Summary
Vitamin D has known effects on lung development and the immune system that may be important in the development, severity and course of allergic diseases (asthma, eczema and food allergy). Vitamin D deficiency is prevalent worldwide and may partly explain the increases in asthma and allergic diseases that have occurred over the last 50–60 years. In this review we explore past and current knowledge on the effect of vitamin D on lung development and immunomodulation and present the evidence of its role in allergic conditions. While there is growing observational and experimental evidence for the role of vitamin D, well-designed and well-powered clinical trials are needed to determine whether supplementation of vitamin D should be recommended in these disorders.
Keywords: Vitamin D, Immunomodulation, Development, Asthma and Allergy
Introduction
Allergic (atopic) diseases result from an interaction between individual genetic susceptibility and exposure to environmental factors. According to twin studies, the genetic contribution to allergic disease has been estimated to be about 50%, with heritability estimates ranging from 36–79% [1]. Since the beginning of the 20th century, allergic disease has shown a continuous upward trend in prevalence such that asthma, atopic dermatitis, and food allergies currently are common chronic conditions in westernized societies [2]. A multitude of environmental factors, have triggered the steep rise in this trend over the last five decades [3]. Accordingly, sensitization rates to one or more common allergens among school children and adults have globally increased and now approach 40%–50% [3]. As a result, allergic conditions are the sixth most costly chronic disease category in the United States [4]. Worldwide, health care costs have reached approximately US$ 21, AU$ 7.8 (US$ 7.32), £1 (US$ 1.7) billion annually in the US, Australia and UK, respectively [4–6]. In Europe, the costs of health care for asthma alone amounts to more than 25 (US$ 34) billion [7]. The number of disability-adjusted life years (DALYs) lost due to asthma worldwide has been estimated to be about 15 million per year. Worldwide, asthma accounts for around 1% of all DALYs lost [8].
Along with a clear genetic basis in allergic disease, environmental factors, including early neonatal nutrition, may have an important influence on allergy development and, thus, present an opportunity to prevent or delay the onset of the disease [1, 9]. Vitamin D may be an important environmental factor. Evolutionarily, early humans evolved in sun-rich environments and the increased efficiency of vitamin D production in the skin is thought to be a major driving factor in human skin depigmentation as humans migrated away from the equatorial areas [10, 11]. 7-Dehydrocholesterol (7-DHC) in the skin is converted to previtamin D3 after exposure to UVB rays, and is then transformed to vitamin D3 (cholecalciferol) by a thermally induced isomerization. Vitamin D3 can also come from the gut, via diet or supplements. Vitamin D3 in circulation then undergoes hydroxylation in the liver to 25-hydroxyvitamin D3 (25OHD), which then is hydroxylated further in the kidney to its biologically active form 1,25-dihydroxyvitamin D3 (1,25[OH]2D or calcitriol) [12, 13]. Henceforth in this review, the term “vitamin D” will refer to 1,25[OH]2D, unless otherwise stated. Vitamin D mediates its biological effect through the vitamin D receptor (VDR) which was discovered to be present in a variety of tissues, suggesting the importance of the vitamin D system in various cellular and tissue functions [13–17]. Hence, over the past few years, researchers have paid a great deal of attention to the effect of vitamin D on immunologic mechanisms as one potentially modifiable environmental factor [18, 19]. This is mostly due to the advent of the ability to easily measure 25OHD levels and other vitamin D metabolites in serum or plasma and the growing recognition that vitamin D insufficiency (defined as 25OHD levels of less than 30 ng/ml) exists in most populations around the world [20]. In the US, there is evidence to show that vitamin D insufficiency has increased almost 2-fold over a span of about 10 years, from the late 1980s and early 1990s through the early 2000s [21]. Indeed, it has been proposed that vitamin D insufficiency has contributed to the rise of asthma and allergic disease, [22] and various biological mechanisms for how vitamin D may play a role in the development and treatment of asthma and allergies have been proposed [23]. Historically, Rappaport and colleagues [24, 25] investigated the modifying effect of vitamin D on allergic conditions as early as the 1930s and reported relief of symptoms in a majority of allergic patients treated with viosterol (a vitamin D preparation produced by the irradiation of ergostrol) compared to controls, a finding subsequently supported by several studies [23, 26–29]. These studies have also shown that the determinants of vitamin D status are multifactorial and a nonlinear relationship has been shown between serum levels and biological effects [23, 30]. However, these studies need to be replicated using more rigorous and modern methods. There is controversy over what levels are optimal for overall health. However, the serum level of 25OHD 30–40 ng/mL (75–100 nmol/L) has been suggested as a lower threshold of an optimal serum level for the immune effects of vitamin D [12, 31, 32]. According to the lower defined threshold value for bone health [33, 34], more than one-third of the population worldwide may have low levels of vitamin D (25OHD < 20 ng/mL [50 nmol/L]) [35]. This fact highlights the potential for risk modification of low vitamin D on the worldwide increase in rates of allergic sensitization.
Vitamin D and immunomodulation related to allergy
Vitamin D has immunomodulatory effects on allergen-induced inflammatory pathways [19] by acting on VDR expressed on a variety of immune cells, including B cells, T cells, dendritic cells and macrophages [36, 37]. Many of these cells, such as activated macrophages and dendritic cells, are capable of synthesizing biologically active vitamin D from circulating 25OHD [38–40]. This mechanism, extrarenal expression of CP27B, enables immune cells to rapidly increase local levels of vitamin D, potentially needed to shape adaptive immune responses [39–41]. In this context, a clinical study involving mild allergic asthmatics who underwent segmental allergen challenge showed a significant increase in vitamin D receptor binding protein (VDBP) and 25OHD in bronchoalveolar lavage fluid (but not in their serum levels) 24hrs after allergen challenge, thus, suggesting a role for vitamin D mediated immune responses in the local asthmatic late-phase reaction [42].
Vitamin D has shown the ability to inhibit both Th1- and Th2-type responses by suppressing both the production of IL-12-generated IFN-γ as well as IL-4 and IL-4-induced expression of IL-13 [43]. This ability could be of importance since the balance of Th1 and Th2 affects the pattern of immune response. While asthma is thought to be a Th2 dominant condition and is largely characterized by the production of cytokines such as IL-4, IL-5, IL10 and IL-13, and the production of IgE by B cells [44–46], some experimental and human studies do not support a unidirectional effect (either inhibition alone or enhancement alone) of vitamin D on adaptive T cell responses [23, 47–50]. For example, one study investigated the associations of circulating 1,25[OH]2D on Th1/Th2 serum markers in patients with concomitant nasal polyps and allergic rhinitis. They found a negative correlation between plasma 1,25[OH]2D with IgE and IL-4 levels, and a positive correlation between 1,25[OH]2D with IFN-γ [51].
The differences in the observations on Th1-Th2 balance among allergic conditions has been attributed to variation in the baseline vitamin D status, timing of exposure to allergen, and chronicity of vitamin D administration relative to sensitization [23]. The type and concentration of allergen as well as the balance of immune responses (Th1 cytokines/Th2 cytokines) to different types of allergens are additional factors contributing to variability in response [52, 53]. How the absolute change in vitamin D levels from the baseline vitamin D status over a time period might affect the balance of Th1/Th2 cytokines in response to various allergens is a matter for further investigation. It has been proposed that in the absence of vitamin D signaling, the T cell compartment has a potentially stronger Th1 phenotype and that at pharmacological levels, vitamin D suppresses both Th1 and Th2 immune responses [54, 55].
Vitamin D has potent antiproliferative effects on CD4+ T cells as well as the ability to inhibit T lymphocyte function, both directly, and via effects on antigen-presenting cells (APCs) [49, 56]. Th1-cell responses are responsible for some of the pathogenic features in allergic patients particularly chronic features, including epithelial cell apoptosis, smooth-muscle-cell activation and may contribute to mucus secretion [45]. In addition to inhibiting Th1-associated pro-inflammatory cytokines [37, 49, 56–58], vitamin D has been shown to act on Th17 cells to suppress the production of IL-17 [22, 59]. Additionally, Rausch-Fan et al. has shown that vitamin D modulates cytokine production in human peripheral blood mononuclear cells and allergen-specific Th cell clones; an effect which was time and concentration dependent [60].
Regulatory T cells (Tregs), including naturally occurring and induced Tregs, play an important role in maintaining immune homeostasis in response to allergen exposure by suppressing Th2 mediated inflammation such as airway eosinophilia, mucous hyper-secretion, and airway hyper-responsiveness [53, 61, 62]. Tregs use multiple suppressive mechanisms, including IL-10 and TGF-beta as well as cytotoxic T lymphocyte antigen 4 and programmed death 1 as surface molecules to regulate downstream immune activation [63]. Vitamin D can induce antigen specific IL-10–producing Tregs, that express low levels of the CD4+CD25+ Treg-associated transcription factor FoxP3 [64, 65]. Consequently, secreted IL-10 inhibits inappropriate allergen specific Th2-driven immune responses and regulates allergic sensitization [61, 66]. Similarly, allergen immunotherapy and glucocorticoid therapy for allergy and asthma have shown increased IL-10 synthesis by Tregs [66–68]. Vitamin D supplementation of cholecalciferol (140,000 IU) has been also associated with an increased Tregs frequency (%Tregs) in apparently healthy individuals with vitamin D insufficiency after 4 weeks [69]. However, Chi et al. demonstrated that cord blood 25OHD levels were inversely associated with the proportion of CD25+, CD25Bright, and CD25+FoxP3 T-regulatory cells [46]. Thus, it is likely that the effect of vitamin D on Tregs is complex and depends on the contextual cellular milieu and factors such as the age of the individual, pregnancy, as well as health and disease status.
Vitamin D and its receptor (VDR) are both essential for development of Natural Killer (NK) cells and the expression of IL-4 and IFN-γ production [70]. NK cells contribute to the development of T-cell mediated allergic airway inflammation [71–73] and are capable of producing numerous pro-inflammatory cytokines such as IFN-γ, TNF-α, GM-CSF, and MIP-1a upon IgE stimulation and exhibit cytotoxicity against IgE-coated target cells through FcγRIII [74]. Natural killer T (NKT) cells are a distinct subset of T lymphocytes that can produce both Th1 (IFN-γ) and Th2 (IL-4) cytokines and have been investigated for their role in asthma and allergy [75–77]. Du et al. studied the interaction of low vitamin D levels on asthma exacerbations [78]. They identified three associated common variants in the class I MHC–restricted T cell–associated molecule gene (CRTAM), which is highly expressed in activated human CD8+ and NKT cells. Their findings implicate a mechanism by which vitamin D might prevent asthma exacerbations through CD8+ and NKT cells, particularly during viral infections.
Effect of vitamin D on development (fetal lung & immune system) and normal function
Vitamin D in pregnancy and fetal development
The Barker hypothesis first posited that environmental influences early in development and during intrauterine life, could increase the risk of chronic disease later in life [79]. Numerous epidemiological studies have shown strong associations between maternal diet and altered risk of chronic disease, particularly asthma [80–82]. Active vitamin D (1,25[OH]2D) dramatically rises during pregnancy, with levels reaching up to 124% to 135% of normal values [83, 84]. As a result, the role of vitamin D during pregnancy has recently come to the forefront as studies have documented an increased risk to maternal and fetal health in relation to low serum levels of 25OHD [85].
In addition to the well-described skeletal effects of vitamin D, such as calcium homeostasis [86, 87] and bone development, it may promote proper fetal implantation and regulates placental development [88, 89]. Moreover, vitamin D regulates immune responsiveness by enhancing the activity of immunosuppressive Tregs and suppresses pro-inflammatory Interleukin-17 expressing Th17 cells [64, 90]. In addition, vitamin D is well known for its role in regulating the expression of the neutrophil associated calethicidin antimicrobial protein (CAMP) as well as numerous defensins molecules, particularly beta defensin 1 [91]. Collectively, these findings suggest that vitamin D has immunosuppressive effects in placental tissue and promotes enhanced protection of the mother and fetus against infection.
Studies in animals and humans have shown that circulating levels of maternal 25OHD crosses the placenta freely. Importantly, placental trophoblast cells express the VDR and CYP27B1 (1aOHase) and hence are capable of converting 25OHD into the active hormonal form[92, 93]. Several observational studies suggest that low 25OHD level promotes intra-uterine growth restriction and low birth weight and has modest effects on pre-term birth [94, 95]. Thus, mounting evidence indicates that vitamin D is necessary for normal fetal development and organ function, although the molecular mechanism by which fetal vitamin D levels influence the complex fetal development pathways remain unclear [96].
Association with lung development
In addition to immunomodulatory effects during fetal development, vitamin D also has known genomic effects and thus the ability to influence fetal lung development [85, 97]. Experimental evidence in rats show that fetal alveolar type II epithelial cells express VDR, suggesting that pulmonary maturation is responsive to vitamin D exposure[98–100]. In humans, Kho et al. recently examined gene expression profiles during human fetal lung development and identified a number of genes associated with the vitamin D pathway whose expression was developmentally regulated [97]. Although the exact role these vitamin D related genes play in fetal lung development remains to be fully explored, several genes (LAMP3, PIP5K1B, SCRAB2 and TXNIP) were also found to be significantly overexpressed in cells derived from asthmatic children, thus, suggesting a link between vitamin D pathway genes, fetal development and asthma [97]. Studies in mice and human performed by Zosky et al. further support the notion that maternal vitamin D deficiency during fetal lung development may impact early life lung structure and function and increase the risk of chronic lung disease later in life [101, 102].
Correlation of serum vitamin D of mother and newborn
Studies show a strong correlation between maternal 25OHD levels and cord blood 25OHD levels of neonates. In a recent study of 107 pregnant mother-infant pairs, cord blood levels of 25OHD were shown to be approximately 62 ± 16% of maternal levels and correlated positively with maternal 25OHD levels (r=0.83, P<0.001) [103]. The influence of vitamin D on early childhood health and growth has emerged as an area of great interest [104]. In this context, infections, including respiratory infections, are an important cause of neonatal childhood morbidity and mortality and, in this regard, several studies have documented a correlation between deficiencies in 25OHD levels in early life with increased rates of respiratory infections [105]. This is of particular interest, as respiratory infections in the first years of life have been associated with an increased risk of allergic asthma in young children [106].
Asthma and allergy
The prenatal and early life period have been identified as “windows of opportunity” during which immune responses can be permanently programed. The role of vitamin D during this period in the prevention of asthma and allergies in children remains controversial. Positive and negative results of several epidemiologic studies have been published [23]. Reasons for these differing results are numerous, and can be gleaned from patterns of the results that have emerged. Firstly, all the published studies to date that have reported a protective effect of vitamin D have all assessed vitamin D intake in pregnancy from food frequency questionnaires [107–110], which are known to be an indicator of long-term diet pattern [111, 112], hence long-term intakes of vitamin D. Next, studies that have assessed either 2nd and 3rd trimester maternal vitamin D status (via 25OHD levels) [110, 113–116] or cord blood 25OHD levels [117–122] have not shown a consistently protective effect on asthma and allergies. Since 25OHD is known to fluctuate by season [123–125] and the intraclass correlation coefficient over several years of 25OHD levels is only on the order of 0.3 [126], it is likely that a one-time assessment of vitamin D status is not sufficient for the question of whether vitamin D can prevent asthma or allergies. Additionally, the ineffective trial of vitamin D supplementation in the 3rd trimester in showing effect modification of clinical characteristics of asthma and allergies, underscoring the fact that intervening in the 3rd trimester may be too late for these outcomes. The only observational study that showed that maternal vitamin D status was protective for asthma measured 25OHD levels in the first trimester [102]. The studies that used Food Frequency Questionnaires (FFQs) are consistent with this notion since these are markers of long-term intakes encompassing the first trimester. Thus, vitamin D supplementation trials that begin earlier in pregnancy are needed. One such trial, The Vitamin D Antenatal Asthma Reduction Trial (VDAART): a randomized controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children, is currently underway (ClinicalTrials.gov: NCT00920621).
Role of vitamin D in prevention and modification of asthma and allergy
After pregnancy and during early life
I. Asthma
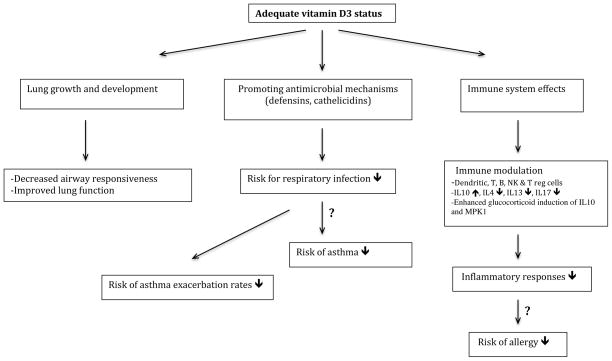
Vitamin D may potentially decrease the severity of asthma and allergies through a variety of mechanisms (figure 1) including effects on immune cells, improved handling or prevention of predisposing infections [19, 127–129], decreased inflammatory responses, improved lung function [130–132], effects on airway smooth muscle function and mass, reduced airway remodeling [19, 133] and reversal of steroid resistance (by IL-10 production and modifying ligand-induced down-regulation of glucocorticoid receptors [66]). Several studies have investigated the relationship between vitamin D deficiency and asthma exacerbations. A low level of vitamin D at age 6 was associated with increased allergies and asthma at age 14 in one study from Australia and serum 25OHD levels in children of both ages were negatively associated with concurrent allergic phenotypes in males [134]. In 616 Costa Rican children with asthma aged 6–14 years, Brehm et al. found that 28% of the children had insufficient 25OHD levels. On a log scale, 25OHD level was associated with reduced risks of any hospitalization for asthma in the previous year (odds ratio [OR], 0.05) and any use of anti-inflammatory medications in the previous year (OR, 0.18). In addition, 25OHD levels were significantly and inversely associated with total IgE and eosinophil count [135]. Brehm et al, using collected data in 1024 participants of the Childhood Asthma Management Program (CAMP), a placebo-controlled randomized trial of inhaled budesonide vs. nedocromil, showed that vitamin D insufficiency (25OHD <30 ng/ml) was associated with higher risks for severe asthma exacerbations leading to ED visits or hospitalizations [136]. There was a greater effect among children who were randomized to the inhaled budesonide arm, suggesting an interaction between vitamin D and corticosteroid use. Searing et al [137] in a cross-sectional study of 100 asthmatic children, showed that 25OHD levels were inversely associated with serum IgE, number of skin prick tests to perennial aeroallergens, lung function, and use of inhaled or oral corticosteroids. In-vitro studies using peripheral blood mononuclear cells showed that vitamin D amplified glucocorticoid induction of mitogen-activated protein kinase phosphatase (MPK)-1 and IL10, which are critical for anti-inflammatory and immunosuppressive effects. In a study of 54 adult asthmatics, subjects with vitamin D insufficiency had lower lung function and increased airway hyperresponsiveness [138]. In addition, 25OHD levels in this study were inversely correlated with tumor necrosis factor (TNF)-α expression and positively correlated with dexamethasone-induced MPK-1 expression. Other studies have also found that lower 25OHD levels are associated with poor asthma control [139], lower lung function and the presence of exercise-induced bronchoconstriction [140] in asthmatic children, and the severity of atopic dermatitis [141].
Figure 1.
Potential effects of vitamin D in asthma and allergy development or modification Several studies support the modification role of vitamin D in asthma and allergy. However, preventive effects of vitamin D in risk reduction of asthma and allergy development during pregnancy and early life requires more well designed longitudinal studies.
Bosse et al. [142] have shown in vitro that vitamin D increases glucocorticoid bioavailability in bronchial smooth muscle cells suggesting a further beneficial role for vitamin D in the prevention and treatment of asthma. This interaction between vitamin D and corticosteroids was investigated in a recent clinical trial. In “Vitamin D Add-on Therapy Enhances Corticosteroid Responsiveness in Asthma (VIDA)”, Castro et al. investigated if taking vitamin D in addition to a standard asthma medication would prevent the worsening of asthma symptoms or attacks [143]. In their study, adult asthmatics were randomly received an initial dose of 100,000 IU of oral cholecalciferol followed by 4000 IU/day for 28 weeks or placebo (n = 201 vs. 207) while using inhaled ciclesonide (Alvesco). After a 12-week inhaled corticosteroids stability phase with 320 μg/d of ciclesonide, the dose of ciclesonide was reduced to 160 μg/day for 8 weeks, followed by maintenance dose of 80 μg/day for another 8 weeks. Although they found that vitamin D supplementation had no significant effect on the overall rate of first treatment failure or exacerbation in patients with asthma and low vitamin D levels, at 28 weeks, there was a significant difference in cumulative ciclesonide dosing between the vitamin D and placebo groups (111.3 vs. 126.2 μg/day; P = .02). In addition, there was significant reduction in the overall asthma treatment failure and the exacerbations in subjects that achieved a normal vitamin D level. This trial was likely underpowered for the primary outcomes of first treatment failure, as the authors acknowledged that there was a lower than expected event rate in the control group. Furthermore, several observational studies have already established that the interaction between corticosteroids and vitamin D in asthma is much stronger in children than in adults with asthma [144–146], thus well-designed and well-powered trials in children are needed.
II. Atopic dermatitis, allergic rhinitis and food allergy
Several clinical, genetic and experimental studies suggest that prior history of atopic dermatitis (AD) and its severity are a major risk factors for the development of allergic rhinitis, asthma and specific sensitization, highlighting the importance of the epidermal barrier in the pathogenesis of these allergic disorders [147]. Low levels of vitamin D appear to be inversely correlated with AD severity, and vitamin D deficiency at birth is associated with higher risk of developing AD. Also, a pilot randomized trial of vitamin D supplementation in children demonstrated a favorable effect on AD symptoms during winter months [148]. It is possible that this effect was mediated by the induction of endogenous antimicrobial peptides in the skin in AD by oral vitamin D supplementation [149].
Few studies have investigated vitamin D status in allergic rhinitis and food allergy. Allergic rhinitis has been shown to be a risk factor for developing asthma [150–152]. Ciprandi et al. [152] showed that nasal symptoms, airflow, and markers of inflammation directly correlate with lower airway markers including forced expiratory volume in 1 second (FEV1). Leynaert et al. [153] found that approximately 75% of asthmatics report rhinitis; patients with rhinitis have increased risk for asthma and lower airway reactivity compared to patients without rhinitis; and the risk for asthma increases from 2.0% in subjects without rhinitis to 18.8% in subjects with allergic rhinitis when exposed to either pollen or animal dander. Mai et al. [154] also investigated the relationship between serum 25OHD and the incidence of allergic rhinitis in adults. The study included a random sample (N=1351) from an adult population who participated in Nord-Trøndelag Health Study (HUNT). In the 11-year follow-up of the subjects, they showed that 9% of men and 15% of women developed allergic rhinitis. Among men, serum 25OHD level <50 nmol/L at baseline was associated with an increased risk of allergic rhinitis (adjusted OR 2.55, p=0.001); each 25 nmol/L reduction in 25OHD level was associated with an adjusted OR of 1.84. However, women had lower risk of allergic rhinitis with adjusted OR of 0.83 (p>0.05) for each 25 nmol/L reduction in serum 25OHD level.
It has been suggested vitamin D deficiency might impair epithelial barrier integrity, that in turn leads to increased and inappropriate mucosal exposure to food antigens and also a pro-sensitization immune imbalance that compromises immunological tolerance [155]. Consequently, early correction of vitamin D deficiency might promote mucosal defense, maintain healthy microbial ecology and allergen tolerance, and decrease risk of food allergies in children [149, 156]. Food allergy is a known provoking cause of AD and the prevalence of IgE-mediated food allergy is about 35% in children affected with AD [38]. Kull et al. showed vitamin D in water-soluble form increased the risk of allergic disease in children of less than 4 years old compared with supplementation of vitamin D given in peanut oil [157]. This study justifiably raised questions about the varying results of vitamin D supplementation on risk modification of food allergy and other allergic diseases [158]. However, the study did not measure baseline nor follow-up vitamin D levels. Sharief et al. [159] showed that higher levels of IgE sensitization were associated with vitamin D deficiency in children and adolescents. Accordingly, 25(OH)D levels of less than 15 ng/mL were associated with evidence of sensitization to various allergens. For example the odds ratios of allergy for peanut, ragweed, and oak were 2.39, 1.83 and 4.75, respectively [159]. Similarly, mean 25(OH)D serum levels have been lower in moderate and severe atopic dermatitis (AD) children, suggesting potential benefit of serum level correction using vitamin D supplementation in AD [141]. Allen et al. [160] demonstrated that infants of Australian-born parents with vitamin D insufficiency (25OHD ≤50 nmol/L) had higher risk of peanut (OR, 11.51) and/or egg (OR, 3.79) allergy than those with adequate vitamin D levels. They were also more likely to have multiple food allergies (≥2) rather than a single food allergy (OR, 10.48 vs. OR, 1.82) [160]. Moreover, Mullins et al. [161], similar to Vassallo et al. [162], reported significantly higher rates of food allergy in children born in the autumn/winter, suggesting a relationship between relative food allergy rates and monthly sun exposure.
Future Directions
There remains a great deal of controversy as to the role of vitamin D in overall health and what represents adequate levels of vitamin D in the blood for human health generally [163] and specifically for each of the reviewed allergic conditions. However, there is sufficient suggestion of a benefit of raising vitamin D levels to encourage more studies in this field. Future interventional and longitudinal cohort studies are needed to establish whether changes in maternal nutrient intake during pregnancy can be used as a healthy low-cost public health measure to reduce the incidence of childhood asthma and atopy [164]. Such studies will be very important in shedding light on the role of vitamin D in fetal development. More studies in established asthma are also necessary. Clinical trials investigating the role of vitamin D in modifying the severity of asthma and allergies and in controlling exacerbations need to be adequately powered, use the appropriate dose, and need to be of sufficient duration. Furthermore, a focus on children with persistent disease also seems to be appropriate. These trials should perform additional analyses that attempt to identify the appropriate vitamin D level that provides the maximal beneficial effects.
Vitamin D may regulate epigenetic events [165] that promote allergic conditions [166]. Hence, studies on molecular genetic and epigenetic mechanisms of vitamin D in allergic diseases and disease severity (immunoinflammatory responses, steroid resistance, host defense) particularly in response to high and low vitamin D supplementation would provide mechanistic insights in the management and prevention of allergic diseases. As an example, a study in healthy adults who received either 400 or 2000 IU/d of vitamin D3 for 3 months in winter demonstrated the up or down-regulation of 291 genes by vitamin D intake. That these genes effected as many as 80 different metabolic pathways, including immune modulation and enhanced antioxidant activity, emphasizes the potential importance of vitamin D status on transcriptional regulation [19, 167]. Thus, these clinical trials will also need to collect the appropriate specimens and consent to perform these genomic studies. These clinical and genomic studies will help to clarify the potential role of vitamin D on both the development and modulation of asthma and allergies.
Footnotes
Conflict of interest
Drs. Scott Weiss and Augusto Litonjua have received research support for Vitamin D Antenatal Asthma Reduction Trial (VDAART) by U01 HL091528 from the National Heart, Lung, and Blood Institute.
References
- 1.Jenerowicz D, Silny W, Danczak-Pazdrowska A, Polanska A, Osmola-Mankowska A, Olek-Hrab K. Environmental factors and allergic diseases. Annals of agricultural and environmental medicine : AAEM. 2012;19:475–81. [PubMed] [Google Scholar]
- 2.Calderon MA, Demoly P, Gerth van Wijk R, Bousquet J, Sheikh A, Frew A, Scadding G, Bachert C, Malling HJ, Valenta R, Bilo B, Nieto A, Akdis C, Just J, Vidal C, Varga EM, Alvarez-Cuesta E, Bohle B, Bufe A, Canonica WG, Cardona V, Dahl R, Didier A, Durham SR, Eng P, Fernandez-Rivas M, Jacobsen L, Jutel M, Kleine-Tebbe J, Klimek L, Lotvall J, Moreno C, Mosges R, Muraro A, Niggemann B, Pajno G, Passalacqua G, Pfaar O, Rak S, Senna G, Senti G, Valovirta E, van Hage M, Virchow JC, Wahn U, Papadopoulos N. EAACI: A European Declaration on Immunotherapy. Designing the future of allergen specific immunotherapy Clinical and translational allergy. 2012;2:20. doi: 10.1186/2045-7022-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawankar R, Canonica GW, Holgate STLR. White Book on Allergy: Executive Summary. 2011–2012. [Google Scholar]
- 4.American Academy of Allergy, Asthma and Immunology (AAAAI) The Allergy Report: Science Based Findings on the Diagnosis & Treatment of Allergic Disorders. 1996–2005. [Google Scholar]
- 5.Gupta R, Sheikh A, Strachan DP, Anderson HR. Burden of allergic disease in the UK: secondary analyses of national databases. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2004;34:520–6. doi: 10.1111/j.1365-2222.2004.1935.x. [DOI] [PubMed] [Google Scholar]
- 6.Australian Society of Clinical Immunology and Allergy (ASCIA) Report published by Access Economics Pty Ltd for ASCIA. Balgowlah, NSW, Australia: 2007. The Economic Impact of Allergic Disease in Australia: Not To Be Sneezed at. [Google Scholar]
- 7.European Respiratory Society (ERS) The First Comprehensive Survey on Respiratory Health in Europe. 2003. European Lung White Book. [Google Scholar]
- 8.Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma P. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–78. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 9.Greer FR, Sicherer SH, Burks AW. American Academy of Pediatrics Committee on N, American Academy of Pediatrics Section on A, Immunology, Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121:183–91. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 10.Jablonski NG, Chaplin G. The evolution of human skin coloration. Journal of human evolution. 2000;39:57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- 11.Chaplin G, Jablonski NG. The human environment and the vitamin D compromise: Scotland as a case study in human biocultural adaptation and disease susceptibility. Human biology. 2013;85:529–52. doi: 10.3378/027.085.0402. [DOI] [PubMed] [Google Scholar]
- 12.Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 13.Lips P. Vitamin D physiology. Progress in biophysics and molecular biology. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nature reviews Drug discovery. 2010;9:941–55. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 15.Borradale D, Kimlin M. Vitamin D in health and disease: an insight into traditional functions and new roles for the ‘sunshine vitamin’. Nutrition research reviews. 2009;22:118–36. doi: 10.1017/S0954422409990102. [DOI] [PubMed] [Google Scholar]
- 16.Kato S. The function of vitamin D receptor in vitamin D action. Journal of biochemistry. 2000;127:717–22. doi: 10.1093/oxfordjournals.jbchem.a022662. [DOI] [PubMed] [Google Scholar]
- 17.Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: physiology and biomarkers. The American journal of clinical nutrition. 2008;88:500S–06S. doi: 10.1093/ajcn/88.2.500S. [DOI] [PubMed] [Google Scholar]
- 18.Wacker M, Holick MF. Sunlight and Vitamin D: A global perspective for health. Dermato-endocrinology. 2013;5:51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clinic proceedings Mayo Clinic. 2013;88:720–55. doi: 10.1016/j.mayocp.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lips P. Worldwide status of vitamin D nutrition. The Journal of steroid biochemistry and molecular biology. 2010;121:297–300. doi: 10.1016/j.jsbmb.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Archives of internal medicine. 2009;169:626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? The Journal of allergy and clinical immunology. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Litonjua A. The Role of Vitamin D in the Development, Exacerbation, and Severity of Asthma and Allergic Diseases. Vitamin D and the Lung: Mechanisms and Disease Associations. In: Litonjua AA, editor. Respiratory medicine. Vol. 3. 2012. pp. 201–38. [Google Scholar]
- 24.Rappaport BZ, Reed CE. Viosterol of high potency in seasonal hay fever and related conditions. JAMA : the journal of the American Medical Association. 1933;101(2):105–9. [Google Scholar]
- 25.Rappaport BZ, Reed CI, Hathaway ML, Struck HC. The treatment of hay fever and asthma with viosterol of high potency. J Allergy. 1934;5(6):541–53. [Google Scholar]
- 26.Canon P. The therapy of nasal allergy; results obtained by high dose vitamin D therapy and calcium. Acta oto-rhino-laryngologica Belgica. 1951;5:495–508. [PubMed] [Google Scholar]
- 27.Jakso G. Studies on the anti-allergic effects of vitamin D2. Borgyogyaszati es venerologiaia szemle. 1950;4:223–5. [PubMed] [Google Scholar]
- 28.Reeve J, Meunier PJ, Parsons JA, Bernat M, Bijvoet OL, Courpron P, Edouard C, Klenerman L, Neer RM, Renier JC, Slovik D, Vismans FJ, Potts JT., Jr Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. British medical journal. 1980;280:1340–4. doi: 10.1136/bmj.280.6228.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Utz G, Hauck AM. Oral application of calcium and vitamin D2 in allergic bronchial asthma. MMW, Munchener medizinische Wochenschrift. 1976;118:1395–8. [PubMed] [Google Scholar]
- 30.Hypponen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE - a significant but nonlinear relationship. Allergy. 2009;64:613–20. doi: 10.1111/j.1398-9995.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- 31.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, Norman AW, Scragg R, Whiting SJ, Willett WC, Zittermann A. The urgent need to recommend an intake of vitamin D that is effective. The American journal of clinical nutrition. 2007;85:649–50. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 32.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. The American journal of clinical nutrition. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 33.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 34.Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Manson JE, Mayne ST, Ross AC, Shapses SA, Taylor CL. IOM committee members respond to Endocrine Society vitamin D guideline. The Journal of clinical endocrinology and metabolism. 2012;97:1146–52. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, Pierroz DD, Weber P, Hoffmann K. A systematic review of vitamin D status in populations worldwide. The British journal of nutrition. 2014;111:23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 36.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 37.Adorini L, Penna G, Giarratana N, Roncari A, Amuchastegui S, Daniel KC, Uskokovic M. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. The Journal of steroid biochemistry and molecular biology. 2004;89–90:437–41. doi: 10.1016/j.jsbmb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nature clinical practice Endocrinology & metabolism. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbour GL, Coburn JW, Slatopolsky E, Norman AW, Horst RL. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. The New England journal of medicine. 1981;305:440–3. doi: 10.1056/NEJM198108203050807. [DOI] [PubMed] [Google Scholar]
- 40.Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. The Journal of clinical investigation. 1983;72:1856–60. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Current opinion in pharmacology. 2010;10:482–96. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Bratke K, Wendt A, Garbe K, Kuepper M, Julius P, Lommatzsch M, Virchow JC. Vitamin D binding protein and vitamin D in human allergen-induced endobronchial inflammation. Clinical and experimental immunology. 2014 doi: 10.1111/cei.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pichler J, Gerstmayr M, Szepfalusi Z, Urbanek R, Peterlik M, Willheim M. 1 alpha,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatric research. 2002;52:12–8. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Holgate ST. Pathogenesis of asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2008;38:872–97. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 45.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nature reviews Immunology. 2008;8:218–30. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 46.Vasiliou JE, Lui S, Walker SA, Chohan V, Xystrakis E, Bush A, Hawrylowicz CM, Saglani S, Lloyd CM. Vitamin D deficiency induces Th2 skewing and eosinophilia in neonatal allergic airways disease. Allergy. 2014 doi: 10.1111/all.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lange NE, Litonjua A, Hawrylowicz CM, Weiss S. Vitamin D, the immune system and asthma. Expert review of clinical immunology. 2009;5:693–702. doi: 10.1586/eci.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. Journal of immunology. 2001;167:4974–80. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 49.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. Journal of cellular biochemistry. 2003;89:922–32. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 50.Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-gamma and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. Journal of immunology. 2002;168:1181–9. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- 51.Ozkara S, Keles E, Ilhan N, Gungor H, Kaygusuz I, Alpay HC. The relationship between Th1/Th2 balance and 1alpha,25-dihydroxyvitamin D(3) in patients with nasal polyposis. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2012;269:2519–24. doi: 10.1007/s00405-012-1967-x. [DOI] [PubMed] [Google Scholar]
- 52.Tiemessen MM, Van Ieperen-Van Dijk AG, Bruijnzeel-Koomen CA, Garssen J, Knol EF, Van Hoffen E. Cow’s milk-specific T-cell reactivity of children with and without persistent cow’s milk allergy: key role for IL-10. The Journal of allergy and clinical immunology. 2004;113:932–9. doi: 10.1016/j.jaci.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 53.Larche M. Regulatory T cells in allergy and asthma. Chest. 2007;132:1007–14. doi: 10.1378/chest.06-2434. [DOI] [PubMed] [Google Scholar]
- 54.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. The American journal of clinical nutrition. 2004;80:1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 55.Searing DA, Leung DY. Vitamin D in atopic dermatitis, asthma and allergic diseases. Immunology and allergy clinics of North America. 2010;30:397–409. doi: 10.1016/j.iac.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annual review of nutrition. 2003;23:117–45. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 57.Reichel H, Koeffler HP, Tobler A, Norman AW. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:3385–9. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matheu V, Back O, Mondoc E, Issazadeh-Navikas S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. The Journal of allergy and clinical immunology. 2003;112:585–92. doi: 10.1016/s0091-6749(03)01855-4. [DOI] [PubMed] [Google Scholar]
- 59.Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. The Journal of allergy and clinical immunology. 2009;123:1004–11. doi: 10.1016/j.jaci.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Rausch-Fan X, Leutmezer F, Willheim M, Spittler A, Bohle B, Ebner C, Jensen-Jarolim E, Boltz-Nitulescu G. Regulation of cytokine production in human peripheral blood mononuclear cells and allergen-specific th cell clones by 1alpha,25-dihydroxyvitamin D3. International archives of allergy and immunology. 2002;128:33–41. doi: 10.1159/000058001. [DOI] [PubMed] [Google Scholar]
- 61.Hawrylowicz CM. Regulatory T cells and IL-10 in allergic inflammation. The Journal of experimental medicine. 2005;202:1459–63. doi: 10.1084/jem.20052211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson DS. Regulatory T cells and asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2009;39:1314–23. doi: 10.1111/j.1365-2222.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- 63.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig H, Kegel C, Disch R, Schmidt-Weber CB, Blaser K, Akdis CA. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. The Journal of experimental medicine. 2004;199:1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. The Journal of experimental medicine. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baris S, Kiykim A, Ozen A, Tulunay A, Karakoc-Aydiner E, Barlan IB. Vitamin D as an adjunct to subcutaneous allergen immunotherapy in asthmatic children sensitized to house dust mite. Allergy. 2014;69:246–53. doi: 10.1111/all.12278. [DOI] [PubMed] [Google Scholar]
- 66.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, Loke TK, Robinson DS, Barrat FJ, O’Garra A, Lavender P, Lee TH, Corrigan C, Hawrylowicz CM. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. The Journal of clinical investigation. 2006;116:146–55. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. The Journal of clinical investigation. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hawrylowicz C, Richards D, Loke TK, Corrigan C, Lee T. A defect in corticosteroid-induced IL-10 production in T lymphocytes from corticosteroid-resistant asthmatic patients. The Journal of allergy and clinical immunology. 2002;109:369–70. doi: 10.1067/mai.2002.121455. [DOI] [PubMed] [Google Scholar]
- 69.Prietl B, Pilz S, Wolf M, Tomaschitz A, Obermayer-Pietsch B, Graninger W, Pieber TR. Vitamin D supplementation and regulatory T cells in apparently healthy subjects: vitamin D treatment for autoimmune diseases? The Israel Medical Association journal : IMAJ. 2010;12:136–9. [PubMed] [Google Scholar]
- 70.Yu S, Cantorna MT. The vitamin D receptor is required for iNKT cell development. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5207–12. doi: 10.1073/pnas.0711558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Korsgren M, Persson CG, Sundler F, Bjerke T, Hansson T, Chambers BJ, Hong S, Van Kaer L, Ljunggren HG, Korsgren O. Natural killer cells determine development of allergen-induced eosinophilic airway inflammation in mice. The Journal of experimental medicine. 1999;189:553–62. doi: 10.1084/jem.189.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wingett D, Nielson CP. Divergence in NK cell and cyclic AMP regulation of T cell CD40L expression in asthmatic subjects. Journal of leukocyte biology. 2003;74:531–41. doi: 10.1189/jlb.0303103. [DOI] [PubMed] [Google Scholar]
- 73.Ple C, Barrier M, Amniai L, Marquillies P, Bertout J, Tsicopoulos A, Walzer T, Lassalle P, Duez C. Natural killer cells accumulate in lung-draining lymph nodes and regulate airway eosinophilia in a murine model of asthma. Scandinavian journal of immunology. 2010;72:118–27. doi: 10.1111/j.1365-3083.2010.02419.x. [DOI] [PubMed] [Google Scholar]
- 74.Karimi K, Forsythe P. Natural killer cells in asthma. Frontiers in immunology. 2013;4:159. doi: 10.3389/fimmu.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–5. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 76.Akbari O, Stock P, DeKruyff RH, Umetsu DT. Role of regulatory T cells in allergy and asthma. Current opinion in immunology. 2003;15:627–33. doi: 10.1016/j.coi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 77.Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo LM, Hachem P, Fourneau JM, Sidobre S, Kronenberg M, Taniguchi M, Van Endert P, Dy M, Askenase P, Russo M, Vargaftig BB, Herbelin A, Leite-de-Moraes MC. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. Journal of immunology. 2003;171:1637–41. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 78.Du R, Litonjua AA, Tantisira KG, Lasky-Su J, Sunyaev SR, Klanderman BJ, Celedon JC, Avila L, Soto-Quiros ME, Weiss ST. Genome-wide association study reveals class I MHC-restricted T cell-associated molecule gene (CRTAM) variants interact with vitamin D levels to affect asthma exacerbations. The Journal of allergy and clinical immunology. 2012;129:368–73. 73 e1–5. doi: 10.1016/j.jaci.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barker DJ, Osmond C. Diet and coronary heart disease in England and Wales during and after the second world war. Journal of epidemiology and community health. 1986;40:37–44. doi: 10.1136/jech.40.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peters JL, Boynton-Jarrett R, Sandel M. Prenatal environmental factors influencing IgE levels, atopy and early asthma. Current opinion in allergy and clinical immunology. 2013;13:187–92. doi: 10.1097/ACI.0b013e32835e82d3. [DOI] [PubMed] [Google Scholar]
- 81.Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, Britton JR, McKeever TM. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129:735–44. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 82.Montefort S, Ellul P, Montefort M, Caruana S, Grech V, Agius Muscat H. The effect of cigarette smoking on allergic conditions in Maltese children (ISAAC) Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2012;23:472–8. doi: 10.1111/j.1399-3038.2012.01276.x. [DOI] [PubMed] [Google Scholar]
- 83.Brannon PM, Picciano MF. Vitamin D in pregnancy and lactation in humans. Annual review of nutrition. 2011;31:89–115. doi: 10.1146/annurev.nutr.012809.104807. [DOI] [PubMed] [Google Scholar]
- 84.Papapetrou PD. The interrelationship of serum 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D in pregnancy at term: a meta-analysis. Hormones. 2010;9:136–44. doi: 10.14310/horm.2002.1263. [DOI] [PubMed] [Google Scholar]
- 85.Liu NQ, Hewison M. Vitamin D, the placenta and pregnancy. Archives of biochemistry and biophysics. 2012;523:37–47. doi: 10.1016/j.abb.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 86.Miller SC, Halloran BP, DeLuca HF, Jee WS. Role of vitamin D in maternal skeletal changes during pregnancy and lactation: a histomorphometric study. Calcified tissue international. 1982;34:245–52. doi: 10.1007/BF02411245. [DOI] [PubMed] [Google Scholar]
- 87.Wasserman RH, Smith CA, Brindak ME, De Talamoni N, Fullmer CS, Penniston JT, Kumar R. Vitamin D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology. 1992;102:886–94. doi: 10.1016/0016-5085(92)90174-w. [DOI] [PubMed] [Google Scholar]
- 88.Rebut-Bonneton C, Demignon J. Effects of 1,25-dihydroxyvitamin D3 on in vitro lymphocyte reactions: arguments for a role at the maternofetal interface. Gynecologic and obstetric investigation. 1991;32:134–8. doi: 10.1159/000293014. [DOI] [PubMed] [Google Scholar]
- 89.Halhali A, Acker GM, Garabedian M. 1,25-Dihydroxyvitamin D3 induces in vivo the decidualization of rat endometrial cells. Journal of reproduction and fertility. 1991;91:59–64. doi: 10.1530/jrf.0.0910059. [DOI] [PubMed] [Google Scholar]
- 90.Joshi S, Pantalena LC, Liu XK, Gaffen SL, Liu H, Rohowsky-Kochan C, Ichiyama K, Yoshimura A, Steinman L, Christakos S, Youssef S. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Molecular and cellular biology. 2011;31:3653–69. doi: 10.1128/MCB.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. Journal of immunology. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 92.Henry HL, Norman AW. Vitamin D: metabolism and biological actions. Annual review of nutrition. 1984;4:493–520. doi: 10.1146/annurev.nu.04.070184.002425. [DOI] [PubMed] [Google Scholar]
- 93.Liu NQ, Kaplan AT, Lagishetty V, Ouyang YB, Ouyang Y, Simmons CF, Equils O, Hewison M. Vitamin D and the regulation of placental inflammation. Journal of immunology. 2011;186:5968–74. doi: 10.4049/jimmunol.1003332. [DOI] [PubMed] [Google Scholar]
- 94.Gernand AD, Simhan HN, Klebanoff MA, Bodnar LM. Maternal serum 25-hydroxyvitamin D and measures of newborn and placental weight in a U.S. multicenter cohort study. The Journal of clinical endocrinology and metabolism. 2013;98:398–404. doi: 10.1210/jc.2012-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bodnar LM, Klebanoff MA, Gernand AD, Platt RW, Parks WT, Catov JM, Simhan HN. Maternal vitamin D status and spontaneous preterm birth by placental histology in the US Collaborative Perinatal Project. American journal of epidemiology. 2014;179:168–76. doi: 10.1093/aje/kwt237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brannon PM. Vitamin D and adverse pregnancy outcomes: beyond bone health and growth. The Proceedings of the Nutrition Society. 2012;71:205–12. doi: 10.1017/S0029665111003399. [DOI] [PubMed] [Google Scholar]
- 97.Kho AT, Sharma S, Qiu W, Gaedigk R, Klanderman B, Niu S, Anderson C, Leeder JS, Weiss ST, Tantisira KG. Vitamin D related genes in lung development and asthma pathogenesis. BMC medical genomics. 2013;6:47. doi: 10.1186/1755-8794-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nguyen TM, Guillozo H, Marin L, Dufour ME, Tordet C, Pike JW, Garabedian M. 1,25-dihydroxyvitamin D3 receptors in rat lung during the perinatal period: regulation and immunohistochemical localization. Endocrinology. 1990;127:1755–62. doi: 10.1210/endo-127-4-1755. [DOI] [PubMed] [Google Scholar]
- 99.Marin L, Dufour ME, Nguyen TM, Tordet C, Garabedian M. Maturational changes induced by 1 alpha,25-dihydroxyvitamin D3 in type II cells from fetal rat lung explants. The American journal of physiology. 1993;265:L45–52. doi: 10.1152/ajplung.1993.265.1.L45. [DOI] [PubMed] [Google Scholar]
- 100.Marin L, Dufour ME, Tordet C, Nguyen M. 1,25(OH)2D3 stimulates phospholipid biosynthesis and surfactant release in fetal rat lung explants. Biology of the neonate. 1990;57:257–60. doi: 10.1159/000243200. [DOI] [PubMed] [Google Scholar]
- 101.Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. American journal of respiratory and critical care medicine. 2011;183:1336–43. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]
- 102.Zosky GR, Hart PH, Whitehouse AJ, Kusel MM, Ang W, Foong RE, Chen L, Holt PG, Sly PD, Hall GL. Vitamin D deficiency at 16 to 20 weeks’ gestation is associated with impaired lung function and asthma at 6 years of age. Annals of the American Thoracic Society. 2014;11:571–7. doi: 10.1513/AnnalsATS.201312-423OC. [DOI] [PubMed] [Google Scholar]
- 103.Vieth Streym S, Kristine Moller U, Rejnmark L, Heickendorff L, Mosekilde L, Vestergaard P. Maternal and infant vitamin D status during the first 9 months of infant life-a cohort study. European journal of clinical nutrition. 2013;67:1022–8. doi: 10.1038/ejcn.2013.152. [DOI] [PubMed] [Google Scholar]
- 104.Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatric and perinatal epidemiology. 2012;26 (Suppl 1):75–90. doi: 10.1111/j.1365-3016.2012.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bergman P, Lindh AU, Bjorkhem-Bergman L, Lindh JD. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PloS one. 2013;8:e65835. doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sly PD, Kusel M, Holt PG. Do early-life viral infections cause asthma? The Journal of allergy and clinical immunology. 2010;125:1202–5. doi: 10.1016/j.jaci.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 107.Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippila C, Ahonen S, Nevalainen J, Veijola R, Pekkanen J, Ilonen J, Simell O, Knip M, Virtanen SM. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2009;39:875–82. doi: 10.1111/j.1365-2222.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- 108.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. The American journal of clinical nutrition. 2007;85:788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, Helms PJ, Seaton A, Weiss ST. Maternal vitamin D intake during pregnancy and early childhood wheezing. The American journal of clinical nutrition. 2007;85:853–9. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 110.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. The European respiratory journal. 2010;35:1228–34. doi: 10.1183/09031936.00100609. [DOI] [PubMed] [Google Scholar]
- 111.Willett WC. Nutritional epidemiology. 2. New York (NY): Oxford University Press; 1998. [Google Scholar]
- 112.Willett WC, Hu FB. The food frequency questionnaire. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:182–3. doi: 10.1158/1055-9965.EPI-06-0843. [DOI] [PubMed] [Google Scholar]
- 113.Thomas SD, Fudge AN, Whiting M, Coates PS. The correlation between third-trimester maternal and newborn-serum 25-hydroxy-vitamin D in a selected South Australian group of newborn samples. BMJ open. 2011;1:e000236. doi: 10.1136/bmjopen-2011-000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maslova E, Hansen S, Jensen CB, Thorne-Lyman AL, Strom M, Olsen SF. Vitamin D intake in mid-pregnancy and child allergic disease - a prospective study in 44,825 Danish mother-child pairs. BMC pregnancy and childbirth. 2013;13:199. doi: 10.1186/1471-2393-13-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wills AK, Shaheen SO, Granell R, Henderson AJ, Fraser WD, Lawlor DA. Maternal 25-hydroxyvitamin D and its association with childhood atopic outcomes and lung function. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2013;43:1180–8. doi: 10.1111/cea.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cremers E, Thijs C, Penders J, Jansen E, Mommers M. Maternal and child’s vitamin D supplement use and vitamin D level in relation to childhood lung function: the KOALA Birth Cohort Study. Thorax. 2011;66:474–80. doi: 10.1136/thx.2010.151985. [DOI] [PubMed] [Google Scholar]
- 117.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, Town GI, Pattemore PK, Espinola JA, Crane J, New Zealand A. Allergy Cohort Study G, Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127:e180–7. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 118.Rothers J, Wright AL, Stern DA, Halonen M, Camargo CA., Jr Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. The Journal of allergy and clinical immunology. 2011;128:1093–9. e1–5. doi: 10.1016/j.jaci.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chawes BL, Bonnelykke K, Jensen PF, Schoos AM, Heickendorff L, Bisgaard H. Cord Blood 25(OH)-Vitamin D Deficiency and Childhood Asthma, Allergy and Eczema: The COPSAC2000 Birth Cohort Study. PloS one. 2014;9:e99856. doi: 10.1371/journal.pone.0099856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu X, Wang G, Hong X, Wang D, Tsai HJ, Zhang S, Arguelles L, Kumar R, Wang H, Liu R, Zhou Y, Pearson C, Ortiz K, Schleimer R, Holt PG, Pongracic J, Price HE, Langman C, Wang X. Gene-vitamin D interactions on food sensitization: a prospective birth cohort study. Allergy. 2011;66:1442–8. doi: 10.1111/j.1398-9995.2011.02681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jones AP, Palmer D, Zhang G, Prescott SL. Cord blood 25-hydroxyvitamin D3 and allergic disease during infancy. Pediatrics. 2012;130:e1128–35. doi: 10.1542/peds.2012-1172. [DOI] [PubMed] [Google Scholar]
- 122.Weisse K, Winkler S, Hirche F, Herberth G, Hinz D, Bauer M, Roder S, Rolle-Kampczyk U, von Bergen M, Olek S, Sack U, Richter T, Diez U, Borte M, Stangl GI, Lehmann I. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy. 2013;68:220–8. doi: 10.1111/all.12081. [DOI] [PubMed] [Google Scholar]
- 123.Kasahara AK, Singh RJ, Noymer A. Vitamin D (25OHD) Serum Seasonality in the United States. PloS one. 2013;8:e65785. doi: 10.1371/journal.pone.0065785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, Reid IR. The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. The American journal of clinical nutrition. 2007;86:959–64. doi: 10.1093/ajcn/86.4.959. [DOI] [PubMed] [Google Scholar]
- 125.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. The American journal of clinical nutrition. 2008;87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 126.Lasky-Su J, Lange N, Brehm JM, Damask A, Soto-Quiros M, Avila L, Celedon JC, Canino G, Cloutier MM, Hollis BW, Weiss ST, Litonjua AA. Genome-wide association analysis of circulating vitamin D levels in children with asthma. Human genetics. 2012;131:1495–505. doi: 10.1007/s00439-012-1185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. The American journal of clinical nutrition. 2010;91:1255–60. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 128.Majak P, Olszowiec-Chlebna M, Smejda K, Stelmach I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. The Journal of allergy and clinical immunology. 2011;127:1294–6. doi: 10.1016/j.jaci.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 129.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PloS one. 2010;5:e11088. doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–8. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 131.Devereux G, Wilson A, Avenell A, McNeill G, Fraser WD. A case-control study of vitamin D status and asthma in adults. Allergy. 2010;65:666–7. doi: 10.1111/j.1398-9995.2009.02220.x. [DOI] [PubMed] [Google Scholar]
- 132.Li F, Peng M, Jiang L, Sun Q, Zhang K, Lian F, Litonjua AA, Gao J, Gao X. Vitamin D deficiency is associated with decreased lung function in Chinese adults with asthma. Respiration; international review of thoracic diseases. 2011;81:469–75. doi: 10.1159/000322008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Umland SP, Schleimer RP, Johnston SL. Review of the molecular and cellular mechanisms of action of glucocorticoids for use in asthma. Pulmonary pharmacology & therapeutics. 2002;15:35–50. doi: 10.1006/pupt.2001.0312. [DOI] [PubMed] [Google Scholar]
- 134.Hollams EM, Hart PH, Holt BJ, Serralha M, Parsons F, de Klerk NH, Zhang G, Sly PD, Holt PG. Vitamin D and atopy and asthma phenotypes in children: a longitudinal cohort study. The European respiratory journal. 2011;38:1320–7. doi: 10.1183/09031936.00029011. [DOI] [PubMed] [Google Scholar]
- 135.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, Weiss ST, Litonjua AA. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. American journal of respiratory and critical care medicine. 2009;179:765–71. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, Weiss ST, Litonjua AA. Childhood Asthma Management Program Research G, Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. The Journal of allergy and clinical immunology. 2010;126:52–8. e5. doi: 10.1016/j.jaci.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Aller Clin Immun. 2010;125:995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chinellato I, Piazza M, Sandri M, Peroni D, Piacentini G, Boner AL. Vitamin D serum levels and markers of asthma control in Italian children. The Journal of pediatrics. 2011;158:437–41. doi: 10.1016/j.jpeds.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 140.Chinellato I, Piazza M, Sandri M, Peroni DG, Cardinale F, Piacentini GL, Boner AL. Serum vitamin D levels and exercise-induced bronchoconstriction in children with asthma. Eur Respir J. 2011;37:1366–70. doi: 10.1183/09031936.00044710. [DOI] [PubMed] [Google Scholar]
- 141.Peroni DG, Piacentini GL, Cametti E, Chinellato I, Boner AL. Correlation between serum 25-hydroxyvitamin D levels and severity of atopic dermatitis in children. Br J Dermatol. 2011;164:1078–82. doi: 10.1111/j.1365-2133.2010.10147.x. [DOI] [PubMed] [Google Scholar]
- 142.Bosse Y, Maghni K, Hudson TJ. 1alpha,25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiological genomics. 2007;29:161–8. doi: 10.1152/physiolgenomics.00134.2006. [DOI] [PubMed] [Google Scholar]
- 143.Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, Kazani SD, Moore WC, Moy J, Sorkness CA, Avila P, Bacharier LB, Bleecker E, Boushey HA, Chmiel J, Fitzpatrick AM, Gentile D, Hundal M, Israel E, Kraft M, Krishnan JA, LaForce C, Lazarus SC, Lemanske R, Lugogo N, Martin RJ, Mauger DT, Naureckas E, Peters SP, Phipatanakul W, Que LG, Sheshadri A, Smith L, Solway J, Sullivan-Vedder L, Sumino K, Wechsler ME, Wenzel S, White SR, Sutherland ER. National Heart L, Blood Institute’s A, Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA : the journal of the American Medical Association. 2014;311:2083–91. doi: 10.1001/jama.2014.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goleva E, Searing DA, Jackson LP, Richers BN, Leung DY. Steroid requirements and immune associations with vitamin D are stronger in children than adults with asthma. The Journal of allergy and clinical immunology. 2012;129:1243–51. doi: 10.1016/j.jaci.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. The Journal of allergy and clinical immunology. 2010;125:995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. American journal of respiratory and critical care medicine. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zheng T, Yu J, Oh MH, Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy, asthma & immunology research. 2011;3:67–73. doi: 10.4168/aair.2011.3.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sidbury R, Sullivan AF, Thadhani RI, Camargo CA., Jr Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: a pilot study. The British journal of dermatology. 2008;159:245–7. doi: 10.1111/j.1365-2133.2008.08601.x. [DOI] [PubMed] [Google Scholar]
- 149.Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, Kanada K, Yamasaki K, Alexandrescu D, Gallo RL. Administration of oral vitamin D induces cathelicidin production in atopic individuals. The Journal of allergy and clinical immunology. 2008;122:829–31. doi: 10.1016/j.jaci.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Spergel JM. Atopic march: link to upper airways. Current opinion in allergy and clinical immunology. 2005;5:17–21. doi: 10.1097/00130832-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 151.Semper AE, Heron K, Woollard AC, Kochan JP, Friedmann PS, Church MK, Reischl IG. Surface expression of Fc epsilon RI on Langerhans’ cells of clinically uninvolved skin is associated with disease activity in atopic dermatitis, allergic asthma, and rhinitis. The Journal of allergy and clinical immunology. 2003;112:411–9. doi: 10.1067/mai.2003.1626. [DOI] [PubMed] [Google Scholar]
- 152.Ciprandi G, Cirillo I, Vizzaccaro A, Milanese M, Tosca MA. Airway function and nasal inflammation in seasonal allergic rhinitis and asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2004;34:891–6. doi: 10.1111/j.1365-2222.2004.01970.x. [DOI] [PubMed] [Google Scholar]
- 153.Leynaert B, Neukirch C, Kony S, Guenegou A, Bousquet J, Aubier M, Neukirch F. Association between asthma and rhinitis according to atopic sensitization in a population-based study. The Journal of allergy and clinical immunology. 2004;113:86–93. doi: 10.1016/j.jaci.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 154.Mai XM, Chen Y, Camargo CA, Jr, Langhammer A. Serum 25-hydroxyvitamin D levels and self-reported allergic rhinitis in Norwegian adults - The HUNT Study. Allergy. 2014;69:488–93. doi: 10.1111/all.12365. [DOI] [PubMed] [Google Scholar]
- 155.Roider E, Ruzicka T, Schauber J. Vitamin d, the cutaneous barrier, antimicrobial peptides and allergies: is there a link? Allergy, asthma & immunology research. 2013;5:119–28. doi: 10.4168/aair.2013.5.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C. Princess Anne Hospital Study G, Maternal vitamin D status during pregnancy and child outcomes. European journal of clinical nutrition. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kull I, Bergstrom A, Melen E, Lilja G, van Hage M, Pershagen G, Wickman M. Early-life supplementation of vitamins A and D, in water-soluble form or in peanut oil, and allergic diseases during childhood. The Journal of allergy and clinical immunology. 2006;118:1299–304. doi: 10.1016/j.jaci.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 158.Reinholz M, Ruzicka T, Schauber J. Vitamin D and its role in allergic disease. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2012;42:817–26. doi: 10.1111/j.1365-2222.2011.03923.x. [DOI] [PubMed] [Google Scholar]
- 159.Sharief S, Jariwala S, Kumar J, Muntner P, Melamed ML. Vitamin D levels and food and environmental allergies in the United States: results from the National Health and Nutrition Examination Survey 2005–2006. The Journal of allergy and clinical immunology. 2011;127:1195–202. doi: 10.1016/j.jaci.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Allen KJ, Koplin JJ, Ponsonby AL, Gurrin LC, Wake M, Vuillermin P, Martin P, Matheson M, Lowe A, Robinson M, Tey D, Osborne NJ, Dang T, Tina Tan HT, Thiele L, Anderson D, Czech H, Sanjeevan J, Zurzolo G, Dwyer T, Tang ML, Hill D, Dharmage SC. Vitamin D insufficiency is associated with challenge-proven food allergy in infants. The Journal of allergy and clinical immunology. 2013;131:1109–16. 16 e1–6. doi: 10.1016/j.jaci.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 161.Mullins RJ, Clark S, Katelaris C, Smith V, Solley G, Camargo CA., Jr Season of birth and childhood food allergy in Australia. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2011;22:583–9. doi: 10.1111/j.1399-3038.2011.01151.x. [DOI] [PubMed] [Google Scholar]
- 162.Vassallo MF, Banerji A, Rudders SA, Clark S, Mullins RJ, Camargo CA., Jr Season of birth and food allergy in children. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2010;104:307–13. doi: 10.1016/j.anai.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weiss ST, Litonjua AA. Vitamin D in asthma and allergy: what next? The European respiratory journal. 2011;38:1255–7. doi: 10.1183/09031936.00129811. [DOI] [PubMed] [Google Scholar]
- 164.Bozzetto S, Carraro S, Giordano G, Boner A, Baraldi E. Asthma, allergy and respiratory infections: the vitamin D hypothesis. Allergy. 2012;67:10–7. doi: 10.1111/j.1398-9995.2011.02711.x. [DOI] [PubMed] [Google Scholar]
- 165.Sundar IK, Rahman I. Vitamin d and susceptibility of chronic lung diseases: role of epigenetics. Frontiers in pharmacology. 2011;2:50. doi: 10.3389/fphar.2011.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wjst M. Is vitamin D supplementation responsible for the allergy pandemic? Current opinion in allergy and clinical immunology. 2012;12:257–62. doi: 10.1097/ACI.0b013e3283535833. [DOI] [PubMed] [Google Scholar]
- 167.Hossein-nezhad A, Spira A, Holick MF. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: a randomized double-blind clinical trial. PloS one. 2013;8:e58725. doi: 10.1371/journal.pone.0058725. [DOI] [PMC free article] [PubMed] [Google Scholar]