Abstract
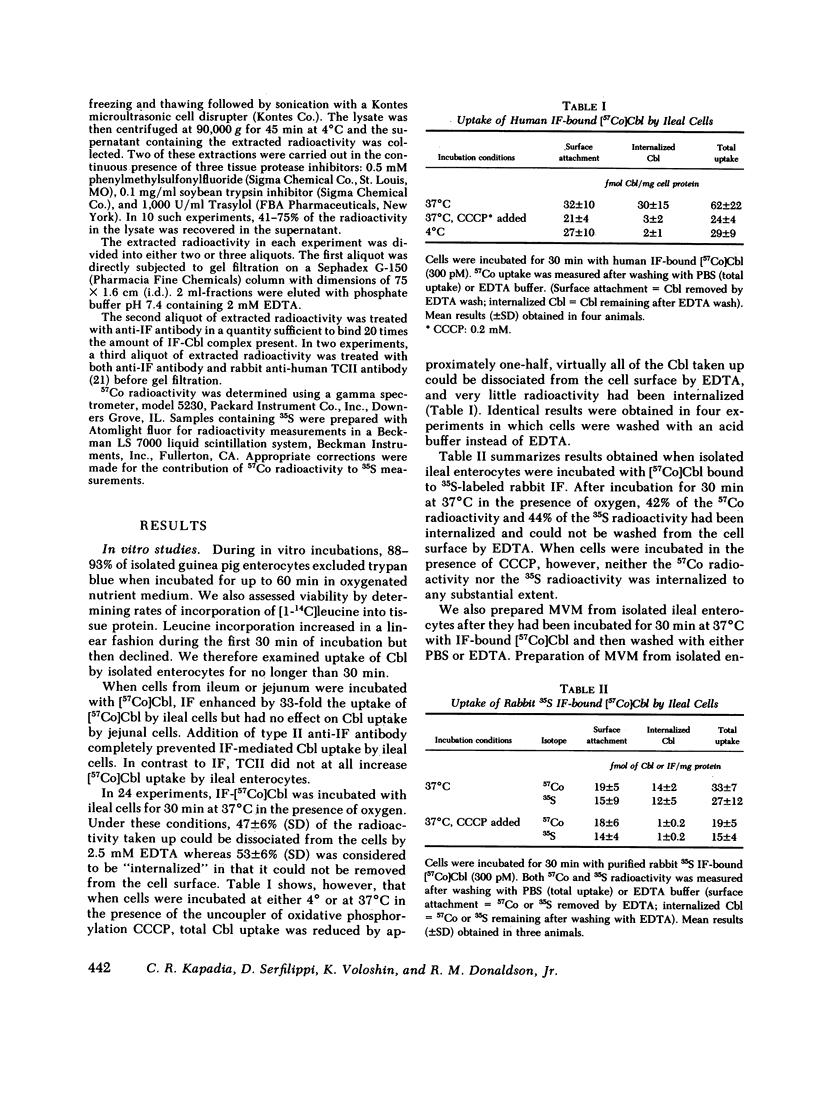
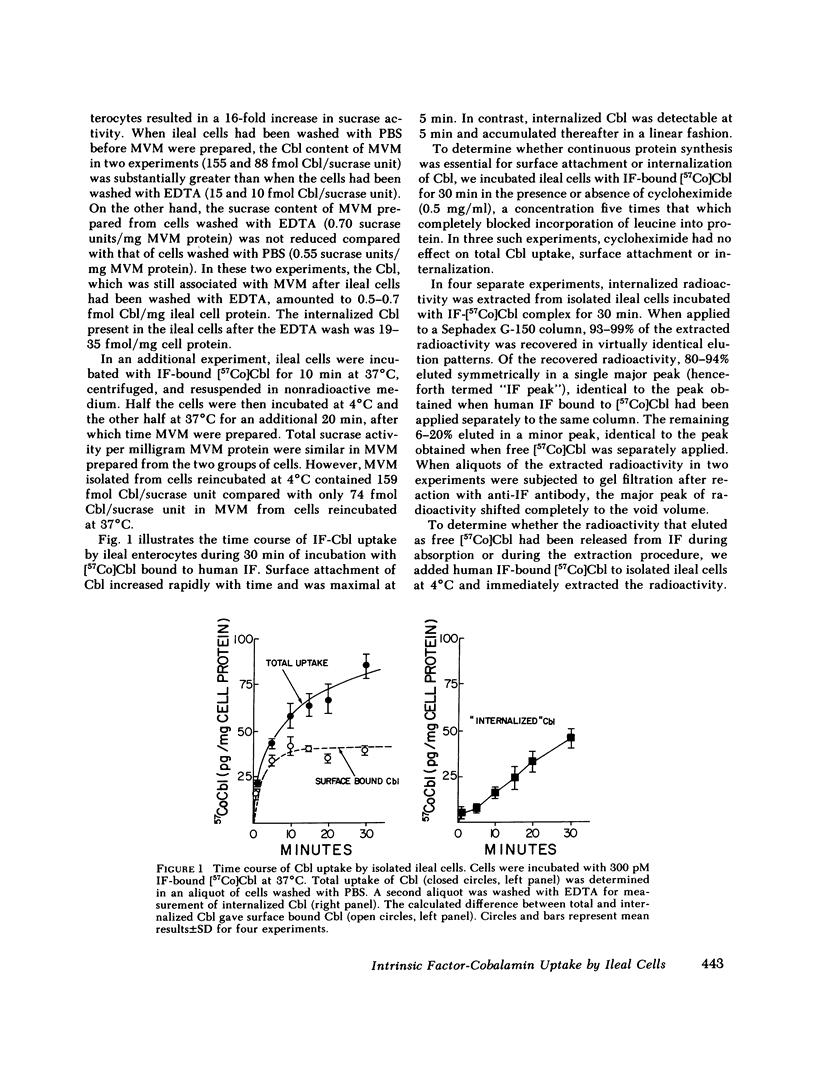
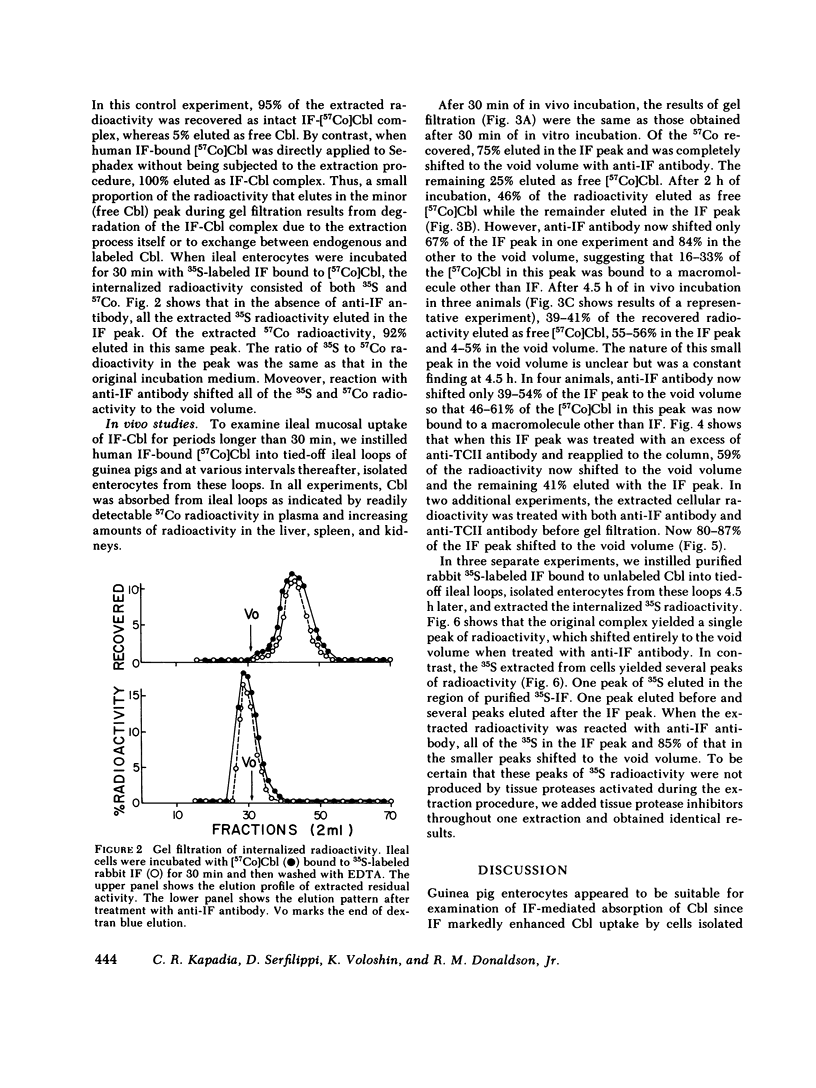
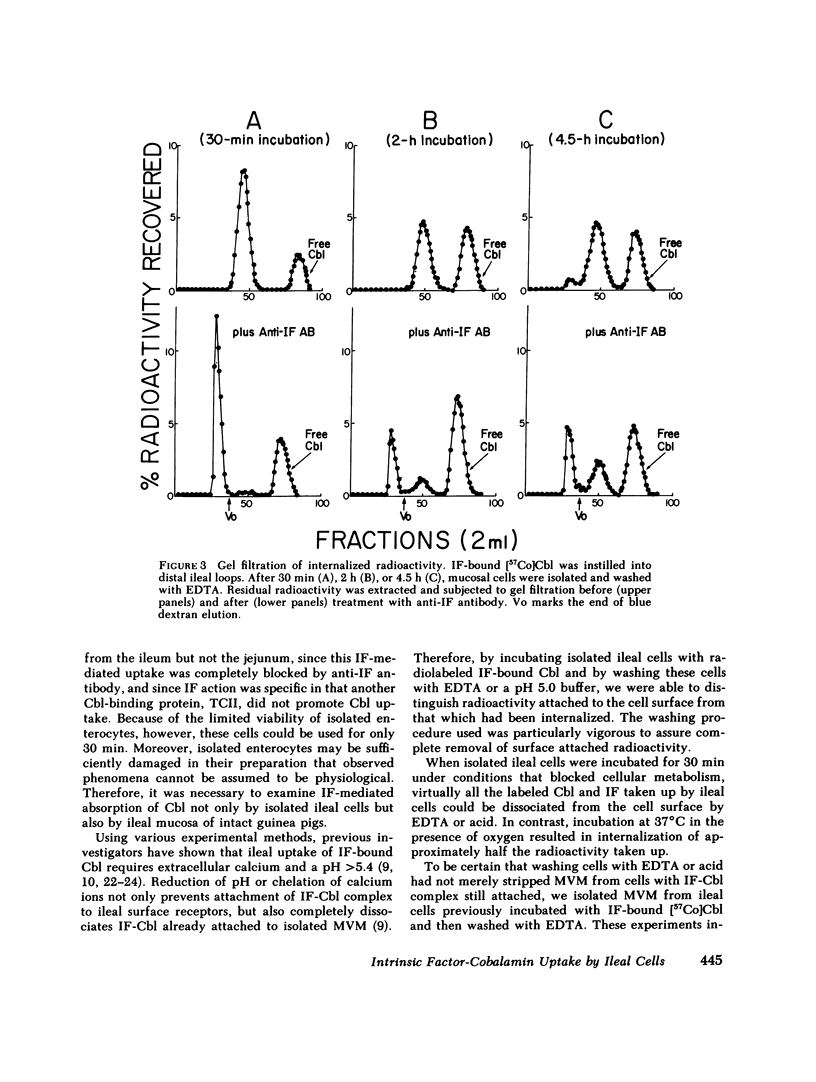
To investigate the fate of intrinsic factor and cobalamin during cobalamin absorption, we incubated enterocytes isolated from guinea pig ileum for periods of up to 30 min with 57Co-labeled cyano-cobalamin bound either to human intrinsic factor or to rabbit intrinsic factor biosynthetically labeled with [35S]methionine. When the labeled complex was incubated for 30 min with isolated ileal cells under conditions that block cellular metabolism, virtually all cellular radioactivity could be removed by washing the cell surface with EDTA or acid. In contrast, washing removed only half the radioactivity from cells incubated at 37°C in O2. When residual cellular radioactivity was extracted and analyzed by gel filtration, 80-94% of both the 35S and 57Co radioactivity eluted in the same fractions as the original complex. The remaining 6-20% eluted as free [57Co]cobalamin or [35S]methionine. To examine events occurring after 30 min, we instilled into tied-off ileal loops of intact guinea pigs radiolabeled intrinsic factor-cobalamin complex and extracted nondissociable radioactivity 2-4.5 h later. The proportion of extracted 57Co eluting as free cobalamin increased to 39-46%, that eluting as intrinsic factor-cobalamin complex declined to 22-45%, and 9-34% now eluted as a macromolecule that reacted with antitranscobalamin II antibody but not antiintrinsic factor antibody. Extracted 35S radioactivity eluted in several peaks in addition to the intrinsic factor peak. These findings suggest that (a) after reversible attachment of intrinsic factor-cobalamin complex to its ileal surface receptor, an energy-dependent process prevents removal of the complex from the cell surface by EDTA or acid; (b) cobalamin dissociates from intrinsic factor and, as suggested by previous workers, binds to a molecule antigenically similar to transcobalamin II; and (c) intrinsic factor is slowly degraded and forms breakdown products that are detectable in ileal extracts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. H., Majerus P. W. Isolation of vitamin B12-binding proteins using affinity chromatography. I. Preparation and properties of vitamin B12-sepharose. J Biol Chem. 1972 Dec 10;247(23):7695–7701. [PubMed] [Google Scholar]
- BOASS A., WILSON T. H. INTESTINAL ABSORPTION OF INTRINSIC FACTOR AND B12-INTRINSIC FACTOR COMPLEX. Am J Physiol. 1964 Jul;207:27–32. doi: 10.1152/ajplegacy.1964.207.1.27. [DOI] [PubMed] [Google Scholar]
- Bloomfield F. J., Scott J. M. Identification of a new vitamin B 12 binder (transcobalamin 3) in normal human serum. Br J Haematol. 1972 Jan;22(1):33–42. doi: 10.1111/j.1365-2141.1972.tb08784.x. [DOI] [PubMed] [Google Scholar]
- COOPER B. A., CASTLE W. B. Sequential mechanisms in the enhanced absorption of vitamin B12 by intrinsic factor in the rat. J Clin Invest. 1960 Jan;39:199–214. doi: 10.1172/JCI104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER B. A., PARANCHYCH W., LOWENSTEIN L. Studies on the absorption by guinea pig intestine of cyanocobalamin incubated with intrinsic factor. J Clin Invest. 1962 Feb;41:370–377. doi: 10.1172/JCI104491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER B. A. THE UPTAKE OF CO57-LABELED VITAMIN B12 BY EVERTED SACS OF INTESTINE IN VITRO. Medicine (Baltimore) 1964 Nov;43:689–696. doi: 10.1097/00005792-196411000-00012. [DOI] [PubMed] [Google Scholar]
- Chanarin I., Muir M., Hughes A., Hoffbrand A. V. Evidence for intestinal origin of transcobalamin II during vitamin B12 absorption. Br Med J. 1978 Jun 3;1(6125):1453–1455. doi: 10.1136/bmj.1.6125.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper B. A. Complex of intrinsic factor and B12 in human ileum during vitamin B12 absorption. Am J Physiol. 1968 Apr;214(4):832–835. doi: 10.1152/ajplegacy.1968.214.4.832. [DOI] [PubMed] [Google Scholar]
- DOSCHERHOLMEN A., HAGEN P. S. A dual mechanism of vitamin B12 plasma absorption. J Clin Invest. 1957 Nov;36(11):1551–1557. doi: 10.1172/JCI103552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- GOTTLIEBLAU K. S., WASSERMAN L. R., HERBERT V. RAPID CHARCOAL ASSAY FOR INTRINSIC FACTOR (IF), GASTRIC JUICE UNSATURATED B12 BINDING CAPACITY, ANTIBODY TO IF, AND SERUM UNSATURATED B12 BINDING CAPACITY. Blood. 1965 Jun;25:875–884. [PubMed] [Google Scholar]
- HERBERT V., CASTLE W. B. Divalent cation and pH dependence of rat intrinsic factor action in everted sacs and mucosal homogenates of rat small intestine. J Clin Invest. 1961 Nov;40:1978–1983. doi: 10.1172/JCI104423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERT V. Mechanism of intrinsic factor action in everted sacs of rat small intestine. J Clin Invest. 1959 Jan 1;38(1 Pt 1):102–109. doi: 10.1172/JCI103779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines J. D., Rosenberg A., Harris J. W. Intrinsic factor-mediated radio-B12 uptake in sequential incubation studies using everted sacs of guinea pig small intestine: evidence that IF in not absorbed into the intestinal cell. Proc Soc Exp Biol Med. 1968 Nov;129(2):653–658. doi: 10.3181/00379727-129-33390. [DOI] [PubMed] [Google Scholar]
- Katz M., O'Brien R. Vitamin B12 absorption studied by vascular perfusion of rat intestine. J Lab Clin Med. 1979 Dec;94(6):817–825. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine J. S., Nakane P. K., Allen R. H. Immunocytochemical localization of intrinsic factor--cobalamin bound to the guinea pig ileum in vivo. Gastroenterology. 1982 Feb;82(2):284–290. [PubMed] [Google Scholar]
- Mackenzie I. L., Donaldson R. M., Jr Effect of divalent cations and pH on intrinsic factor-mediated attachment of vitamin B 12 to intestinal microvillous membranes. J Clin Invest. 1972 Sep;51(9):2465–2471. doi: 10.1172/JCI107060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoullis G., Rothenberg S. P. Intrinsic factor-mediated intestinal absorption of cobalamin in the dog. Am J Physiol. 1981 Oct;241(4):G294–G299. doi: 10.1152/ajpgi.1981.241.4.G294. [DOI] [PubMed] [Google Scholar]
- Rothenberg S. P., Weisberg H., Ficarra A. Evidence for the absorption of immunoreactive intrinsic factor into the intestinal epithelial cell during vitamin B 12 absorption. J Lab Clin Med. 1972 Apr;79(4):587–597. [PubMed] [Google Scholar]
- Rothenberg S. P., Weiss J. P., Cotter R. Formation of transcobalamin II--vitamin B12 complex by guinea-pig ileal mucosa in organ culture after in vivo incubation with intrinsic factor--vitamin B12. Br J Haematol. 1978 Nov;40(3):401–414. doi: 10.1111/j.1365-2141.1978.tb05812.x. [DOI] [PubMed] [Google Scholar]
- Schneider R. J., Burger R. L., Mehlman C. S., Allen R. H. The role and fate of rabbit and human transcobalamin II in the plasma transport of vitamin B12 in the rabbit. J Clin Invest. 1976 Jan;57(1):27–38. doi: 10.1172/JCI108265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton D. R., Donaldson M., Jr Synthesis and secretion of protein and pepsinogen by rabbit gastric mucosa in organ culture. Gastroenterology. 1975 Jul;69(1):166–174. [PubMed] [Google Scholar]
- TAYLOR K. B., ROITT I. M., DONIACH D., COUCHMAN K. G., SHAPLAND C. Autoimmune phenomena in pernicious anaemia: gastric antibodies. Br Med J. 1962 Nov 24;2(5316):1347–1352. doi: 10.1136/bmj.2.5316.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UKYO S., COOPER B. A. INTRINSIC FACTOR-LIKE ACTIVITY IN EXTRACTS OF GUINEA PIG INTESTINE. Am J Physiol. 1965 Jan;208:9–13. doi: 10.1152/ajplegacy.1965.208.1.9. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Will P. C., Hopfer U. Apparent inhibition of active non-electrolyte transport by an increased sodium permeability of the plasma membrane. Mechanism of action of p-chloromercuribenzene sulfonate. J Biol Chem. 1979 May 25;254(10):3806–3811. [PubMed] [Google Scholar]


