affinity.
Although studies on Spo0J (ParB homologue) binding to ten parS sequences in the B. subtilis genome have revealed high and low affinity sites [63] as well as an asymmetry of Spo0J spreading [8], no correlation between nucleotide sequence and Spo0J affinity or direction of the spreading could be observed.
We analyzed the P. aeruginosa parS sequences in terms of 8-bp half sites and generated a sequence logo (Fig. 1C) that is slightly different from the B. subtilis parS [8] especially in the number and location of invariant positions. In P. aeruginosa only two positions are strictly conserved among the twenty parS half sites in contrast to four invariant nucleotides in B. subtilis parS. Additionally, the C at position 5 in the half site is invariant in P. aeruginosa but not in B. subtilis [8]. The significance of the observed species-specificity of chromosomal parS sequences awaits elucidation.
The ParB binding sites in the genome of PAO1161 were sequentially modified and phenotypes of the mutant strains were established to define the role of particular parS sequences in the biology of P. aeruginosa. Before introducing the mutated parS sequences into the PAO1161 chromosome effects of the nucleotide substitutions on ParB affinity were evaluated using two approaches: in vivo, by a test for ParB binding and spreading, so-called “transcriptional silencing” assay in a heterologous system [9], and in vitro, by a mobility shift assay with purified ParB. Whereas the silencing test clearly discriminated between wt and mutated versions of only parS1, parS2 and parS3, the in vitro test demonstrated a decreased ParB affinity towards all mutated parS variants versus their wt counterparts with the exception of parS5. The differences in ParB binding to parS5 and parS5* were difficult to establish due to the low ParB affinity for wt parS5.
For a detailed in vivo analysis of the role of individual parS sites a collection of strains with each single parS impaired (10 mutants) and with combinations of different mutated parS sequences (21 mutants) was constructed and analyzed. To the best of our knowledge, this is the first such comprehensive analysis of parS mutants. We assessed growth kinetics, colony morphology, motility, number of anucleate cells and the localization of ParB in the cells of the mutants. It turned out that the mutants fell into two categories, those with the wild type phenotypes (here referred to as wt-like), and those with phenotypes previously shown for parA and parB mutants [54–55] (here referred to as parAB-like). The defects of the latter group included slower growth (data not shown), at least a hundred-fold increased frequency of anucleate cells, dispersion of ParB foci (typical for parA null [54] and parB mutants producing defective protein [55, 68]), impaired motility (swimming defects in this group of mutants were less pronounced than the defects in swarming, data not shown), and altered colony morphology. Remarkably, mutations in any single parS sequence did not lead to an observable defect, demonstrating functional redundancy of the parSs (Figs. 5 and 6). Also strains with multiple parS sequences modified had a wt-like phenotype as long as at least one of four parS from the oriC proximal region (parS1-parS4) was intact (compare parSmut13 and parSmut14 with parSmut15 or parSmut28 and parSmut30 with parS null, Fig. 5 and Fig. 6). However, when all four oriC-proximal parSs were mutated, a typical parAB-like phenotype was obtained even though the remaining six parS sites were unaltered; additional mutations in any or all of those sites produced no visible changes of the parAB-like phenotype. Since overproduction of ParB results in the same defects as a lack of ParB [9, 55] one could argue that an excess of unbound ParB was responsible for the observed defects when the four “principal” parS sites were impaired. However, mutation of nine out of ten parS sites in PAO1161 parSmut28 and parSmut30, in which only the oriC-proximal parS2 and parS4, respectively, was left intact, still did not lead to the appearance of the parAB-like phenotype.
Since parS2 and parS3 sequences are identical as are parS1 and parS4 (Fig. 1B) and all four sites occupy top positions in the hierarchy of ParB binding in vitro, (even though parS1/parS4 binds ParB in vitro with a slightly lower affinity than parS2/parS3 as shown in Fig. 4), we conclude that i/ a single, high affinity ParB binding site from the cluster of four parS sequences closest to the ori domain (parS1-parS4) is necessary and sufficient for proper chromosome segregation and ii/ none of the remaining six parS sites can substitute for these four oriC-proximal sites in this function.
Since these four parS sequences that enable proper chromosome segregation are bound in vitro by ParB with the highest affinity, it was important to evaluate the role of the ParB binding strength versus the genomic context (localization) of the binding site. The perfect palindromic sequence parS2 was therefore inserted in an ectopic position, 3 Mb from oriC, into the PAO1161 parS null genome to give PAO1161 parSmut29 strain (Figs. 5 and 6). The strain with the ectopic parS2 produced 100-fold more anucleate cells and grew slightly slower than the wt strain, indicating disturbances in chromosome segregation similar to those in the parS null mutant. Notably, the PAO1161 parSmut29 strain had improved motility and colony morphology similar to wt strain. The single high affinity parS2 site in the genome of P. aeruginosa, regardless of its genome position was also sufficient for ParB to form between one and four foci, as is typical for wt strain, confirming the model of ParB-induced condensation of chromosomal DNA by a combination of spreading and bridging after binding to the single site and undergoing a conformational change [61–62]. However, our data for parSmut28, parSmut29 and parSmu30 (Figs. 5 and 6) clearly indicate that the formation of a condensed ParB-DNA complex around a single parS will promote accurate chromosome segregation only when the complex is formed close to oriC, that is, on a parS from the parS1-parS4 group in its native position.
The differences in functioning of parS2 in chromosome segregation, when either at its oriC proximal position or distal to oriC, may be related to the important role of ParB-parS nucleoprotein complex in spatial orientation and directing the newly replicated oriC regions to the opposite cell poles. The C. crescentus genome has a single parS locus (tandem parS sequences) adjacent to the parAB operon. It has been shown that moving the parS region 100–400 kb away from its original position close to oriC, but still in the ori domain, delayed the initiation of chromosome segregation until the parS region had been replicated [29].
A similar experiment was conducted for B. subtilis with the ectopic parS inserted close to the replication terminus in a genome deprived of the eight parS sites from the ori domain [30], among them all six high affinity sites for Spo0J (ParB). The B. subtilis Δ8parS strain produced 100-fold more anucleate cells than the wt strain. The ectopic insertion of parS in such a mutant caused major disturbances in nucleoid organization, chromosome segregation and its coordination with cell division (a further 10-fold increase in the frequency of anucleate cells and high proportion of cells with chromosomes guillotined by the cell division septum) [30]. Since in B. subtilis one of the parS proximal to the oriC (parS359) is the main loading platform for the SMC condensation complex, it was hypothesized that such gross defects in the ectopic mutant strain were due to inappropriate recruitment of SMC by the Spo0J-parS complex formed at the replication terminus site [30]. Further studies will elucidate whether also in P. aeruginosa ParB directs SMC complex loading and if so why the defects observed in PAO1161 parSmut29 are not as strong as those observed for B. subtilis.
While defective in chromosome segregation and growth the PAO1161 parSmut29 with the single ectopic parS2 demonstrated significantly improved motility and the wt colony morphology. This strongly suggests that various roles of ParB in the cell e.g., chromosome segregation versus regulation of gene expression may be related to its interactions with differently located parS sequences.
Conclusions
The analysis of our collection of P. aeruginosa parS mutants aided by in vitro binding studies has revealed a hierarchy of ParB binding to different sites and a crucial role of the parS sequences closest to the oriC in accurate chromosome segregation. It has also demonstrated that a single parS of these four at its natural location, but not when moved opposite oriC in the genome, is sufficient to support accurate chromosome segregation. The role of other ParB binding sites in the biology of P. aeruginosa awaits elucidation.
Supporting Information
Binding reactions contained 6 pmoles of 5’ Cy3-labelled parS2 oligonucleotide (annealed oligonucleotides #3 and #4, S3 Table) and increasing amounts of His6-ParB (0, 40, 80, 100 pmoles) in 20 μl of binding buffer. Cy5-labelled nonspecific oligonucleotide (annealed oligonucleotides #35 and #36) was used as a control. After incubation at 37°C for 15 min, the complexes were separated on a native 5% polyacrylamide gel in TBE buffer, the DNA was visualized with FluorChemQ MultiImageIII ChemiImager and the images were captured using AlphaView software (Alpha Innotech).
(TIF)
Six pmoles of fluorescently labelled ds oligonucleotides corresponding to wt (Cy3) and mutated version (Cy5) of parS were incubated with 240 pmoles of His6-ParB and increasing amounts (18, 60, 90, 120, 180 pmoles) of the unlabelled ds wt parS2 as competitor. (A) parS5 and parS5*; (B) parS6 and parS6*; (C) parS7 and parS7*; (D) parS8 and parS8*; (E) parS9 and parS9*; (F) parS10 and parS10*.
(TIF)
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
PJ was supported by the International PhD Projects Programme "Studies of nucleic acids and proteins—from basic to applied research" of the Foundation for Polish Science co-financed by the European Union—Regional Development Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gerdes K, Moller-Jensen J, Bugge Jensen R. Plasmid and chromosome partitioning: surprises from phylogeny. Mol Microbiol. 2000;37: 455–466. [DOI] [PubMed] [Google Scholar]
- 2. Gerdes K, Howard M, Szardenings F. Pushing and pulling in prokaryotic DNA segregation. Cell. 2010;141: 927–942. 10.1016/j.cell.2010.05.033 [DOI] [PubMed] [Google Scholar]
- 3. Hayes F. The partition system of multidrug resistance plasmid TP228 includes a novel protein that epitomizes an evolutionarily distinct subgroup of the ParA superfamily. Mol Microbiol. 2000;37: 528–541. [DOI] [PubMed] [Google Scholar]
- 4. Schumacher MA. Bacterial plasmid partition machinery: a minimalist approach to survival. Curr Opin Struct Biol. 2012;22: 72–79. 10.1016/j.sbi.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogasawara N, Yoshikawa H. Genes and their organization in the replication origin region of the bacterial chromosome. Mol Microbiol. 1992;6: 629–634. 10.1111/j.1365-2958.1992.tb01510.x [DOI] [PubMed] [Google Scholar]
- 6. Yamaichi Y, Niki H. Active segregation by the Bacillus subtilis partitioning system in Escherichia coli. Proc Natl Acad Sci USA. 2000;97: 14656–14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Livny J, Yamaichi Y, Waldor MK. Distribution of centromere-like parS sites in bacteria: insights from comparative genomics. J Bacteriol. 2007;189: 8693–8703. 10.1128/JB.01239-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Breier AM, Grossman AD. Whole-genome analysis of the chromosome partitioning and sporulation protein Spo0J (ParB) reveals spreading and origin-distal sites on the Bacillus subtilis chromosome. Mol Microbiol. 2007;64: 703–718. 10.1111/j.1365-2958.2007.05690.x [DOI] [PubMed] [Google Scholar]
- 9. Bartosik AA, Lasocki K, Mierzejewska J, Thomas CM, Jagura-Burdzy G. ParB of Pseudomonas aeruginosa: interactions with its partner ParA and its target parS and specific effects on bacterial growth. J Bacteriol. 2004;186: 6983–6998. 10.1128/JB.186.20.6983-6998.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koonin EV. A common set of conserved motifs in a vast variety of putative nucleic acid-dependent ATPases including MCM proteins involved in the initiation of eukaryotic DNA replication. Nucleic Acids Res. 1993;21: 2541–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leonard TA, Moller-Jensen J, Lowe J. Towards understanding the molecular basis of bacterial DNA segregation. Philos Trans R Soc Lond B Biol Sci. 2005;360: 523–535. 10.1098/rstb.2004.1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ptacin JL, Lee SF, Garner EC, Toro E, Eckart M, Comolli LR, et al. A spindle-like apparatus guides bacterial chromosome segregation. Nat Cell Biol. 2010;12: 791–798. 10.1038/ncb2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ptacin JL, Gahlmann A, Bowman GR, Perez AM, von Diezmann ARS, Eckart MR, et al. Bacterial scaffold directs pole-specific centromere segregation. Proc Natl Acad Sci USA. 2014;111: E2046–E2055. 10.1073/pnas.1405188111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang X, Llopis PM, Rudner DZ. Organization and segregation of bacterial chromosomes. Nat Rev Genet. 2013;14: 191–203. 10.1038/nrg3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee PS, Lin DC-H, Moriya S, Grossman AD. Effects of the chromosome partitioning protein Spo0J (ParB) on oriC positioning and replication initiation in Bacillus subtilis. J Bacteriol. 2003;185: 1326–1337. 10.1128/JB.185.4.1326-1337.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jakimowicz D, Zydek P, Kois A, Zakrzewska-Czerwinska J, Chater KF. Alignment of multiple chromosomes along helical ParA scaffolding in sporulating Streptomyces hyphae. Mol Microbiol. 2007;65: 625–641. 10.1111/j.1365-2958.2007.05815.x [DOI] [PubMed] [Google Scholar]
- 17. Umbarger MA, Toro E, Wright MA, Porreca GJ, Baù D, Hong S-H, et al. The three-dimensional architecture of a bacterial genome and its alteration by genetic perturbation. Mol Cell. 2011;44: 252–264. 10.1016/j.molcel.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Glaser P, Sharpe ME, Raether B, Perego M, Ohlsen K, Errington J. Dynamic, mitotic-like behavior of a bacterial protein required for accurate chromosome partitioning. Genes Dev. 1997;11: 1160–1168. 10.1101/gad.11.9.1160 [DOI] [PubMed] [Google Scholar]
- 19. Lin DC-H, Levin PA, Grossman AD. Bipolar localization of a chromosome partition protein in Bacillus subtilis. Proc Natl Acad Sci USA. 1997;94: 4721–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mohl DA, Gober JW. Cell cycle–dependent polar localization of chromosome partitioning proteins in Caulobacter crescentus. Cell. 1997;88: 675–684. [DOI] [PubMed] [Google Scholar]
- 21. Sharpe ME, Errington J. A fixed distance for separation of newly replicated copies of oriC in Bacillus subtilis: implications for co-ordination of chromosome segregation and cell division. Mol Microbiol. 1998;28: 981–990. [DOI] [PubMed] [Google Scholar]
- 22. Wu LJ, Errington J. A large dispersed chromosomal region required for chromosome segregation in sporulating cells of Bacillus subtilis. EMBO J. 2002;21: 4001–4011. 10.1093/emboj/cdf393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu LJ, Errington J. RacA and the Soj-Spo0J system combine to effect polar chromosome segregation in sporulating Bacillus subtilis. Mol Microbiol. 2003;49: 1463–1475. 10.1046/j.1365-2958.2003.03643.x [DOI] [PubMed] [Google Scholar]
- 24. Viollier PH, Thanbichler M, McGrath PT, West L, Meewan M, McAdams HH, et al. Rapid and sequential movement of individual chromosomal loci to specific subcellular locations during bacterial DNA replication. Proc Natl Acad Sci USA. 2004;101: 9257–9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fogel MA, Waldor MK. Distinct segregation dynamics of the two Vibrio cholerae chromosomes. Mol Microbiol. 2004;55: 125–136. 10.1111/j.1365-2958.2004.04379.x [DOI] [PubMed] [Google Scholar]
- 26. Fogel MA, Waldor MK. A dynamic, mitotic-like mechanism for bacterial chromosome segregation. Genes Dev. 2006;20: 3269–3282. 10.1101/gad.1496506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee PS, Grossman AD. The chromosome partitioning proteins Soj (ParA) and Spo0J (ParB) contribute to accurate chromosome partitioning, separation of replicated sister origins, and regulation of replication initiation in Bacillus subtilis. Mol Microbiol. 2006;60: 853–869. 10.1111/j.1365-2958.2006.05140.x [DOI] [PubMed] [Google Scholar]
- 28. Saint-Dic D, Frushour BP, Kehrl JH, Kahng LS. A ParA homolog selectively influences positioning of the large chromosome origin in Vibrio cholerae. J Bacteriol. 2006;188: 5626–5631. 10.1128/JB.00250-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toro E, Hong S-H, McAdams HH, Shapiro L. Caulobacter requires a dedicated mechanism to initiate chromosome segregation. Proc Natl Acad Sci USA. 2008;105: 15435–15440. 10.1073/pnas.0807448105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sullivan NL, Marquis KA, Rudner DZ. Recruitment of SMC by ParB-parS organizes the origin region and promotes efficient chromosome segregation. Cell. 2009;137: 697–707. 10.1016/j.cell.2009.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gruber S, Errington J. Recruitment of Condensin to replication origin regions by ParB/SpoOJ promotes chromosome segregation in B. subtilis. Cell. 2009;137: 685–696. 10.1016/j.cell.2009.02.035 [DOI] [PubMed] [Google Scholar]
- 32. Minnen A, Attaiech L, Thon M, Gruber S, Veening J-W. SMC is recruited to oriC by ParB and promotes chromosome segregation in Streptococcus pneumoniae. Mol Microbiol. 2011;81: 676–688. 10.1111/j.1365-2958.2011.07722.x [DOI] [PubMed] [Google Scholar]
- 33. Bowman GR, Comolli LR, Zhu J, Eckart M, Koenig M, Downing KH, et al. A polymeric protein anchors the chromosomal origin/ParB complex at a bacterial cell pole. Cell. 2008;134: 945–955. 10.1016/j.cell.2008.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ebersbach G, Briegel A, Jensen GJ, Jacobs-Wagner C. A self-associating protein critical for chromosome attachment, division, and polar organization in Caulobacter. Cell. 2008;134: 956–968. 10.1016/j.cell.2008.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schofield WB, Lim HC, Jacobs-Wagner C. Cell cycle coordination and regulation of bacterial chromosome segregation dynamics by polarly localized proteins. EMBO J. 2010;29: 3068–3081. 10.1038/emboj.2010.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mierzejewska J, Jagura-Burdzy G. Prokaryotic ParA–ParB–parS system links bacterial chromosome segregation with the cell cycle. Plasmid. 2012;67: 1–14. 10.1016/j.plasmid.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 37. Kadoya R, Baek JH, Sarker A, Chattoraj DK. Participation of chromosome segregation protein ParAI of Vibrio cholerae in chromosome replication. J Bacteriol. 2011;193: 1504–1514. 10.1128/JB.01067-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murray H, Errington J. Dynamic control of the DNA replication initiation protein DnaA by Soj/ParA. Cell. 2008;135: 74–84. 10.1016/j.cell.2008.07.044 [DOI] [PubMed] [Google Scholar]
- 39. Scholefield G, Whiting R, Errington J, Murray H. Spo0J regulates the oligomeric state of Soj to trigger its switch from an activator to an inhibitor of DNA replication initiation. Mol Microbiol. 2011;79: 1089–1100. 10.1111/j.1365-2958.2010.07507.x [DOI] [PubMed] [Google Scholar]
- 40. Yamaichi Y, Gerding MA, Davis BM, Waldor MK. Regulatory cross-talk links Vibrio cholerae chromosome ii replication and segregation. PLoS Genet. 2011;7: e1002189 10.1371/journal.pgen.1002189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Venkova-Canova T, Baek JH, FitzGerald PC, Blokesch M, Chattoraj DK. Evidence for two different regulatory mechanisms linking replication and segregation of Vibrio cholerae chromosome II. PLoS Genet. 2013;9: e1003579 10.1371/journal.pgen.1003579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ireton K, Gunther N, Grossman AD. Spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J Bacteriol. 1994;176: 5320–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Quisel JD, Grossman AD. Control of sporulation gene expression in Bacillus subtilis by the chromosome partitioning proteins Soj (ParA) and Spo0J (ParB). J Bacteriol. 2000;182: 3446–3451. 10.1128/JB.182.12.3446-3451.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim H-J, Calcutt MJ, Schmidt FJ, Chater KF. Partitioning of the linear chromosome during sporulation of Streptomyces coelicolor A3(2) involves an oriC-linked parAB locus. J Bacteriol. 2000;182: 1313–1320. 10.1128/JB.182.5.1313-1320.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jakimowicz D, Chater KF, Zakrzewska-Czerwinska J. The ParB protein of Streptomyces coelicolor A3 (2) recognizes a cluster of parS sequences within the origin-proximal region of the linear chromosome. Mol Microbiol. 2002;45: 1365–1377. [DOI] [PubMed] [Google Scholar]
- 46. Jakimowicz P, Cheesman MR, Bishai WR, Chater KF, Thomson AJ, Buttner MJ. Evidence that the Streptomyces developmental protein WhiD, a member of the WhiB family, binds a [4Fe-4S] cluster. J Biol Chem. 2005;280: 8309–8315. 10.1074/jbc.M412622200 [DOI] [PubMed] [Google Scholar]
- 47. Mohl DA, Easter J, Gober JW. The chromosome partitioning protein, ParB, is required for cytokinesis in Caulobacter crescentus. Mol Microbiol. 2001;42: 741–755. [DOI] [PubMed] [Google Scholar]
- 48. Autret S, Errington J. A role for division-site-selection protein MinD in regulation of internucleoid jumping of Soj (ParA) protein in Bacillus subtilis. Mol Microbiol. 2003;47: 159–169. [DOI] [PubMed] [Google Scholar]
- 49. Real G, Autret S, Harry EJ, Errington J, Henriques AO. Cell division protein DivIB influences the Spo0J/Soj system of chromosome segregation in Bacillus subtilis. Mol Microbiol. 2004;55: 349–367. 10.1111/j.1365-2958.2004.04399.x [DOI] [PubMed] [Google Scholar]
- 50. Thanbichler M, Shapiro L. MipZ, a spatial regulator coordinating chromosome segregation with cell division in Caulobacter. Cell. 2006;126: 147–162. 10.1016/j.cell.2006.05.038 [DOI] [PubMed] [Google Scholar]
- 51. Jakimowicz D, van Wezel GP. Cell division and DNA segregation in Streptomyces: how to build a septum in the middle of nowhere?. Mol Microbiol. 2012;85: 393–404. 10.1111/j.1365-2958.2012.08107.x [DOI] [PubMed] [Google Scholar]
- 52. Ginda K, Bezulska M, Ziolkiewicz M, Dziadek J, Zakrzewska-Czerwinska J, Jakimowicz D. ParA of Mycobacterium smegmatis co-ordinates chromosome segregation with the cell cycle and interacts with the polar growth determinant DivIVA. Mol Microbiol. 2013;87: 998–1012. 10.1111/mmi.12146 [DOI] [PubMed] [Google Scholar]
- 53. Ditkowski B, Holmes N, Rydzak J, Donczew M, Bezulska M, Ginda K, et al. Dynamic interplay of ParA with the polarity protein, Scy, coordinates the growth with chromosome segregation in Streptomyces coelicolor. Open Biol. 2013;3: 130006–130006. 10.1098/rsob.130006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lasocki K, Bartosik AA, Mierzejewska J, Thomas CM, Jagura-Burdzy G. Deletion of the parA (soj) homologue in Pseudomonas aeruginosa causes ParB instability and affects growth rate, chromosome segregation, and motility. J Bacteriol. 2007;189: 5762–5772. 10.1128/JB.00371-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bartosik AA, Mierzejewska J, Thomas CM, Jagura-Burdzy G. ParB deficiency in Pseudomonas aeruginosa destabilizes the partner protein ParA and affects a variety of physiological parameters. Microbiology. 2009;155: 1080–1092. 10.1099/mic.0.024661-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bartosik AA, Glabski K, Jecz P, Mikulska S, Fogtman A, Koblowska M, et al. Transcriptional profiling of parA and parB mutants in actively dividing cells of an opportunistic human pathogen Pseudomonas aeruginosa. PLoS ONE. 2014;9: e87276 10.1371/journal.pone.0087276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Baek JH, Rajagopala SV, Chattoraj DK. Chromosome segregation proteins of Vibrio cholerae as transcription regulators. mBio. 2014;5: e01061–14. 10.1128/mBio.01061-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bartosik AA, Glabski K, Jecz P, Lasocki K, Mikosa M, Plochocka D, et al. Dissection of the region of Pseudomonas aeruginosa ParA that is important for dimerization and interactions with its partner ParB. Microbiology. 2014;160: 2406–2420. 10.1099/mic.0.081216-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leonard TA, Butler PJ, Lowe J. Bacterial chromosome segregation: structure and DNA binding of the Soj dimer—a conserved biological switch. EMBO J. 2005;24: 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Murray H, Ferreira H, Errington J. The bacterial chromosome segregation protein Spo0J spreads along DNA from parS nucleation sites. Mol Microbiol. 2006;61: 1352–1361. 10.1111/j.1365-2958.2006.05316.x [DOI] [PubMed] [Google Scholar]
- 61. Broedersz CP, Wang X, Meir Y, Loparo JJ, Rudner DZ, Wingreen NS. Condensation and localization of the partitioning protein ParB on the bacterial chromosome. Proc Natl Acad Sci USA. 2014;111: 8809–8814. 10.1073/pnas.1402529111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Graham TGW, Wang X, Song D, Etson CM, van Oijen AM, Rudner DZ, et al. ParB spreading requires DNA bridging. Genes Dev. 2014;28: 1228–1238. 10.1101/gad.242206.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lin DC-H, Grossman AD. Identification and characterization of a bacterial chromosome partitioning site. Cell. 1998;92: 675–685. [DOI] [PubMed] [Google Scholar]
- 64. Dubarry N, Pasta F, Lane D. ParABS systems of the four replicons of Burkholderia cenocepacia: new chromosome centromeres confer partition specificity. J Bacteriol. 2006;188: 1489–1496. 10.1128/JB.188.4.1489-1496.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamaichi Y, Fogel MA, McLeod SM, Hui MP, Waldor MK. Distinct centromere-like parS sites on the two chromosomes of Vibrio spp. J Bacteriol. 2007;189: 5314–5324. 10.1128/JB.00416-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lobocka M, Yarmolinsky M. P1 plasmid partition: a mutational analysis of ParB. J Mol Biol. 1996;259: 366–382. [DOI] [PubMed] [Google Scholar]
- 67. Mierzejewska J, Bartosik AA, Macioszek M, Plochocka D, Thomas CM, Jagura-Burdzy G. Identification of C-terminal hydrophobic residues important for dimerization and all known functions of ParB of Pseudomonas aeruginosa. Microbiology. 2012;158: 1183–1195. 10.1099/mic.0.056234-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kusiak M, Gapczynska A, Plochocka D, Thomas CM, Jagura-Burdzy G. Binding and spreading of ParB on DNA determine its biological function in Pseudomonas aeruginosa. J Bacteriol. 2011;193: 3342–3355. 10.1128/JB.00328-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406: 959–964. [DOI] [PubMed] [Google Scholar]
- 70. Sambrook J, Fritsch EF, Maniatis T. Molecular cloning:a laboratory manual, 2nd edition Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 71. Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51 Pt 1: 263–273. [DOI] [PubMed] [Google Scholar]
- 72. El-Sayed AK, Hothersall J, Thomas CM. Quorum-sensing-dependent regulation of biosynthesis of the polyketide antibiotic mupirocin in Pseudomonas fluorescens NCIMB 10586. Microbiology. 2001;147: 2127–2139. [DOI] [PubMed] [Google Scholar]
- 73. Irani VR, Rowe JJ. Enhancement of transformation in Pseudomonas aeruginosa PAO1 by Mg2+ and heat. Biotechniques. 1997;22: 54–6. [DOI] [PubMed] [Google Scholar]
- 74. Ringgaard S, Lowe J, Gerdes K. Centromere pairing by a plasmid-encoded type I ParB protein. J Biol Chem. 2007;282: 28216–28225. 10.1074/jbc.M703733200 [DOI] [PubMed] [Google Scholar]
- 75. Ringgaard S, Ebersbach G, Borch J, Gerdes K. Regulatory cross-talk in the double par locus of plasmid pB171. J Biol Chem. 2007;282: 3134–3145. 10.1074/jbc.M609092200 [DOI] [PubMed] [Google Scholar]
- 76. Churchward G, Belin D, Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984;31: 165–171. 10.1016/0378-1119(84)90207-5 [DOI] [PubMed] [Google Scholar]
- 77. Jagura-Burdzy G, Ibbotson JP, Thomas CM. The korF region of broad-host-range plasmid RK2 encodes two polypeptides with transcriptional repressor activity. J Bacteriol. 1991;173: 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rashid MH, Kornberg A. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2000;97: 4885–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Resnekov O, Alper S, Losick R. Subcellular localization of proteins governing the proteolytic activation of a developmental transcription factor in Bacillus subtilis. Genes Cells. 1996;1: 529–542. 10.1046/j.1365-2443.1996.d01-262.x [DOI] [PubMed] [Google Scholar]
- 80. Rodionov O, Lobocka M, Yarmolinsky M. Silencing of genes flanking the P1 plasmid centromere. Science. 1999;283: 546–549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Binding reactions contained 6 pmoles of 5’ Cy3-labelled parS2 oligonucleotide (annealed oligonucleotides #3 and #4, S3 Table) and increasing amounts of His6-ParB (0, 40, 80, 100 pmoles) in 20 μl of binding buffer. Cy5-labelled nonspecific oligonucleotide (annealed oligonucleotides #35 and #36) was used as a control. After incubation at 37°C for 15 min, the complexes were separated on a native 5% polyacrylamide gel in TBE buffer, the DNA was visualized with FluorChemQ MultiImageIII ChemiImager and the images were captured using AlphaView software (Alpha Innotech).
(TIF)
Six pmoles of fluorescently labelled ds oligonucleotides corresponding to wt (Cy3) and mutated version (Cy5) of parS were incubated with 240 pmoles of His6-ParB and increasing amounts (18, 60, 90, 120, 180 pmoles) of the unlabelled ds wt parS2 as competitor. (A) parS5 and parS5*; (B) parS6 and parS6*; (C) parS7 and parS7*; (D) parS8 and parS8*; (E) parS9 and parS9*; (F) parS10 and parS10*.
(TIF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.