Abstract
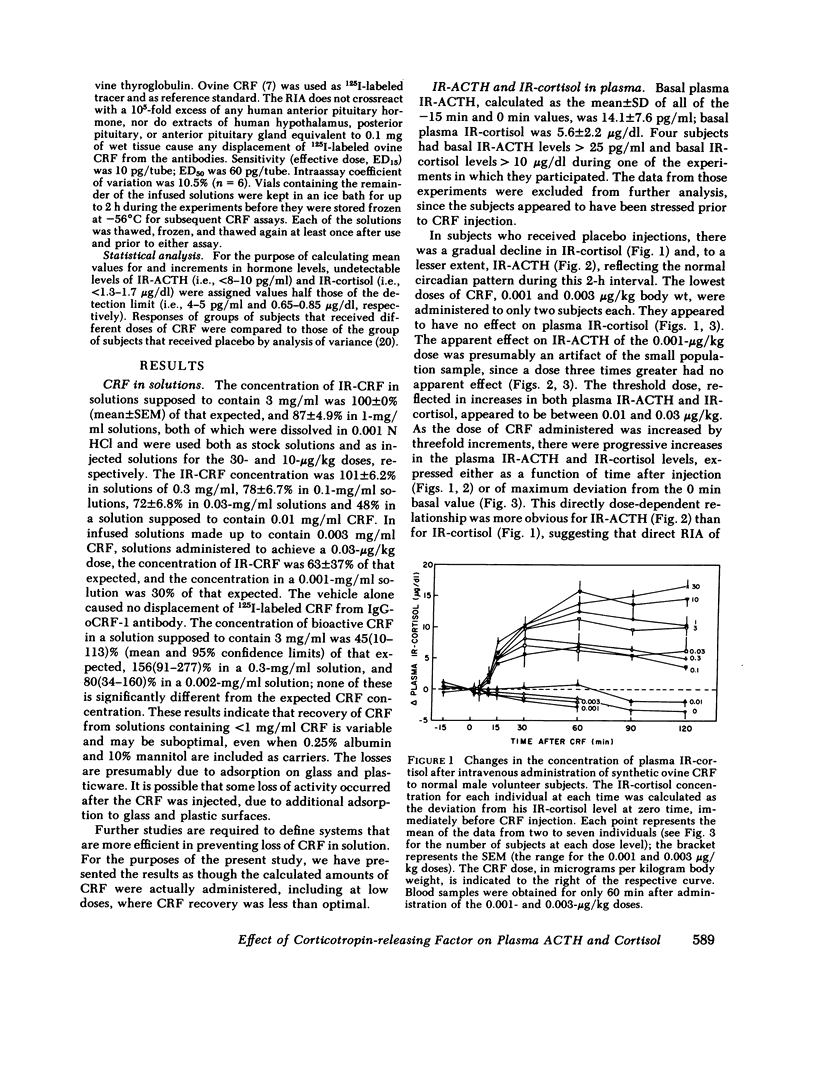
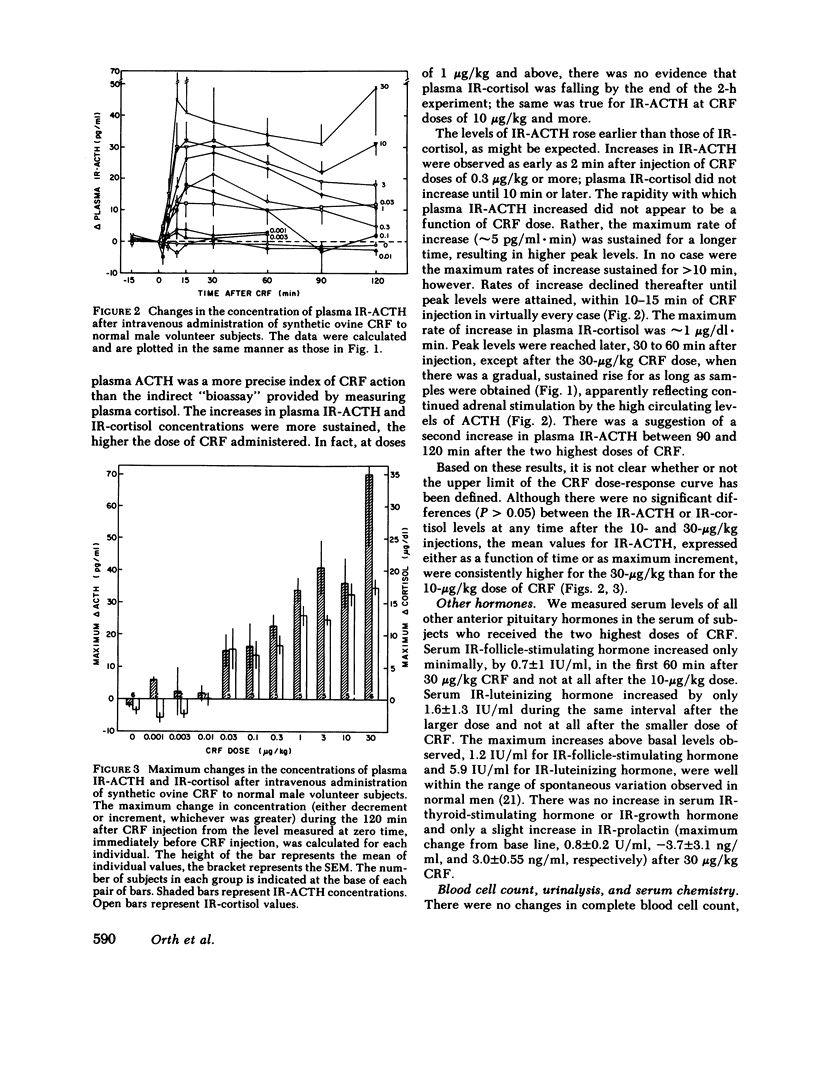
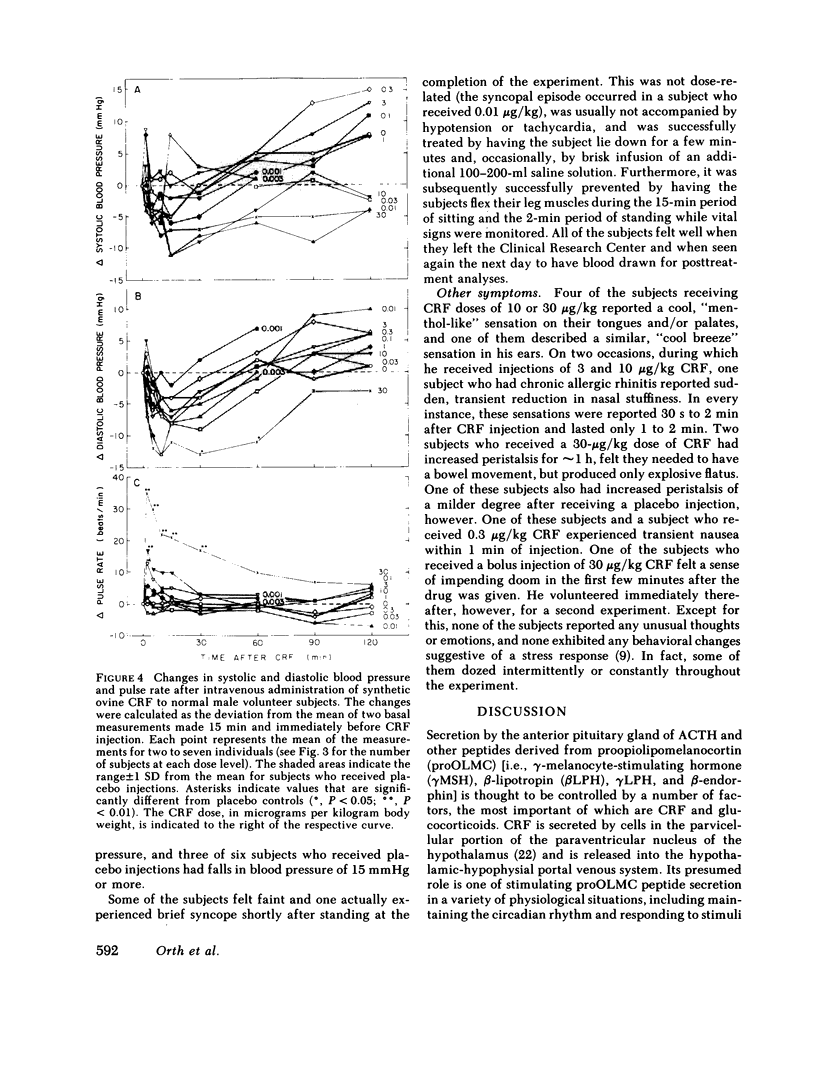
Synthetic ovine corticotropin-releasing factor (CRF) was administered to normal male volunteer subjects as an intravenous bolus or 30-s infusion. Doses of CRF ranging from 0.001 to 30 micrograms/kg body wt were administered, and plasma immunoreactive (IR)-ACTH and IR-cortisol concentrations were measured. The threshold dose appeared to be 0.01-0.03 micrograms/kg, the half-maximal dose 0.3-1 micrograms/kg, and the maximally effective dose 3-10 micrograms/kg. Basal concentrations of IR-ACTH and IR-cortisol were 14 +/- 7.6 pg/ml (mean +/- SD) and 5.6 +/- 2.2 micrograms/dl, respectively. IR-ACTH rose as early as 2 min after CRF injection, reached peak levels in 10-15 min, and declined slowly thereafter. IR-cortisol rose at 10 min or later and reached peak levels in 30-60 min. At a dose of 30 micrograms/kg, neither IR-ACTH nor IR-cortisol fell from peak levels of 82 +/- 21 pg/ml (mean +/- SE) and 23 +/- 1.4 micrograms/dl, respectively, during the 2-h course of the experiment, indicating that CRF has a sustained effect on ACTH release and/or a prolonged circulating plasma half-life. There was little or no increase in the levels of other anterior pituitary hormones. At doses of 1 microgram/kg and higher, facial flushing, tachycardia, and, in some subjects, a 15-29-mmHg decline in systemic arterial blood pressure were observed, even though blood volume was replaced and the subjects remained supine. These data indicate that synthetic ovine CRF is a very potent and specific ACTH secretagogue in man. Administered with caution until its vasomotor effects are more fully defined, CRF promises to be a safe and very useful investigative, diagnostic, and, possibly, therapeutic agent in man.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besser G. M., Orth D. N., Nicholson W. E., Byyny R. L., Abe K., Woodham J. P. Dissociation of the disappearance of bioactive and radioimmunoreactive ACTH from plasma in man. J Clin Endocrinol Metab. 1971 May;32(5):595–603. doi: 10.1210/jcem-32-5-595. [DOI] [PubMed] [Google Scholar]
- Bloom F. E., Battenberg E. L., Rivier J., Vale W. Corticotropin releasing factor (CRF): immunoreactive neurones and fibers in rat hypothalamus. Regul Pept. 1982 Jun;4(1):43–48. doi: 10.1016/0167-0115(82)90107-0. [DOI] [PubMed] [Google Scholar]
- Boyar R., Perlow M., Hellman L., Kapen S., Weitzman E. Twenty-four hour pattern of luteinizing hormone secretion in normal men with sleep stage recording. J Clin Endocrinol Metab. 1972 Jul;35(1):73–81. doi: 10.1210/jcem-35-1-73. [DOI] [PubMed] [Google Scholar]
- Britton D. R., Koob G. F., Rivier J., Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci. 1982 Jul 26;31(4):363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Fisher L. A., Spiess J., Rivier C., Rivier J., Vale W. Corticotropin-releasing factor: actions on the sympathetic nervous system and metabolism. Endocrinology. 1982 Sep;111(3):928–931. doi: 10.1210/endo-111-3-928. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Fisher L. A., Spiess J., Rivier J., Rivier C., Vale W. Comparison of the biologic actions of corticotropin-releasing factor and sauvagine. Regul Pept. 1982 Jul;4(2):107–114. doi: 10.1016/0167-0115(82)90101-x. [DOI] [PubMed] [Google Scholar]
- Fisher L. A., Rivier J., Rivier C., Spiess J., Vale W., Brown M. R. Corticotropin-releasing factor (CRF): central effects on mean arterial pressure and heart rate in rats. Endocrinology. 1982 Jun;110(6):2222–2224. doi: 10.1210/endo-110-6-2222. [DOI] [PubMed] [Google Scholar]
- Fleischer N., Vale W. Inhibition of vasopressin-induced ACTH release from the pituitary by glucocorticoids in vitro. Endocrinology. 1968 Dec;83(6):1232–1236. doi: 10.1210/endo-83-6-1232. [DOI] [PubMed] [Google Scholar]
- GUILLEMIN R., ROSENBERG B. Humoral hypothalamic control of anterior pituitary: a study with combined tissue cultures. Endocrinology. 1955 Nov;57(5):599–607. doi: 10.1210/endo-57-5-599. [DOI] [PubMed] [Google Scholar]
- Grossman A., Kruseman A. C., Perry L., Tomlin S., Schally A. V., Coy D. H., Rees L. H., Comaru-Schally A. M., Besser G. M. New hypothalamic hormone, corticotropin-releasing factor, specifically stimulates the release of adrenocorticotropic hormone and cortisol in man. Lancet. 1982 Apr 24;1(8278):921–922. doi: 10.1016/s0140-6736(82)91929-8. [DOI] [PubMed] [Google Scholar]
- Hook V. Y., Heisler S., Sabol S. L., Axelrod J. Corticotropin releasing factor stimulates adrenocorticotropin and beta-endorphin release from AtT-20 mouse pituitary tumor cells. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1364–1371. doi: 10.1016/0006-291x(82)91264-5. [DOI] [PubMed] [Google Scholar]
- Labrie F., Veilleux R., Lefevre G., Coy D. H., Sueiras-Diaz J., Schally A. V. Corticotropin-releasing factor stimulates accumulation of adenosine 3', 5'-monophosphate in rat pituitary corticotrophs. Science. 1982 May 28;216(4549):1007–1008. doi: 10.1126/science.6281886. [DOI] [PubMed] [Google Scholar]
- Orth D. N., DeBold C. R., DeCherney G. S., Jackson R. V., Alexander A. N., Rivier J., Rivier C., Spiess J., Vale W. Pituitary microadenomas causing Cushing's disease respond to corticotropin-releasing factor. J Clin Endocrinol Metab. 1982 Nov;55(5):1017–1019. doi: 10.1210/jcem-55-5-1017. [DOI] [PubMed] [Google Scholar]
- Quigley M. E., Yen S. S. A mid-day surge in cortisol levels. J Clin Endocrinol Metab. 1979 Dec;49(6):945–947. doi: 10.1210/jcem-49-6-945. [DOI] [PubMed] [Google Scholar]
- Rivier C., Brownstein M., Spiess J., Rivier J., Vale W. In vivo corticotropin-releasing factor-induced secretion of adrenocorticotropin, beta-endorphin, and corticosterone. Endocrinology. 1982 Jan;110(1):272–278. doi: 10.1210/endo-110-1-272. [DOI] [PubMed] [Google Scholar]
- SAFFRAN M., SCHALLY A. V. The release of corticotrophin by anterior pituitary tissue in vitro. Can J Biochem Physiol. 1955 May;33(3):408–415. [PubMed] [Google Scholar]
- Schulte H. M., Chrousos G. P., Gold P. W., Oldfields E. H., Phillips J. M., Munson P. J., Cutler G. B., Jr, Loriaux D. L. Metabolic clearance rate and plasma half-life of radioiodinated corticotropin releasing factor in a primate. J Clin Endocrinol Metab. 1982 Nov;55(5):1023–1025. doi: 10.1210/jcem-55-5-1023. [DOI] [PubMed] [Google Scholar]
- Schulte H. M., Chrousos G. P., Oldfield E. H., Gold P. W., Cutler G. B., Jr, Loriaux D. L. The effects of corticotropin releasing factor on the anterior pituitary function of stalk-sectioned cynomolgus macaques: dose response of cortisol secretion. J Clin Endocrinol Metab. 1982 Oct;55(4):810–812. doi: 10.1210/jcem-55-4-810. [DOI] [PubMed] [Google Scholar]
- Slag M. F., Ahmad M., Gannon M. C., Nuttall F. Q. Meal stimulation of cortisol secretion: a protein induced effect. Metabolism. 1981 Nov;30(11):1104–1108. doi: 10.1016/0026-0495(81)90055-x. [DOI] [PubMed] [Google Scholar]
- Spiess J., Rivier J., Rivier C., Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6517–6521. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Nicholson W. E., Orth D. N. Diurnal rhythm and disappearance half-time of endogenous plasma immunoreactive beta-MSH (LPH) and ACTH in man. J Clin Endocrinol Metab. 1978 Jun;46(6):883–890. doi: 10.1210/jcem-46-6-883. [DOI] [PubMed] [Google Scholar]
- Vale W., Grant G., Amoss M., Blackwell R., Guillemin R. Culture of enzymatically dispersed pituitary cells: functional validation of a method. Endocrinology. 1972 Aug;91(2):562–572. doi: 10.1210/endo-91-2-562. [DOI] [PubMed] [Google Scholar]
- Vale W., River C. Substances modulating the secretion of ACTH by cultured anterior pituitary cells. Fed Proc. 1977 Jul;36(8):2094–2099. [PubMed] [Google Scholar]
- Vale W., Rivier C., Brown M., Chan L., Ling N., Rivier J. Applications of adenohypophyseal cell cultures to neuroendocrine studies. Curr Top Mol Endocrinol. 1976;3:397–429. doi: 10.1007/978-1-4684-2598-7_23. [DOI] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Wilkin J. K. Flushing reactions: consequences and mechanisms. Ann Intern Med. 1981 Oct;95(4):468–476. doi: 10.7326/0003-4819-95-4-468. [DOI] [PubMed] [Google Scholar]
- Wilson M. G., Nicholson W. E., Holscher M. A., Sherrell B. J., Mount C. D., Orth D. N. Proopiolipomelanocortin peptides in normal pituitary, pituitary tumor, and plasma of normal and Cushing's horses. Endocrinology. 1982 Mar;110(3):941–954. doi: 10.1210/endo-110-3-941. [DOI] [PubMed] [Google Scholar]
- Yasuda N., Greer M. A., Aizawa T. Corticotropin-releasing factor. Endocr Rev. 1982 Spring;3(2):123–140. doi: 10.1210/edrv-3-2-123. [DOI] [PubMed] [Google Scholar]