Abstract
Peripheral mechanisms in appetite regulation include the motor functions of the stomach, such as the rate of emptying and accommodation, which convey symptoms of satiation to the brain. The rich repertoire of peripherally released peptides and hormones provides feedback from the arrival of nutrients in different regions of the gut from where they are released to exert effects on satiation, or regulate metabolism through their incretin effects. Ultimately, these peripheral factors provide input to the highly organized hypothalamic circuitry and vagal complex of nuclei to determine cessation of energy intake during meal ingestion, and the return of appetite and hunger after fasting. Understanding these mechanisms is key to the physiological control of feeding and the derangements that occur in obesity and their restoration with treatment (as demonstrated by the effects of bariatric surgery).
Keywords: satiation, satiety, stomach, neurohormonal
INTRODUCTION
The objectives are to review the control of gastric motility and digestion; the gastrointestinal mechanisms controlling appetite including gut-brain communication, the effects of peripherally-released peptides and hormones on hypothalamic and brainstem control; and the effects of macronutrients on the functions associated with appetite and gastric functions.
Definitions of Satiation and Satiety
Satiation refers to the postprandial feeling of fullness that may be one of pleasure or distress and manifests other symptoms such as fullness, nausea or bloating. Operationally, satiation can be defined as the maximum tolerated volume of a liquid nutrient meal, and by intra- and postprandial symptoms experienced with the challenge.
Satiety reflects the appetite to ingest meals, and it may be operationally defined by the kilocalories ingested at a subsequent ad libitum buffet meal after a standard period of fasting or a specified time from a prior standard meal, typically ingested 4 hours previously (e.g., a 300kcal meal). It is relevant to distinguish these two terms, as satiation reflects the early postprandial experience, whereas satiety reflects the appetite to ingest food at a subsequent meal after a period of fasting.
Since obesity results from the imbalance between energy consumed and expended, postprandial satiation and satiety are critically relevant to the development of obesity.
Gastric Motility and Phases of Digestion
Gastric motility is critically involved in the cephalic, gastric and enteric phases of digestion; these functions are integrated.
Taste and other visceral sensations project into the nucleus of the solitary tract (NTS). The NTS is a sensory complex linked through the action of neurons secreting acetylcholine (Ach), neuropeptide Y (NPY) and thyrotrophin-releasing hormone (TRH) to the dorsal motor nucleus of the vagus (DMV) in the brainstem to activate the processes of digestion. The vagus nerve is the major mediator of the cephalic phase of gastric motility and digestion, and the main neurotransmitter is acetylcholine. Cholecystokinin (CCK) and gastrin are additional modulators that control a coordinated digestive response, including gastric acid and pepsin secretion, gastric motility and emptying, and integrated pancreatico-biliary secretion. Descending projections from higher senses, such as olfaction and vision, and from the forebrain also modulate these brainstem reflexes.
It is estimated that the cephalically-stimulated motor and secretory activities in the upper and mid-gut contribute >50% of the overall postprandial response.1 Food ingestion releases gastrointestinal hormones and activates gastrointestinal motility, gastric and pancreatico-biliary secretion and absorption (Fig. 1). Gastrin, CCK, glucose-dependent insulinotropic peptide (GIP), ghrelin, gastric leptin and pancreatic polypeptide are secreted from the upper gut. Incretins such as GLP-1, peptide YY and oxyntomodulin, which are secreted from the proximal and distal small intestine, generally inhibit the cephalic phase mediated through the vagus nerve. Upper gut-derived hormones, such as CCK and GIP, also inhibit gastric motility by relaxing the fundus and antrum or stimulating pyloric contraction to inhibit gastric emptying. This results in slowing of meal digestion, and induction of satiation resulting in reduced ingestion of calories.
Figure 1.
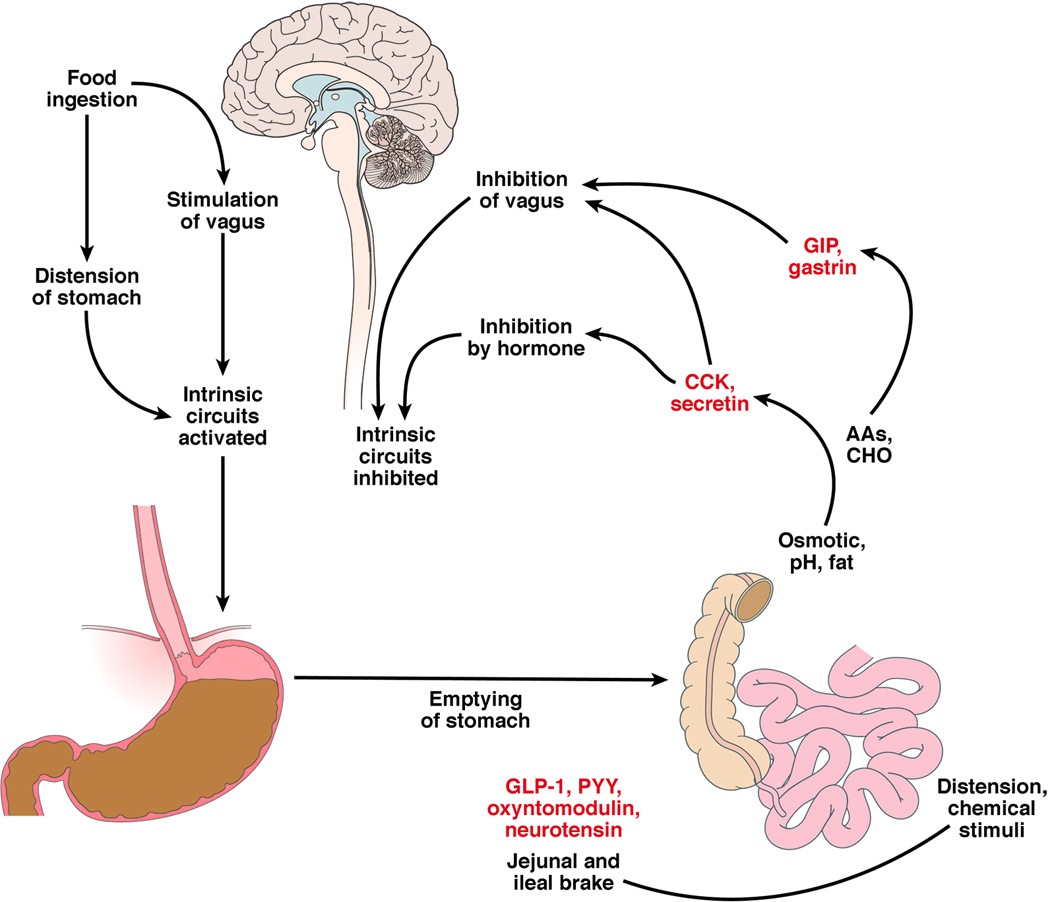
Integrated neurohormonal response to the ingestion of food. Sensing of different nutrients by enteroendocrine cells results in the release of diverse hormones and peptides that result in gastric accommodation to the meal, stimulation of gastric contractions that lead to emptying and, when the nutrients reach different levels of the small intestine, the release of substances that provide generally negative feedback that delays gastric emptying (e.g. CCK in the duodenum, GLP-1 and PYY in the more distal small intestine and colon).
Role of Gastric Motor Functions in Postprandial Symptoms
There has been little attention to the role of gastric motor functions and development of postprandial symptoms in obesity.2 However, a vast literature based on hundreds of patients with dyspepsia convincingly shows that gastric motor functions (such as emptying and accommodation) and gastric sensation are important determinants of intraprandial and postprandial symptoms.3 Moreover, the proportion of fats with at least 12-carbon chain length influences satiation in health and dyspepsia.4 In obese individuals, apart from BMI, age and gender, the following factors contribute to postprandial symptoms: smaller fasting or postprandial gastric volume, accelerated gastric emptying at 1 hour and delayed gastric emptying at 4 hours.5 The univariate association of postprandial gastric volume with symptoms is not independent of the fasting gastric volume. However, these data support the notion that gastric motor functions are important predictors of the sensation of fullness or satiation and may determine the individual’s choice to stop ingesting food. The relevance of these factors is demonstrated by the effects of sleeve gastrectomy on appetite and weight loss (discussed below).
GUT-BRAIN COMMUNICATION
The vagal nuclei and the vagus nerve innervate most of the gastrointestinal tract involved in energy intake, satiation and digestion.6 Vagal afferents are stimulated directly by change in viscus tension when food, or later chyme, passes through the gut gastrointestinal tract, and indirectly by chemical stimuli activating taste receptors (see below) releasing peptides from mucosal enteroendocrine cells. Some of these peptides act on vagal or other pathways to induce appetite (an orexigenic effect, e.g. ghrelin) whereas others (e.g. gastric leptin, CCK, GLP-1 or PYY) induce satiety – an anorexigenic effect.
Circulating nutrients, reflecting levels of nutrients in the periphery, influence brainstem nuclei after being sensed in the area postrema in the floor of the 4th ventricle, where there is a thin blood brain barrier.7 In response, the brain stem controls the enteric nervous system,8,9 modulating upper gut function or signalling to the hypothalamic circuits to reduce calorie intake. On the other hand, partial vagotomy or total sub-diaphragmatic vagotomy or intermittent vagal nerve electrical stimulation to inhibit vagal function in humans10 decreases food intake and induces early satiety and weight loss, possibly by reducing gastric emptying and inducing satiation.
MECHANISMS REGULATING APPETITE
Several interacting control mechanisms (Fig. 2) that involve peripherally-released mediators are involved in the regulation of appetite.6
Figure 2.

Peripheral and central factors modulating appetite centers in the brain. Gastrointestinal and fat-derived hormones stimulate specific areas of the hypothalamus and brainstem that sense nutrients, and coordinate the response to hunger and the intake of food. The arcuate nucleus in the hypothalamus receives input from brainstem (e.g., vagal) nuclei as well as direct stimulation by circulating hormones through an incomplete blood brain barrier. Neurons in the arcuate nucleus are either orexigenic [e.g., contain neuropeptide Y via Y1 receptors or agouti-related peptide (AgRP)] or anorexigenic [e.g., contain pro-opiomelanocortin (POMC)], cocaine- and amphetamine-related transcript (CART)]. POMC is a precursor of α-melanocyte stimulating hormone (α-MSH). Ultimately, other regions of the hypothalamus (the paraventricular nucleus and lateral hypothalamus) and higher centers (such as amygdala, limbic system and cerebral cortex) are stimulated to change feeding behavior by influencing the functions of the same hypothalamic nuclei. Redrawn from Camilleri M, Grudell AB. Appetite and obesity: a gastroenterologist's perspective. Neurogastroenterol Motil 2007;19:333–341.
A. Hypothalamic and Brainstem Mechanisms
Hypothalamic circuits involve several peptide receptors that control appetite and food intake. These mechanisms include cannabinoid (CB1),11 neuropeptide Y (NPY), pro-opiomelanocortin (POMC), melanin-concentrating hormone (MCH), α-melanocyte stimulating hormone (α-MSH), agouti-related peptide (AgRP), cocaine- and amphetamine-regulated transcript (CART), cholecystokinin (CCK), and glucagon-like peptide 1 (GLP-1) (Figs. 1 and 2).
Neural pathways link the hypothalamic nuclei to higher centers (that produce food reward or feeling of well-being) and to the brainstem nuclei. Through the brainstem nuclei, such as the NTS and DMV, the hypothalamus can slow gastric emptying by stimulating vagal fibers that activate intramural gastric nitrergic neurons to decrease gastric motility,12,13 retard gastric emptying, and, as a consequence, decrease calorie consumption.14–16
The precise pathways and centers involved in reward appear to involve NPY and dopaminergic receptors,17 and eating for reward value is a primitive behavior observed in species such as Drosophila.18
Preliminary data in humans using MRI with pulsed arterial spin labeling (PASL) in response to a nutrient drink (Ensure ®) show that the sensation of satiation is associated with decreased cerebral blood flow (CBF) in the hypothalamus compared to a control brain region (posterior frontal cortex); using repeat measurements at 15-minute intervals, hypothalamic PASL MRI signal decreased significantly after ingesting the maximum tolerated volume (MTV) of the liquid meal, and this decreased signal persisted 30 minutes later.19 The effect of nutrients in Ensure® may reflect the greater reduction in CBF in the hypothalamus to the monosaccharide glucose compared to fructose ingestion,20 as well as increased functional connectivity between the hypothalamus and the thalamus and striatum with glucose, and increased connectivity between the hypothalamus and thalamus, but not the striatum, with fructose. In another study, blood oxygenation level dependent (BOLD) signal in the cortical control areas increased during glucose and decreased during fructose infusion.21 The significance of these findings is unclear; however, fructose ingestion was associated with smaller increase in systemic glucose, insulin, and GLP-1 levels than glucose ingestion.20 The time course of changes in hypothalamic and brainstem fluctuations in BOLD signal on functional MRI in response to sucrose (combination of glucose and fructose) is consistent with a rapid, vagally-mediated mechanism due to nutrient absorption, rather than sweet taste receptor activation.22
Gastrointestinal hormones including leptin, cholecystokinin, PYY, oxyntomodulin (OXM), and GLP-1 may affect nuclei in the hypothalamus and brainstem where the blood brain barrier allows direct interaction between these hormones with the nuclei, just as described above for nutrients. The circulating peptides inhibit the AgRP/NPY pathway in the arcuate nucleus of the hypothalamus thereby reducing appetite and stimulate the pro-opiomelanocortin / α-melanocyte stimulation hormone (POMC/αMSH) pathway thereby increasing satiety and indirectly reducing appetite (Figs. 2 and 3).23
Figure 3.
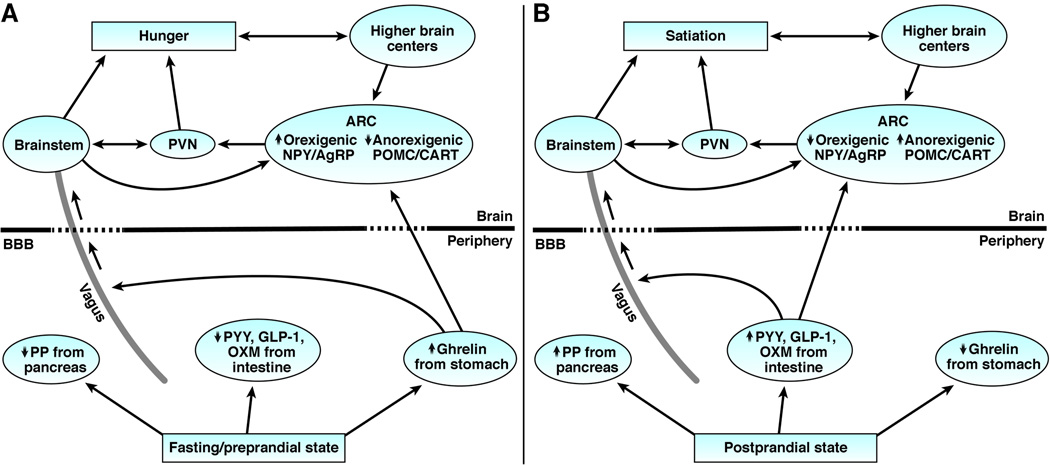
The gut hormone signaling to the brain to mediate sensations of hunger and satiation. Left panel: During the fasting/preprandial state, ghrelin release from the stomach acts upon the arcuate nucleus of the hypothalamus and vagus nucleus in the brainstem to stimulate hunger. Right panel: In the postprandial state, release of anorectic hormones, PYY, GLP-1, OXM, and PP from the intestine act upon the ARC, brainstem, and vagus to cause satiation.
B. Taste Receptors for Sweet or Amino Acids (umami)
Similarities between the chemosensory machinery in neuroepithelial taste receptor cells of the tongue and the molecular transducers localized in enteroendocrine cells in the gut that sense the chemical composition of the luminal contents of the gut24 led to the concept of non-lingual taste receptors. Molecular sensing by gastrointestinal cells controls multiple fundamental functions in digestion and initiates hormonal and/or afferent neural (predominantly vagal) pathways leading to satiation, the regulation of caloric intake, pancreatic insulin secretion, and changes in metabolism. For example, glucose-sensing by gut enteroendocrine cells results in the release of incretin peptides, activation of vagal afferents and glucose homeostasis. Vagal afferent nerve terminals in the intestinal mucosa express receptors for a number of different regulatory peptides and neurotransmitters, including those released by glucose (GLP-1, GLP-2, 5-HT).
There are three distinct subunits of the G protein coupled taste receptors, T1Rs. These form heterodimers that mediate either sweet taste (T1R2 and T1R3) or sense amino acids or umami (T1R1 and T1R3). The subunits of T1R2 and T1R3, as well as other elements of the sweet taste transduction pathway found in the lingual epithelium including Gα-gustducin, are expressed in intestinal enteroendocrine cells. Gα-gustducin is co-localized in enteroendocrine cells that express PYY in the colon or GLP-1 and 5-HT in the small intestine. T1Rs upregulate the sodium-coupled transporters, SGLT-1 and GLUT2, in the intestinal epithelium in response to glucose, and T1Rs also regulate GLP-1 secretion. The T1R system has broad specificity for sweet sensing and can be activated by natural sugars, sweet proteins and artificial sweeteners.25 There are also fat-sensing receptors, FFAR1 and GPR120, in enteroendocrine cells,26 and the receptors sense both medium- and long-chain fatty acids.27,28 Binding of basic tastants (sweet, bitter, umami, or fat) initiates second-messenger cascades that result in the release of peptides or neurotransmitters (e.g., ghrelin, CCK, GLP-1, PYY), resulting in physiological events. Each receptor is coupled to distinct gustatory G proteins; gustducin is the most common taste G protein.27,28 Gut endocrine cells may also detect microbiota, as they also express toll-like receptor molecules that recognize bacterial breakdown products.25
C. Gut Peptidergic and Hormonal Control of the Response to Feeding and Satiation
Ingested nutrients and their digestion products initiate local actions in the upper gut, producing signals that initiate digestion and absorption. Other signals lead to the feeling of satiation, either directly or indirectly through effects on gastric function, and lead to meal termination. Even during ingestion of the meal, gastric emptying of liquids results in the rapid delivery of nutrients into the intestine. Gastric and duodenal vagal afferents are stimulated by the mechanical, chemical and osmotic effects of ingested nutrients, stimulating the release of a variety of peptides and hormones,29 which mediate or modulate digestion, sensation after feeding and appetite.30
Following is a brief summary of the main effects of the predominant hormones or peptides on food intake or appetite. Ghrelin is an orexigen that is important in short-term food intake; in contrast, leptin (gastric) is a minor orexigen, and obestatin is a peptide encoded by the ghrelin gene that opposes ghrelin’s effects on food intake, delays gastric emptying and inhibits jejunal motility.31 CCK is a major mediator of satiation, providing negative feedback to the stomach (delaying emptying, at least in part by fundic relaxation and antral inhibition). GLP-1 is an incretin that modulates glucose control and provides similar negative feedback to the stomach. Peptide YY is involved in appetite control, the ileal brake, and negative feedback to the stomach. Table 1 expands on this summary, reviewing the role of gastrointestinal peptides and hormones released in response to feeding and their potential physiological effects of relevance to satiation or satiety in humans.32–112
Table 1.
Peptides, hormones and receptors involved in the peripheral gastrointestinal control of upper gastrointestinal functions associated with satiation and appetite.
| Peptide/ Hormone/ Receptor |
Predominant Site of Synthesis/ Release |
Main Functions | Refs. |
|---|---|---|---|
| Gastrins | 17-AA peptide from gastric mucosa |
|
32–34 |
| CCK | I cells in duodenal mucosa, particularly ≥12 carbon chain long fatty acids; multiple molecular forms |
|
35–38 |
| CCK1R | Vagal afferents |
|
39 |
| CCK2R | CNS, hypothalamic (e.g. arcuate) nuclei |
|
40–42 |
| Ghrelin | 28-AA peptide expressed mostly in stomach |
|
43–45 46 47–49 50 51–63 64–66 |
| GHS-R | G protein-coupled receptor |
|
43 |
| Leptin | Circulating 16-kDa protein (167 AA) secreted by adipose tissue, placenta, skeletal muscle |
|
67–73 74 75–78 |
| Gastric leptin | Fundic glands, and chief cells | Reduced during fasting, rapidly released after food intake by vagal cholinergic stimulation, CCK and secretin, or in response to satiety factors (e.g., CCK and insulin) | 79 |
| GLP-1 | Co-secreted with PYY from intestinal L cells: GLP-17–36 amide is located in the PVN, DMV, NTS, pituitary, and thalamus |
|
80–82 83 84–87 |
| NPY | 36-AA peptide in hypo-thalamus, and intestinal neurons |
|
88 89 |
| NPY (Y) receptors | Multiple receptor subtypes Y1 to Y5 | Y1, Y2, and Y5 receptor subtypes mediate NPY-induced feeding; central NPY delays GE in rats (through Y2 receptors); in humans, i.v. NPY had no effect on GE | 90,91 |
| PP | 36-AA peptide from D cells in pancreas | Experimentally, anorectic effects with peripheral PP, central PP stimulates food intake; Circulating PP levels inversely proportional to adiposity; higher levels with anorexia nervosa, reductions in circulating PP in some studies in obese subjects; PP may delay GE | 92–96 |
| PYY | Co-secreted with GLP-1 from ileocolonic L cells; active form PYY3–36 |
|
97 98–103 104–107 |
| OXM | 37-AA peptide, from intestinal L cells | Acts via GLP-1 receptors to decrease food intake and inhibit gastric acid secretion and GE | 108,109 |
| Apo A-IV | 46kDa protein from small bowel enterocytes | synthesized and secreted in response to lipid absorption; exogenous apo A-IV decreased food intake (mediated centrally) and inhibited gastric acid secretion and motility in rodents | 110–112 |
AA: amino acid; Apo A-IV: apolipoprotein A-IV; CCK: cholecystokinin; CNS: central nervous system; DMV: dorsal motor nucleus of the vagus nerve; EC: enterochromaffin cells; GE: gastric emptying; GHS-R: growth hormone secretagogue receptor; GLP-1: glucagon like peptide-1; NPY: neuropeptide Y; NTS: nucleus of the tractus solitaries; OXM: oxyntomodulin; PP: pancreatic polypeptide; PVN: paraventricular nucleus of the hypothalamus; PVN-ARC: paraventricular and arcuate nuclei of the hypothalamus; PYY: peptide tyrosine-tyrosine;
italics refers to receptors of peptides/hormones
Adiposity- and Glycemia-related Hormones
Insulin from the pancreatic β cells and leptin from white adipocytes (as well as the stomach and other tissues) are each secreted in direct proportion to body fat. Both hormones are transported through the blood-brain barrier113,114 and access neurons in the hypothalamus and other regions of the brain to influence energy homeostasis. In contrast to satiation signals, which primarily influence calories eaten during individual meals, adiposity signals are more directly related to how much fat the body carries and maintains. Insulin systemically elicits hypoglycemia, which increases food intake. In obesity, there is insulin and leptin resistance; thus, more of each hormone is required to achieve the same physiological effect when compared to lean individuals.
EFFECTS OF MEAL PROPERTIES ON FOOD INTAKE, SATIETY, AND SATIATION
The nutrient content of a meal influences the rate of emptying from the stomach. For example, the maximum emptying rate of liquid food from the stomach is ~200 kcal/hour.115 Compared to calorie density, the volume, carbohydrate and protein content of the meal have minor effects on the rate of gastric emptying, whereas fat has a major effect. CCK-mediated reflexes are activated by chylomicrons or by fatty acids with at least 12-carbon chain lengths in the upper small intestine and result in inhibition of antral motility,116 thereby retarding gastric emptying. The energy density, meal volume and physical properties of a meal influence food intake, satiety and satiation, predominantly through effects on gastric emptying. The next section addresses the role of macronutrient content of food in food intake.
Does Macronutrient Class Influence Gastric or Pancreatic Function, or Food Intake?
Several studies have addressed whether intake of nutrients results in adaptation of gastric functions and satiation mechanisms. The adaptation of these mechanisms to different nutrients has been tested by means of nutrient “preloads”, typically for a two-week period. In these studies, calories in a macronutrient class were given in excess of caloric needs, and the effects on gastric or pancreatic function were assessed. Whereas, such studies can be done in animals with 90% or 100% of the calories designated in a certain class,117 there are limitations to the degree of enrichment with a specific macronutrient class in diets ingested by humans. Hence, animal data are less relevant to the human situation.
Carbohydrate tends to reduce pancreatic enzyme output, and fat has the greatest potential to perturb digestive functions including interdigestive and postprandial outputs of pancreatic enzymes118 and gastric emptying.
Nutrient preloads of four different classes of macronutrients (protein, carbohydrate, fat or mixed/balanced) did not significantly affect satiation, appetite (food ingested at an ad libitum meal) and gastric emptying.119,120 These studies were conducted in 10 male, normal weight healthy volunteers119 and in 52 healthy normal-weight, overweight, or obese participants (14 males, 38 females with BMI 19.4–47.0 kg/m2).119,120 However, nutrient pre-load with a high-fat diet (4800kcal) increased hunger ratings during duodenal lipid infusion compared with the same infusion after a lower fat (2670kcal) diet.121 In contrast, a more physiological, balanced nutrient, orally administered meal (Ensure®) was associated with very similar postprandial gastric volumes after the four diets enriched with a specific macronutrient class.120
Carbohydrate influence on appetite was elucidated in classical studies.122 Compared with soup that was not supplemented with carbohydrate, Blundell et al. found that carbohydrate supplemented soup significantly reduced subjective hunger and subsequent food intake by an amount that compensated for the higher carbohydrate content of the supplemented soup. Such studies provided evidence for the physiological regulation of short-term appetite by carbohydrates.
The effects of different fat molecules (i.e., MCFA, LCFA, saturated and unsaturated FA, as well as omega-3 FAs) are summarized in Table 2.123–130
Table 2.
Summary of effects of different fat molecules on appetite, food intake and weight
| Fat variation | Effects on appetite/satiety, food intake or weight | Reference |
|---|---|---|
| LCFA | C18 fatty acids reduce food intake; effect not related to rate of absorption but partly by CCK release | 123 |
| MCFA | No significant effect of fatty acid chain length (LCFA vs. MCFA for 3 days) on ratings of hunger, fullness, satisfaction or current thoughts of food, nor did energy or macronutrient intake at next meal differ between diets |
124 125 |
| Triacylglycerol (olestra) | Olestra does not influence signals of satiation including cholecystokinin and stomach emptying; most studies of olestra on human satiation found no additional energy consumption when olestra was substituted for dietary fat | 126 |
| MUFAs, PUFAs, or SFAs | Short-term studies indicate that PUFA may exert a relatively stronger control over appetite than MUFA and SFA | 127 |
| SFA-rich meal elicited greater subjective feelings of fullness compared to MUFA-and PUFA-rich meals; postprandial PYY response (area under the curve) was significantly lower for the MUFA-rich meal vs. the SFA-rich or PUFA-rich meals | 128 | |
| Long-chain omega-3 PUFAs | Observational studies (Health Professional Follow-up Study and Nurses’ Health Study) and RCTs provide conflicting evidence of weight gain or loss | 129 |
| High amount (>1300 mg/day; n = 121) associated with lower hunger sensations immediately after test dinner (fullness) and after 120 min (fullness and hunger) compared to low amount (<260 mg/day; n = 112) | 130 |
MUFA=Monounsaturated fatty acids; PUFAs=,polyunsaturated fatty acids; SFAs=saturated fatty
The influence of protein and amino acid on the control of food intake remains incompletely understood. In studies performed in normal weight and obese people, food intake enriched with 500kcal protein for two weeks, had no demonstrable effect on gastric motor function, satiation or satiety.120 The potential for oral protein and duodenal fat to influence satiety was evaluated in healthy volunteers131 who were studied in a randomized, double-blind, four-period, cross-over design. An oral protein preload significantly reduced caloric intake (mean 19%). Simultaneous administration of an oral protein preload and intraduodenal fat did not result in a synergistic effect with respect to reduced caloric consumption.
However, after oral protein ingestion, only small quantities of intact proteins enter the duodenum because of the extensive intragastric digestion and, therefore, the total impact of protein intake on appetite may not be fully appreciated with oral protein supplement. Indeed, intraduodenal administration of protein results in higher CCK and GLP-1 levels, and reduced kcal intake at an ad libitum meal compared to oral protein administration, with greater effects observed in obese than in lean male volunteers.132
Potential Role of Functional Foods in Dietary Intake and Appetite
Functional foods are foods that, in addition to providing essential nutrients including proteins, carbohydrates, fats and vitamins, also improve the general well-being or health status; examples are fermented milk and orange juices.133 Polyphenols are example of natural products that may provide health benefits and are widely present in plants and are ingested daily with food. Not all polyphenols and related compounds are absorbed in the small intestine; some of them reach the large intestine where they undergo metabolic transformation by the colonic microbiota.134 Among the different functional foods and extracts, polyphenols seem to be yielding the most promising results on dietary intake or metabolic effects associated with obesity. Polyphenols reduce metabolic disturbances such as hyperglycemia, insulin resistance, and hyperleptinemia,135,136 and they directly modulate neuropeptides involved in appetite regulation (reviewed by Panickar137). Polyphenols can cross the blood-brain barrier, and they reduced body weight and fat mass in rodent models of obesity, along with suppressing hypothalamic neuropeptide Y levels.138,139 Clinical trials in humans point to beneficial effects of some polyphenol-rich foods (such as tea containing catechins, apple juice, cocoa, red berries, other fruits, and resveratrol in red wine) in the management of body fat.140 Polyphenols may influence the composition of the gut microbiota, for example consumption of blueberry141 and grape142 juices, and red wine or gin143 generally increased Bifidobacterium spp. in fecal samples from human volunteers.
DYSREGULATION OF GASTRIC FUNCTION, SATIETY, AND SATIATION MECHANISMS IN OBESITY
Comparisons of gastric emptying in normal weight and obese persons have shown inconsistent results with rapid, normal or slow gastric emptying (reviewed in ref. 144).
Autopsy studies in obese subjects showed that some intraabdominal organs such as the liver, small intestine and pancreas are heavier than in normal weight individuals.145 Other studies showed wide variation of stomach size with no significant relationship to body weight.146 Obese subjects have been shown to choose more food and to consume more food per minute than non-obese subjects.147 Obese people with binge eating disorder also demonstrate greater motor impulsivity,148 choosing small, immediately available rewards over larger, delayed rewards and/or the inclination to respond rapidly without forethought and/or attention to consequences.149 Gastric capacity was larger in obese persons with binge eating disorder when tested with an intragastric latex balloon filled with water.150,151 Other studies using the barostat or imaging (SPECT) techniques reported no differences in gastric volume or compliance between obese and lean subjects.152,153
However, all these reports are based on relatively small numbers of patients (<40). Recent studies in a prospective cohort of 328 participants across the spectrum of BMI from normal weight to class II or III obesity show that obesity is associated with higher fasting gastric volume, accelerated gastric emptying of solids and liquids, lower postprandial PYY, and higher postprandial GLP-1 levels (the latter being consistent with the accelerated gastric emptying of nutrients).154 Obesity was also associated delayed satiation manifested as larger volume of liquid nutrient ingested at a steady state to induce fullness,154 and larger maximum tolerated volume;155 also, the total caloric intake at an ad libitum meal was greater in people with abnormal (high) waist circumference.154
Fasting gastric volume influences intraprandial satiation, and the rate of gastric emptying also influences postprandial fullness.156,157 Principal components analysis identified latent dimensions accounting for ~81% of overweight-obesity variation, including satiety/satiation (21%), gastric motility (14%), psychological (13%), and gastric sensorimotor (11%). These observations suggest that quantitative traits of satiation, satiety and gastric functions are associated with higher BMI.154
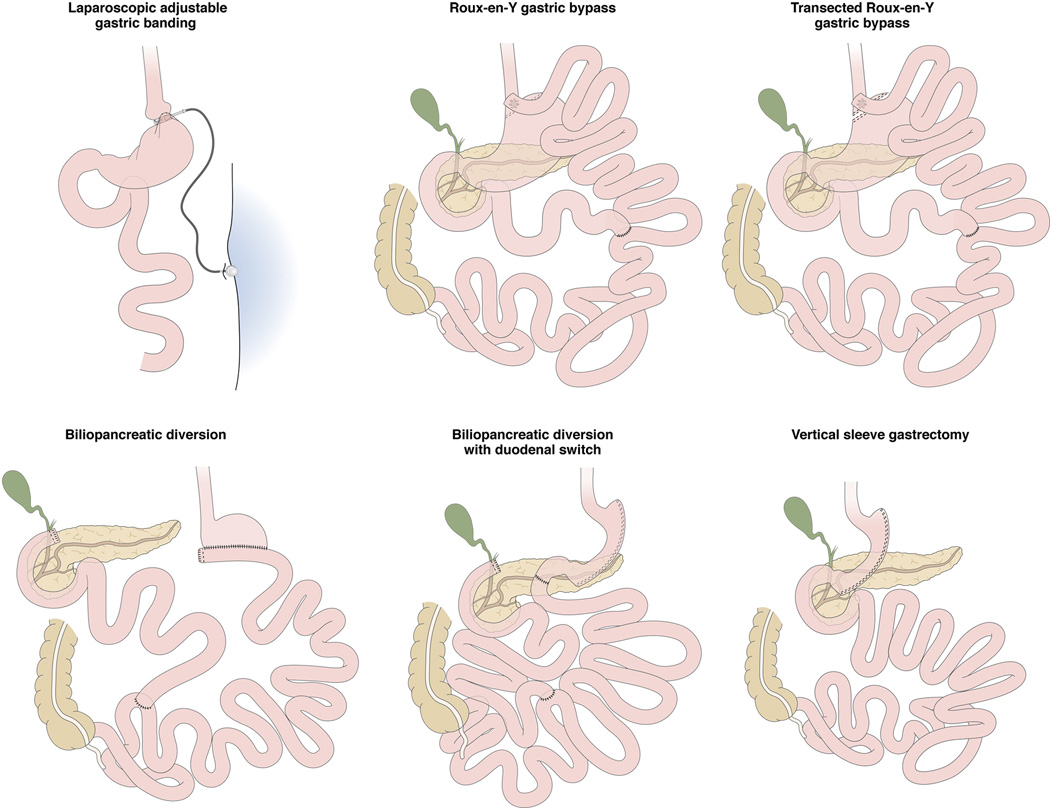
LESSONS FROM EFFECTS OF BARIATRIC SURGERY ON GUT HORMONES, APPETITE, AND WEIGHT LOSS
The most commonly performed bariatric procedures for obesity are illustrated schematically in Figure 4. The impact of gastric volume on weight loss and dietary intake is illustrated by the success of vertical sleeve gastrectomy in inducing weight loss. In fact, in the absence of any direct effect on absorptive mechanisms [as would occur following Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion],158 the restriction of gastric volume results in comparable 5-year outcomes of percent excess weight loss, daily energy intake, as well as the proportion of energy from carbohydrates, protein, and fat with vertical sleeve gastrectomy and RYGB.159 The impact of restriction in gastric volume on dietary intake is demonstrated by the observation in an adjusted multivariate regression model that energy intake and lipid intake independently predicted deficiency of vitamin D (based on plasma vitamin 25hydroxy-D levels) in the same study.159 Clearly, other adaptive responses are also stimulated by the sleeve gastrectomy, and restoration of glycemic control is comparable to that of RYGB,160 and recent data suggest an important activation of the farnesoid X receptor pathway in the small bowel.161
Figure 4.
Most frequently performed operations to treat obesity (figure adapted from Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ. 2014 Aug 27;349:g3961. doi: 10.1136/bmj.g3961).
The main changes in gut hormones after bariatric surgery affect ghrelin, leptin, GLP-1 and PYY.74 The lack of significant changes in these gut hormones and the modest restriction of overall gastric volume with banding gastroplasty are likely explanations for the lower weight loss documented in the long term with banding gastroplasty compared with the other restrictive or malabsorptive procedures.162
Initial studies suggested that ghrelin levels were much reduced after RYGB,163 since the part of the stomach that secretes this hormone is removed; weight loss and appetite loss were partly attributed to the reduced plasma levels of ghrelin. Subsequent studies showed increased ghrelin after RYGB,164 and that signaling of ghrelin which induces hunger may be attenuated in vagotomized patients.164 Thus, the role of ghrelin in appetite control has not been clarified by experience in patients following bariatric surgery.
Leptin levels typically drop after RYGB and sleeve gastrectomy,165,166 possibly reflecting reduced gastric production or reduced body fat mass. It is unclear whether this reduction in leptin levels affects energy intake in bariatric surgery patients.
Increased GLP-1 (associated in some studies with a blunted GIP or ghrelin response) is one mechanism whereby RYGB induces weight loss and enhances glucose control.167–169 Patients after RYGB had higher levels of GLP-1 than those losing a comparable amount of weight through diet.169 GLP-1 levels were higher after RYGB than after gastric banding.170,171 Increased GLP-1 levels and measurements of insulin sensitivity are comparable among patients who underwent sleeve gastrectomy and biliopancreatic diversion for obesity in the short term.172 However, recent data question whether the increased GLP-1 levels are sufficient to maintain glycemic control in obese type 2 diabetics in the longer term.173
PYY levels increase after RYGB and sleeve gastrectomy.174,175 However, it is unclear whether this contributes to weight loss, dietary intake or glycemic control relative to concomitant hormonal changes in GLP-1, GIP, and other hormones.
The type of gastric bypass surgery influences nutrient preference. For example, RYGB patients consumed less energy from fat (with no differences in carbohydrate or protein intake) compared with vertical-banded gastroplasty patients 1 and 6 years after surgery.176 Attenuated appetite after RYGB is associated with elevated PYY and GLP-1 concentrations. Appetite increased when the release of gut hormones was inhibited by the somatostatin analog, octreotide.177 Reduced preference for fat has been reported after sleeve gastrectomy in rats, but there are no reports to date in humans.178
In summary, different bariatric procedures impact a variety of the components involved in appetite, such as, total caloric tolerance, nutrient preference, as well as the hormonal and peptide responses that impact not only the intended effects on weight loss and glycemic control but also affect appetite, satiation and satiety. In addition to the relevance of these different mechanisms that result in weight loss after bariatric surgery, the lessons learned suggest novel opportunities to achieve the same physiological changes without bariatric surgery, as with endoscopic sleeve gastroplasty179 or pharmacological agents such as GLP-1 agonists.180
CONCLUSION
This review illustrates the important role of peripheral “taste” of nutrients and the rich and diverse mechanisms involved in communicating between the gut and the centers in the hypothalamus and brainstem that mediate food intake and digestion. Importantly, the hedonic (pleasures and desires related to food) and higher center responses to food are superimposed on these peripheral mechanisms and are the subject of the accompanying article.181
Acknowledgments
Grant support: Dr. Camilleri receives support for studies in obesity from NIH RO1-DK67071.
Abbreviations used
- 5-HT
serotonin
- α-MSH
α-melanocyte-stimulating hormone
- AA
amino acid
- Ach
acetylcholine
- AgRP
agouti-related protein
- Apo A-IV
apolipoprotein A-IV
- BMI
body mass index
- BOLD
blood oxygenation level dependent
- CART
cocaine- and amphetamine-regulated transcript
- CB1
cannabinoid 1 receptor
- CBF
cerebral blood flow
- CCK
cholecystokinin
- CNS
central nervous system
- DMV
dorsal motor nucleus of the vagus nerve
- EC
enterochromaffin cells
- GE
gastric emptying
- GIP
glucose-dependent insulinotropic peptide
- GH
growth hormone
- GHS-R
growth hormone secretagogue receptor
- GLP
glucagon like peptide (1 or 2)
- GLUT
glucose transporter
- MCH
melanin-concentrating hormone
- NPY
neuropeptide Y
- NTS
nucleus of the tractus solitarius
- OXM
oxyntomodulin
- POMC
pro-opiomelanocortin
- PP
pancreatic polypeptide
- PVN
paraventricular nucleus of the hypothalamus
- PVN-ARC
paraventricular and arcuate nuclei of the hypothalamus
- PYY
peptide tyrosine-tyrosine
- RYGB
Roux-en-Y gastric bypass
- SGLT
sodium-coupled glucose transporter
- T1R, T1R2, T1R3
G protein coupled taste receptors
- TRH
thyrotrophin-releasing hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No conflicts of interest related to the subject matter of this manuscript.
Author’s contributions: The author conceived, researched and wrote the entire manuscript.
REFERENCES
- 1.Camilleri M. Integrated upper gastrointestinal response to food intake. Gastroenterology. 2006;131:640–658. doi: 10.1053/j.gastro.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Csendes A, Burgos AM. Size, volume and weight of the stomach in patients with morbid obesity compared to controls. Obes Surg. 2005;15:1133–1136. doi: 10.1381/0960892055002158. [DOI] [PubMed] [Google Scholar]
- 3.Tack J, Bisschops R. Mechanisms underlying meal-induced symptoms in functional dyspepsia. Gastroenterology. 2004;127:1844–1847. doi: 10.1053/j.gastro.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Feinle-Bisset C, Horowitz M. Dietary factors in functional dyspepsia. Neurogastroenterol Motil. 2006;18:608–618. doi: 10.1111/j.1365-2982.2006.00790.x. [DOI] [PubMed] [Google Scholar]
- 5.Delgado-Aros S, Camilleri M, Castillo EJ, et al. Effect of gastric volume or emptying on meal-related symptoms after liquid nutrients in obesity: a pharmacologic study. Clin Gastroenterol Hepatol. 2005;3:997–1006. doi: 10.1016/s1542-3565(05)00285-5. [DOI] [PubMed] [Google Scholar]
- 6.Korner J, Leibel RL. To eat or not to eat - how the gut talks to the brain. N Engl J Med. 2003;349:926–928. doi: 10.1056/NEJMp038114. [DOI] [PubMed] [Google Scholar]
- 7.Fry M, Hoyda TD, Ferguson AV. Making sense of it: roles of the sensory circumventricular organs in feeding and regulation of energy homeostasis. Exp Biol Med. 2007;232:14–26. [PubMed] [Google Scholar]
- 8.Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity. 2006;14(Suppl 5):216S–221S. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- 9.Berthoud H-R. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil. 2008;20(Suppl 1):64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camilleri M, Toouli J, Herrera MF, et al. Intra-abdominal vagal blocking (VBLOC therapy): clinical results with a new implantable medical device. Surgery. 2008;143:723–731. doi: 10.1016/j.surg.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Horvath TL. Endocannabinoids and the regulation. J Clin Invest. 2003;112:323–326. doi: 10.1172/JCI19376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith G. The direct and indirect controls of meal size. Neurosci Biobehav Rev. 1996;20:41–46. doi: 10.1016/0149-7634(95)00038-g. [DOI] [PubMed] [Google Scholar]
- 13.Berthoud H-R. The vagus nerve, food intake and obesity. Regul Pept. 2008;149:15–25. doi: 10.1016/j.regpep.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azzara A, Sokolnicki J, Schwartz G. Central melanocortin receptor agonist reduces spontaneous and scheduled meal size but does not augment duodenal preload-induced feeding inhibition. Physiol Behav. 2002;77:411–416. doi: 10.1016/s0031-9384(02)00883-1. [DOI] [PubMed] [Google Scholar]
- 15.Ste Marie L, Miura GI, Marsh DJ, et al. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. PNAS. 2000;97:12339–12344. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams D, Kaplan J, Grill H. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology. 2000;141:1332–1337. doi: 10.1210/endo.141.4.7410. [DOI] [PubMed] [Google Scholar]
- 17.Huang XF, Yu Y, Zavitsanou K, et al. Differential expression of dopamine D2 and D4 receptor and tyrosine hydroxylase mRNA in mice prone, or resistant, to chronic high-fat diet-induced obesity. Brain Res Mol Brain Res. 2005;135:150–161. doi: 10.1016/j.molbrainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Pu Y, Shen P. Neuropeptide-gated perception of appetitive olfactory inputs in Drosophila larvae. Cell Rep. 2013;3:820–830. doi: 10.1016/j.celrep.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Abu Dayyeh BK, Port J, Vijayvargiya P, Camilleri M. Characterization of hypothalamic hunger and satiety signals with pulsed arterial spin labeling MRI. Gastroenterology. 2014;146:S845. [Google Scholar]
- 20.Page KA, Chan O, Arora J, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA. 2013;309:63–70. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purnell JQ, Klopfenstein BA, Stevens AA, et al. Brain functional magnetic resonance imaging response to glucose and fructose infusions in humans. Diabetes Obes Metab. 2011;13:229–234. doi: 10.1111/j.1463-1326.2010.01340.x. [DOI] [PubMed] [Google Scholar]
- 22.Kilpatrick LA, Coveleskie K, Connolly L, Labus JS, Ebrat B, Stains J, Jiang Z, Suyenobu BY, Raybould HE, Tillisch K, Mayer EA. Influence of sucrose ingestion on brainstem and hypothalamic intrinsic oscillations in lean and obese women. Gastroenterology. 2014;146:1212–1221. doi: 10.1053/j.gastro.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hillebrand JJ, de Wied D, Adan RA. Neuropeptides, food intake and body weight regulation: a hypothalamic focus. Peptides. 2002;23:2283–2306. doi: 10.1016/s0196-9781(02)00269-3. [DOI] [PubMed] [Google Scholar]
- 24.Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol. 2006;291:G171–G177. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- 25.Raybould HE. Gut chemosensing: interactions between gut endocrine cells and visceral afferents. Auton Neurosci. 2010;153:41–46. doi: 10.1016/j.autneu.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cartoni C, Yasumatsu K, Ohkuri T, et al. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30:8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssen S, Depoortere I. Nutrient sensing in the gut: new roads to therapeutics? Trends Endocrinol Metab. 2013;24:92–100. doi: 10.1016/j.tem.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Sclafani A, Ackroff K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1119–R1133. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirkup AJ, Brunsden AM, Grundy D. Receptors and transmission in the brain-gut axis: potential for novel therapies. I. Receptors on visceral afferents. Am J Physiol. 2001;280:G787–G794. doi: 10.1152/ajpgi.2001.280.5.G787. [DOI] [PubMed] [Google Scholar]
- 30.Strader AD, Woods SC. Gastrointestinal hormones and food intake. Gastroenterology. 2005;128:175–191. doi: 10.1053/j.gastro.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 31.Zhang JV, Ren PG, Avsian-Kretchmer O, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science. 2005;310:996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]
- 32.Dockray G, Dimaline R, Varro A. Gastrin: old hormone, new functions. Pflugers Arch. 2005;449:344–355. doi: 10.1007/s00424-004-1347-5. [DOI] [PubMed] [Google Scholar]
- 33.Dockray GJ, Varro A, Dimaline R, et al. The gastrins: their production and biological activities. Annu Rev Physiol. 2001;63:119–139. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- 34.Kinoshita Y, Ishihara S, Kadowaki Y, et al. Reg protein is a unique growth factor of gastric mucosal cells. J Gastroenterol. 2004;39:507–513. doi: 10.1007/s00535-004-1354-5. [DOI] [PubMed] [Google Scholar]
- 35.Raybould HE, Lloyd KC. Integration of postprandial function in the proximal gastrointestinal tract. Role of CCK and sensory pathways. Ann NY Acad Sci. 1994;713:143–156. doi: 10.1111/j.1749-6632.1994.tb44061.x. [DOI] [PubMed] [Google Scholar]
- 36.Lal S, McLaughlin J, Barlow J, et al. Cholecystokinin pathways modulate sensations induced by gastric distension in humans. Am J Physiol. 2004;287:G72–G79. doi: 10.1152/ajpgi.00351.2003. [DOI] [PubMed] [Google Scholar]
- 37.Moran TH, Ladenheim EE, Schwartz GJ. Within-meal gut feedback signaling. Int J Obes Relat Metab Disord. 2001;25(Suppl. 5):S39–S41. doi: 10.1038/sj.ijo.0801910. [DOI] [PubMed] [Google Scholar]
- 38.Beglinger C, Degen L. Fat in the intestine as a regulator of appetite--role of CCK. Physiol Behav. 2004;83:617–621. doi: 10.1016/j.physbeh.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 39.Feinle C, D'Amato M, Read NW. Cholecystokinin-A receptors modulate gastric sensory and motor responses to gastric distension and duodenal lipid. Gastroenterology. 1996;110:1379–1385. doi: 10.1053/gast.1996.v110.pm8613041. [DOI] [PubMed] [Google Scholar]
- 40.Schick RR, Yaksh TL, Roddy DR, et al. Release of hypothalamic cholecystokinin in cats: effects of nutrient and volume loading. Am J Physiol. 1989;256:R248–R254. doi: 10.1152/ajpregu.1989.256.1.R248. [DOI] [PubMed] [Google Scholar]
- 41.Burdakov D, Ashcroft FM. Cholecystokinin tunes firing of an electrically distinct subset of arcuate nucleus neurons by activating A-Type potassium channels. J Neurosci. 2002;22:6380–6387. doi: 10.1523/JNEUROSCI.22-15-06380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobelt P, Tebbe JJ, Tjandra I, et al. CCK inhibits the orexigenic effect of peripheral ghrelin. Am J Physiol. 2005;288:R751–R758. doi: 10.1152/ajpregu.00094.2004. [DOI] [PubMed] [Google Scholar]
- 43.Dass NB, Munonyara M, Bassil AK, et al. Growth hormone secretagogue receptors in rat and human gastrointestinal tract and the effects of ghrelin. Neuroscience. 2003;120:443–453. doi: 10.1016/s0306-4522(03)00327-0. [DOI] [PubMed] [Google Scholar]
- 44.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 45.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 46.Korner J, Leibel RL. To eat or not to eat - how the gut talks to the brain. N Engl J Med. 2003;349:926–928. doi: 10.1056/NEJMp038114. [DOI] [PubMed] [Google Scholar]
- 47.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 48.Cowley MA, Smith RG, Diano S, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 49.Toshinai K, Date Y, Murakami N, et al. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology. 2003;144:1506–1512. doi: 10.1210/en.2002-220788. [DOI] [PubMed] [Google Scholar]
- 50.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 51.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 52.Murray CDR, Martin NM, Patterson M, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double-blind, placebo-controlled, crossover study. Gut. 2005;54:1693–1698. doi: 10.1136/gut.2005.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Binn M, Albert C, Gougeon A, et al. Ghrelin gastrokinetic action in patients with neurogenic gastroparesis. Peptides. 2006;27:1603–1606. doi: 10.1016/j.peptides.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Tack J, DePoortere I, Bisschops R, et al. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Aliment Pharm Ther. 2005;22:847–853. doi: 10.1111/j.1365-2036.2005.02658.x. [DOI] [PubMed] [Google Scholar]
- 55.Tack J, Depoortere I, Bisschops R, et al. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327–333. doi: 10.1136/gut.2004.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levin F, Edholm T, Schmidt PT, et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab. 2006;91:3296–3302. doi: 10.1210/jc.2005-2638. [DOI] [PubMed] [Google Scholar]
- 57.Cremonini F, Camilleri M, Vazquez Roque M, et al. Obesity does not increase effects of synthetic ghrelin on human gastric motor functions. Gastroenterology. 2006;131:1431–1439. doi: 10.1053/j.gastro.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 58.Ejskjaer N, Vestergaard ET, Hellström PM, et al. Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis. Aliment Pharmacol Ther. 2009;29:1179–1187. doi: 10.1111/j.1365-2036.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 59.Ejskjaer N, Wo JM, Esfandyari T, et al. A phase 2a, randomized, double-blind 28-day study of TZP-102 a ghrelin receptor agonist for diabetic gastroparesis. Neurogastroenterol Motil. 2013;25:e140–e150. doi: 10.1111/nmo.12064. [DOI] [PubMed] [Google Scholar]
- 60.Wo JM, Ejskjaer N, Hellström PM, et al. Randomised clinical trial: ghrelin agonist TZP-101 relieves gastroparesis associated with severe nausea and vomiting--randomised clinical study subset data. Aliment Pharmacol Ther. 2011;33:679–688. doi: 10.1111/j.1365-2036.2010.04567.x. [DOI] [PubMed] [Google Scholar]
- 61.McCallum RW, Lembo A, Esfandyari T, et al. Phase 2b, randomized, double-blind 12-week studies of TZP-102, a ghrelin receptor agonist for diabetic gastroparesis. Neurogastroenterol Motil. 2013 Jul 15; doi: 10.1111/nmo.12184. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 62.Shin A, Camilleri M, Busciglio I, et al. The ghrelin agonist RM-131 accelerates gastric emptying of solids and reduces symptoms in patients with type 1 diabetes mellitus. Clin Gastroenterol Hepatol. 2013 Apr 30; doi: 10.1016/j.cgh.2013.04.019. doi:pii: S1542-3565(13)00578-8. 10.1016/j.cgh.2013.04.019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin A, Camilleri M, Busciglio I, et al. Randomized controlled phase Ib study of ghrelin agonist, RM-131, in type 2 diabetic women with delayed gastric emptying: pharmacokinetics and pharmacodynamics. Diabetes Care. 2013;36:41–48. doi: 10.2337/dc12-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ueno H, Yamaguchi H, Kangawa K, et al. Ghrelin: a gastric peptide that regulates food intake and energy homeostasis. Regul Pept. 2005;126:11–19. doi: 10.1016/j.regpep.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 65.Jarkovska Z, Krsek M, Rosicka M, et al. Endocrine and metabolic activities of a recently isolated peptide hormone ghrelin, an endogenous ligand of the growth hormone secretagogue receptor. Endocr Regul. 2004;38:80–86. [PubMed] [Google Scholar]
- 66.Korbonits M, Grossman AB. Ghrelin: update on a novel hormonal system. Eur J Endocrinol. 2005;151:S67–S70. doi: 10.1530/eje.0.151s067. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 68.Masuzaki H, Ogawa Y, Sagawa N, et al. Nonadipose tissue production of leptin (leptin as a novel placenta-derived hormone in humans) Nat Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 69.Wang J, Liu R, Hawkins M, et al. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- 70.Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Science. 1998;394:90–93. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 71.Lee YH, Magkos F, Mantzoros CS, et al. Effects of leptin and adiponectin on pancreatic β-cell function. Metabolism. 2011;60:1664–1672. doi: 10.1016/j.metabol.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 72.Cinti S, Matteis RD, Pico C, et al. Secretory granules of endocrine and chief cells of human stomach mucosa contain leptin. Int J Obes Relat Metab Disord. 2000;24:789–793. doi: 10.1038/sj.ijo.0801228. [DOI] [PubMed] [Google Scholar]
- 73.Sobhani I, Bado A, Vissuzaine C, et al. Leptin secretion and leptin receptor in the human stomach. Gut. 2000;47:178–183. doi: 10.1136/gut.47.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michalakis K, le Roux C. Gut hormones and leptin: impact on energy control and changes after bariatric surgery--what the future holds. Obes Surg. 2012;22:1648–1657. doi: 10.1007/s11695-012-0698-9. [DOI] [PubMed] [Google Scholar]
- 75.Keim NL, Stern JS, Havel PJ. Relation between circulating leptin concentrations and appetite during a prolonged, moderate energy deficit in women. Am J Clin Nutr. 1998;68:794–801. doi: 10.1093/ajcn/68.4.794. [DOI] [PubMed] [Google Scholar]
- 76.Ahima RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 77.Elmquist JK, Elias CF, Saper CB. From lesions to leptin (hypothalamic control of food intake and body weight) Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 78.Pico C, Oliver P, Sanchez J, et al. Gastric leptin: a putative role in the short-term regulation of food intake. Br J Nutr. 2003;90:735–741. doi: 10.1079/bjn2003945. [DOI] [PubMed] [Google Scholar]
- 79.Pico C, Sanchez J, Oliver P, et al. Leptin production by the stomach is up-regulated in obese (fa/fa) Zucker rats. Obes Res. 2002;10:932–938. doi: 10.1038/oby.2002.127. [DOI] [PubMed] [Google Scholar]
- 80.Kolligs F, Fehmann HC, Göke R, et al. Reduction of the incretin effect in rats by the glucagon-like peptide 1 receptor antagonist exendin (9–39) amide. Diabetes. 1995;44:16–19. doi: 10.2337/diab.44.1.16. [DOI] [PubMed] [Google Scholar]
- 81.Wang Z, Wang RM, Owji AA, et al. Glucagon-like peptide-1 is a physiological incretin in rat. J Clin Invest. 1995;95:417–421. doi: 10.1172/JCI117671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scrocchi L, Brown TJ, MacLusky N, et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nature Med. 1996;2:1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 83.Flint A, Raben A, Astrup A, et al. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schirra J, Wank U, Arnold R, et al. Effects of glucagon-like peptide-1(7–36)amide on motility and sensation of the proximal stomach in humans. Gut. 2002;50:341–348. doi: 10.1136/gut.50.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delgado-Aros S, Vella A, Camilleri M, et al. Effects of glucagon-like peptide-1 and feeding on gastric volumes in diabetes mellitus with cardio-vagal dysfunction. Neurogastroenterol Motil. 2003;15:435–443. doi: 10.1046/j.1365-2982.2003.00422.x. [DOI] [PubMed] [Google Scholar]
- 86.Delgado-Aros S, Kim DY, Burton DD, et al. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. Am J Physiol. 2002;282:G424–G431. doi: 10.1152/ajpgi.2002.282.3.G424. [DOI] [PubMed] [Google Scholar]
- 87.Vella A, Shah P, Basu R, et al. Effect of glucagon-like peptide-1(7–36)-amide on initial splanchnic glucose uptake and insulin action in humans with type 1 diabetes. Diabetes. 2001;50:565–572. doi: 10.2337/diabetes.50.3.565. [DOI] [PubMed] [Google Scholar]
- 88.Kalra SP, Kalra PS. Neuropeptide Y: a physiological orexigen modulated by the feedback action of ghrelin and leptin. Endocrine. 2003;22:49–56. doi: 10.1385/ENDO:22:1:49. [DOI] [PubMed] [Google Scholar]
- 89.Cox HM. Neuropeptide Y receptors; antisecretory control of intestinal epithelial function. Auton Neurosci. 2007;133:76–85. doi: 10.1016/j.autneu.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 90.Ishiguchi T, Amano T, Matsubayashi H, et al. Centrally administered neuropeptide Y delays gastric emptying via Y2 receptors in rats. Am J Physiol. 2001;281:R1522–R1530. doi: 10.1152/ajpregu.2001.281.5.R1522. [DOI] [PubMed] [Google Scholar]
- 91.Allen JM, Fitzpatrick ML, Yeats JC, et al. Effects of peptide YY and neuropeptide Y on gastric emptying in man. Digestion. 1984;30:255–262. doi: 10.1159/000199117. [DOI] [PubMed] [Google Scholar]
- 92.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 93.Uhe AM, Szmukler GI, Collier GR, Hansky J, O’Dea K, Young GP. Potential regulators of feeding behavior in anorexia nervosa. Am J Clin Nutr. 1992;55:28–32. doi: 10.1093/ajcn/55.1.28. [DOI] [PubMed] [Google Scholar]
- 94.Jorde R, Burhol PG. Fasting and postprandial plasma pancreatic polypeptide (PP) levels in obesity. International Journal of Obesity. 1984;8:393–397. [PubMed] [Google Scholar]
- 95.Wisen O, Bjorvell H, Cantor P, Johansson C, Theodorsson E. Plasma concentrations of regulatory peptides in obesity following modified sham feeding (MSF) and a liquid test meal. Regulatory Peptides. 1992;39:43–54. doi: 10.1016/0167-0115(92)90007-h. [DOI] [PubMed] [Google Scholar]
- 96.Lassmann V, Vague P, Vialettes B, Simon MC. Low plasma levels of pancreatic polypeptide in obesity. Diabetes. 1980;29:428–430. doi: 10.2337/diab.29.6.428. [DOI] [PubMed] [Google Scholar]
- 97.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY3–36 physiologically inhibits food intake. Nature. 2002;418:650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 98.Pironi L, Stanghellini V, Miglioli M, et al. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology. 1993;105:733–739. doi: 10.1016/0016-5085(93)90890-o. [DOI] [PubMed] [Google Scholar]
- 99.Wen J, Phillips SF, Sarr MG, et al. PYY and GLP-1 contribute to feedback inhibition from the canine ileum and colon. Am J Physiol. 1995;269:G945–G952. doi: 10.1152/ajpgi.1995.269.6.G945. [DOI] [PubMed] [Google Scholar]
- 100.Chen CH, Rogers RC. Central inhibitory action of peptide YY on gastric motility in rats. Am J Physiol. 1995;269:R787–R792. doi: 10.1152/ajpregu.1995.269.4.R787. [DOI] [PubMed] [Google Scholar]
- 101.Wen J, Luque-de Leon E, Kost LJ, et al. Duodenal motility in fasting dogs: humoral and neural pathways mediating the colonic brake. Am J Physiol. 1998;274:G192–G195. doi: 10.1152/ajpgi.1998.274.1.G192. [DOI] [PubMed] [Google Scholar]
- 102.Chen CH, Stephens RL, Jr, Rogers RC. PYY and NPY: control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterol Motil. 1997;9:109–116. doi: 10.1046/j.1365-2982.1997.d01-26.x. [DOI] [PubMed] [Google Scholar]
- 103.Cuche G, Cuber JC, Malbert CH. Ileal short-chain fatty acids inhibit gastric motility by a humoral pathway. Am J Physiol. 2000;279:G925–G930. doi: 10.1152/ajpgi.2000.279.5.G925. [DOI] [PubMed] [Google Scholar]
- 104.Savage AP, Adrian TE, Carolan G, et al. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut. 1987;28:166–170. doi: 10.1136/gut.28.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pappas TN, Debas HT, Taylor IL. Enterogastrone-like effect of peptide YY is vagally mediated in the dog. J Clin Invest. 1986;77:49–53. doi: 10.1172/JCI112300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Putnam WS, Liddle RA, Williams JA. Inhibitory regulation of rat exocrine pancreas by peptide YY and pancreatic polypeptide. Am J Physiol. 1989;256:G698–G703. doi: 10.1152/ajpgi.1989.256.4.G698. [DOI] [PubMed] [Google Scholar]
- 107.Keller J, Holst JJ, Layer P. Inhibition of human pancreatic and biliary output but not intestinal motility by physiological intraileal lipid loads. Am J Physiol. 2006;290:704–709. doi: 10.1152/ajpgi.00411.2005. [DOI] [PubMed] [Google Scholar]
- 108.Cohen MA, Ellis SM, Le Roux CW, et al. Oxyntomodulin suppresses appetite and reduces food intake in humans. JCEM. 2003;88:4696–4701. doi: 10.1210/jc.2003-030421. [DOI] [PubMed] [Google Scholar]
- 109.Wynne CK, Park AJ, Small CJ, et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomized controlled trial. Int J Obes. 2006;30:1729–1736. doi: 10.1038/sj.ijo.0803344. [DOI] [PubMed] [Google Scholar]
- 110.Fujimoto K, Cardelli JA, Tso P. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am J Physiol. 1992;262:G1002–G1006. doi: 10.1152/ajpgi.1992.262.6.G1002. [DOI] [PubMed] [Google Scholar]
- 111.Tso P, Balint JA. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am J Physiol. 1986;250:G715–G726. doi: 10.1152/ajpgi.1986.250.6.G715. [DOI] [PubMed] [Google Scholar]
- 112.Tso P1, Liu M, Kalogeris TJ, Thomson AB. The role of apolipoprotein A-IV in the regulation of food intake. Annu Rev Nutr. 2001;21:231–254. doi: 10.1146/annurev.nutr.21.1.231. [DOI] [PubMed] [Google Scholar]
- 113.Banks WA. The blood-brain barrier as a regulatory interface in the gut-brain axes. Physiol Behav. 2006;89:472–476. doi: 10.1016/j.physbeh.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 114.Woods SC, Seeley RJ, Baskin DG, et al. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- 115.Hunt JN. Mechanisms and disorders of gastric emptying. Annu Rev Med. 1983;34:219–229. doi: 10.1146/annurev.me.34.020183.001251. [DOI] [PubMed] [Google Scholar]
- 116.Raybould HE, Meyer JH, Tabrizi Y, et al. Inhibition of gastric emptying in response to intestinal lipid is dependent on chylomicron formation. Am J Physiol. 1998;274:R1834–R1838. doi: 10.1152/ajpregu.1998.274.6.R1834. [DOI] [PubMed] [Google Scholar]
- 117.Spannagel AW, Nakano I, Tawil T, et al. Adaptation to fat markedly increases pancreatic secretory response to intraduodenal fat in rats. Am J Physiol. 1996;270:G128–G135. doi: 10.1152/ajpgi.1996.270.1.G128. [DOI] [PubMed] [Google Scholar]
- 118.Boivin M, Lanspa SJ, Zinsmeister AR, et al. Are diets associated with different rates of human interdigestive and postprandial pancreatic enzyme secretion? Gastroenterology. 1990;99:1763–1771. doi: 10.1016/0016-5085(90)90485-j. [DOI] [PubMed] [Google Scholar]
- 119.Cunningham KM, Daly J, Horowitz M, et al. Gastrointestinal adaptation to diets of differing fat composition in human volunteers. Gut. 1991;32:483–486. doi: 10.1136/gut.32.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park MI, Camilleri M, O'Connor H, et al. Effect of different macronutrients on gastric sensory and motor functions and appetite in normal weight, overweight or obese humans. Am J Clin Nutr. 2007;85:411–418. doi: 10.1093/ajcn/85.2.411. [DOI] [PubMed] [Google Scholar]
- 121.Boyd KA, O'Donovan DG, Doran S, et al. High-fat diet effects on gut motility, hormone, and appetite responses to duodenal lipid in healthy men. Am J Physiol. 2003;284:G188–G196. doi: 10.1152/ajpgi.00375.2002. [DOI] [PubMed] [Google Scholar]
- 122.Blundell JE, Green S, Burley V. Carbohydrates and human appetite. Am J Clin Nutr. 1994;59(3 Suppl):728S–734S. doi: 10.1093/ajcn/59.3.728S. [DOI] [PubMed] [Google Scholar]
- 123.French SJ, Conlon CA, Mutuma ST, et al. The effects of intestinal infusion of long-chain fatty acids on food intake in humans. Gastroenterology. 2000;119:943–948. doi: 10.1053/gast.2000.18139. [DOI] [PubMed] [Google Scholar]
- 124.Poppitt SD, Strik CM, MacGibbon AK, et al. Fatty acid chain length, postprandial satiety and food intake in lean men. Physiol Behav. 2010;101:161–167. doi: 10.1016/j.physbeh.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 125.St-Onge MP, Mayrsohn B, O'Keeffe M, et al. Impact of medium and long chain triglycerides consumption on appetite and food intake in overweight men. Eur J Clin Nutr. 2014 Jul 30; doi: 10.1038/ejcn.2014.145. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jandacek RJ. Review of the effects of dilution of dietary energy with olestra on energy intake. Physiol Behav. 2012;105:1124–1131. doi: 10.1016/j.physbeh.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 127.Lawton CL, Delargy HJ, Brockman J, et al. The degree of saturation of fatty acids influences post-ingestive satiety. Br J Nutr. 2000;83:473–482. [PubMed] [Google Scholar]
- 128.Kozimor A, Chang H, Cooper JA. Effects of dietary fatty acid composition from a high fat meal on satiety. Appetite. 2013;69:39–45. doi: 10.1016/j.appet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 129.Buckley JD, Howe PR. Anti-obesity effects of long-chain omega-3 polyunsaturated fatty acids. Obes Rev. 2009;10:648–659. doi: 10.1111/j.1467-789X.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- 130.Parra D, Ramel A, Bandarra N, et al. A diet rich in long chain omega-3 fatty acids modulates satiety in overweight and obese volunteers during weight loss. Appetite. 2008;51:676–680. doi: 10.1016/j.appet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 131.Oesch S, Degen L, Beglinger C. Effect of a protein preload on food intake and satiety feelings in response to duodenal fat perfusions in healthy male subjects. Am J Physiol. 2005;289:R1042–R1047. doi: 10.1152/ajpregu.00039.2005. [DOI] [PubMed] [Google Scholar]
- 132.Geraedts MC, Troost FJ, Munsters MJ, et al. Intraduodenal administration of intact pea protein effectively reduces food intake in both lean and obese male subjects. PLoS One. 2011;6:e24878. doi: 10.1371/journal.pone.0024878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kalra EK. Nutraceutical: definition and introduction. AAPS PharmSci. 2003;5:E25. doi: 10.1208/ps050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jaganath IB, Mullen W, Edwards CA, et al. The relative contribution of the small and large intestine to the absorption and metabolism of rutin in man. Free Radic Res. 2006;40:1035–1046. doi: 10.1080/10715760600771400. [DOI] [PubMed] [Google Scholar]
- 135.Boqué N, Campión J, de la Iglesia R, et al. Screening of polyphenolic plant extracts for antiobesity properties in Wistar rats. J Sci Food Agric. 2012;93:1226–1232. doi: 10.1002/jsfa.5884. [DOI] [PubMed] [Google Scholar]
- 136.Shen CL, Cao JJ, Dagda RY, et al. Green tea polyphenols benefits body composition and improves bone quality in long-term high-fat diet-induced obese rats. Nutr Res. 2012;32:448–457. doi: 10.1016/j.nutres.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 137.Panickar KS. Effects of dietary polyphenols on neuroregulatory factors and pathways that mediate food intake and energy regulation in obesity. Mol Nutr Food Res. 2013;57:34–47. doi: 10.1002/mnfr.201200431. [DOI] [PubMed] [Google Scholar]
- 138.Kim SO, Yun SJ, Lee EH. The water extract of adlay seed (Coix lachrymajobi var. mayuen) exhibits anti-obesity effects through neuroendocrine modulation. Am J Chin Med. 2007;35:297–308. doi: 10.1142/S0192415X07004825. [DOI] [PubMed] [Google Scholar]
- 139.Hamao M, Matsuda H, Nakamura S, et al. Anti-obesity effects of the methanolic extract and chakasaponins from the flower buds of Camellia sinensis in mice. Bioorg Med Chem. 2011;19:6033–6041. doi: 10.1016/j.bmc.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 140.Meydani M, Hasan ST. Dietary polyphenols and obesity. Nutrients. 2010;2:737–751. doi: 10.3390/nu2070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vendrame S, Guglielmetti S, Riso P, et al. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J Agric Food Chem. 2011;59:12815–12820. doi: 10.1021/jf2028686. [DOI] [PubMed] [Google Scholar]
- 142.Jacobs DM, Deltimple N, vanVelzen E, et al. (1) H NMR metabolite profiling of feces as a tool to assess the impact of nutrition on the human microbiome. NMR Biomed. 2008;21:615–626. doi: 10.1002/nbm.1233. [DOI] [PubMed] [Google Scholar]
- 143.Queipo-Ortuño MI, Boto-Ordóñez M, Murri M, et al. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr. 2012;95:1323–1334. doi: 10.3945/ajcn.111.027847. [DOI] [PubMed] [Google Scholar]
- 144.Park M-I, Camilleri M. Gastric motor and sensory functions in obesity. Obesity Res. 2005;13:491–500. doi: 10.1038/oby.2005.51. [DOI] [PubMed] [Google Scholar]
- 145.Naeye RL, Roode P. The sizes and numbers of cells in visceral organs in human obesity. Am J Clin Pathol. 1970;54:251–253. doi: 10.1093/ajcp/54.2.251. [DOI] [PubMed] [Google Scholar]
- 146.Cox AJ. Stomach size and its relation to chronic peptic ulcer. Arch Pathol. 1952;54:407–422. [PubMed] [Google Scholar]
- 147.Stunkard A, Kaplan D. Eating in public places: a review of reports of direct observation of eating behavior. Int J Obes. 1977;1:89–101. [PubMed] [Google Scholar]
- 148.Nasser JA, Gluck ME, Geliebter A. Impulsivity and test meal intake in obese binge eating women. Appetite. 2004;43:303–307. doi: 10.1016/j.appet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 149.Swann AC, Bjork JM, Moeller FG, et al. Two models of impulsivity: relationship of personality traits and psychopathology. Biol Psychiatr. 2002;51:988–994. doi: 10.1016/s0006-3223(01)01357-9. [DOI] [PubMed] [Google Scholar]
- 150.Granstrom L, Backman L. Stomach distension in extremely obese and in normal subjects. Acta Chir Scand. 1985;151:367–370. [PubMed] [Google Scholar]
- 151.Geliebter A, Yahav EK, Gluck ME, et al. Gastric capacity, test meal intake, and appetitive hormones in binge eating disorder. Physiol Behav. 2004;81:735–740. doi: 10.1016/j.physbeh.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 152.Kim D-Y, Camilleri M, Murray JA, et al. Is there a role for gastric accommodation and satiety in asymptomatic obese people? Obesity Res. 2001;9:655–661. doi: 10.1038/oby.2001.89. [DOI] [PubMed] [Google Scholar]
- 153.Vazquez Roque MI, Camilleri M, Stephens DA, et al. Gastric sensorimotor functions and hormone profile in normal weight, overweight and obese people. Gastroenterology. 2006;131:1717–1724. doi: 10.1053/j.gastro.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 154.Acosta A, Camilleri M, Shin A, et al. Quantitative gastrointestinal, psychological and behavioral traits in obesity: identifying phenotypic predictors of response to a pharmacotherapy. 2014 submitted. [Google Scholar]
- 155.Delgado-Aros S, Cremonini F, Castillo JE, et al. Independent influences of body mass and gastric volumes on satiation in humans. Gastroenterology. 2004;126:432–440. doi: 10.1053/j.gastro.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 156.Delgado-Aros S, Camilleri M, Cremonini F, et al. Contributions of gastric volumes and gastric emptying to meal size and post-meal symptoms in functional dyspepsia. Gastroenterology. 2004;127:1685–1694. doi: 10.1053/j.gastro.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 157.Delgado-Aros S, Camilleri M, Castillo EJ, et al. Effect of gastric volume or emptying on meal-related symptoms after liquid nutrients in obesity: a pharmacological study. Clin Gastroenterol Hepatol. 2005;3:997–1006. doi: 10.1016/s1542-3565(05)00285-5. [DOI] [PubMed] [Google Scholar]
- 158.Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ. 2014 Aug 27;349:g3961. doi: 10.1136/bmj.g3961. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Moizé V, Andreu A, Flores L, et al. Long-term dietary intake and nutritional deficiencies following sleeve gastrectomy or Roux-En-Y gastric bypass in a Mediterranean population. J Acad Nutr Diet. 2013;113:400–410. doi: 10.1016/j.jand.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 160.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. N Engl J Med. 2014;370:2002–2013. doi: 10.1056/NEJMoa1401329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–234. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 163.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 164.Sundbom M, Holdstock C, Engström BE, et al. Early changes in ghrelin following Rouxen-Y gastric bypass: influence of vagal nerve functionality? Obes Surg. 2007;17:304–310. doi: 10.1007/s11695-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 165.Perathoner A, Weißenbacher A, Sucher R, et al. Significant weight loss and rapid resolution of diabetes and dyslipidemia during short-term follow-up after laparoscopic sleeve gastrectomy. Obes Surg. 2013 Jul 19; doi: 10.1007/s11695-013-1038-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 166.Terra X, Auguet T, Guiu-Jurado E, et al. Long-term changes in leptin, chemerin and ghrelin levels following different bariatric surgery procedures: Roux-en-Y gastric bypass and sleeve gastrectomy. Obes Surg. 2013 Jul 7; doi: 10.1007/s11695-013-1033-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 167.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Korner J, Bessler M, Inabnet W, et al. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rodieux F, Giusti V, D'Alessio DA, et al. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity. 2008;16:298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 170.Reinehr T, Roth CL, Schernthaner GH, et al. Peptide YY and glucagon-like peptide-1 in morbidly obese patients before and after surgically induced weight loss. Obes Surg. 2007;17:1571–1577. doi: 10.1007/s11695-007-9323-8. [DOI] [PubMed] [Google Scholar]
- 171.Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diab Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mallipedhi A, Prior SL, Barry JD, Caplin S, Baxter JN, Stephens JW. Temporal changes in glucose homeostasis and incretin hormone response at 1 and 6 months after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis. 2014 Mar 12; doi: 10.1016/j.soard.2014.02.038. pii: S1550-7289(14)00106-3. doi: 10.1016/j.soard.2014.02.038. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 173.Jiménez A, Mari A, Casamitjana R, Lacy A, Ferrannini E, Vidal J. GLP-1 and Glucose Tolerance After Sleeve Gastrectomy in Morbidly Obese Subjects with Type 2 Diabetes. Diabetes. 2014 May 21; doi: 10.2337/db14-0357. pii: DB_140357. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 174.Dimitriadis E, Daskalakis M, Kampa M, et al. Alterations in gut hormones after laparoscopic sleeve gastrectomy: a prospective clinical and laboratory investigational study. Ann Surg. 2013;257:647–654. doi: 10.1097/SLA.0b013e31826e1846. [DOI] [PubMed] [Google Scholar]
- 175.Ramón JM, Salvans S, Crous X, et al. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg. 2012;16:1116–1122. doi: 10.1007/s11605-012-1855-0. [DOI] [PubMed] [Google Scholar]
- 176.le Roux CW, Bueter M, Theis N, et al. Gastric bypass reduces fat intake and preference. Am J Physiol. 2011;301:R1057–R1066. doi: 10.1152/ajpregu.00139.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 178.Chambers AP, Wilson-Perez HE, McGrath S, et al. Effect of vertical sleeve gastrectomy on food selection and satiation in rats. Am J Physiol Endocrinol Metab. 2012;303:E1076–E1084. doi: 10.1152/ajpendo.00211.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Abu Dayyeh B, Rajan E, Gostout C. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc. 2013;78:530–535. doi: 10.1016/j.gie.2013.04.197. [DOI] [PubMed] [Google Scholar]
- 180.Astrup A, Rössner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 181.Avena N. Food and the brain: How the brain responds to nutrients. Gastroenterology. (in press). [Google Scholar]