Abstract
The TNF receptor superfamily member Fn14 (TNFRSF12A) is the sole signaling receptor for the pro-inflammatory cytokine TWEAK (TNFSF12). TWEAK:Fn14 engagement stimulates multiple signal transduction pathways, including the NF-κB pathway, and this triggers important cellular processes (e.g., growth, differentiation, migration, invasion). The TWEAK/Fn14 axis is thought to be a major physiological mediator of tissue repair after acute injury. Various studies have revealed that Fn14 is highly expressed in many solid tumor types and that Fn14 signaling may play a role in tumor growth and metastasis. Previously it was shown that Fn14 levels are frequently elevated in non-small cell lung cancer (NSCLC) tumors and cell lines that exhibit constitutive EGFR phosphorylation (activation). Furthermore, elevated Fn14 levels increased NSCLC cell invasion in vitro and lung metastatic tumor colonization in vivo. The present study reveals that EGFR-mutant NSCLC cells that express high levels of Fn14 exhibit constitutive activation of the cytoplasmic tyrosine kinase Src and that treatment with the Src family kinase (SFK) inhibitor dasatinib decreases Fn14 gene expression at both the mRNA and protein levels. Importantly, siRNA-mediated depletion of the SFK member Src in NSCLC cells also decreases Fn14 expression. Finally, expression of the constitutively active v-Src oncoprotein in NIH 3T3 cells induces Fn14 gene expression and NIH 3T3/v-Src cells require Fn14 expression for full invasive capacity.
Keywords: Fn14, EGFR, Src, lung cancer, invasion, dasatinib
Introduction
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer-related deaths worldwide; in the USA, it is predicted that ~224,000 people will be diagnosed with lung cancer and ~159,000 patients will die from this disease in 2014 (1). Despite extensive basic and clinical research efforts, the 5-year survival rate for all lung cancer patients is ~17% (1). NSCLC is the most common lung cancer histological subtype (~85% of all lung cancers), and these tumors can be further divided into adenocarcinoma (AC; 50% of all NSCLCs), squamous cell carcinoma (SCC) or large-cell carcinoma (LCC) (2). Treatment options for NSCLC patients depend on disease stage and include surgery for localized tumors and radiation and/or platinum-based chemotherapy for advanced or recurrent metastatic disease (3). Our therapeutic options for certain NSCLC patients have recently expanded due to the discovery that some NSCLC tumors harbor specific gene mutations that drive cancer cell growth and survival (4). The two most frequently mutated genes in NSCLC patients are KRAS (~25% of lung ACs (5)) and epidermal growth factor receptor (EGFR) (~20% of lung ACs (6–8)). Although targeted therapeutic agents have been developed for the EGFR-mutant patient subgroup (e.g., the tyrosine kinase inhibitor (TKI) erlotinib), drug resistance is a major clinical problem (6–8). There are no FDA-approved targeted drugs for the great majority of NSCLC patients; therefore, studies focused on the identification of new molecules that regulate NSCLC cell biology and the development of new therapeutics targeting these molecules are of critical importance.
Tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK; TNFSF12), a member of the TNF superfamily of cytokines, acts on cells via binding to a single TNF receptor superfamily member named fibroblast growth factor-inducible 14 (Fn14; TNFRSF12A) (9, 10). TWEAK:Fn14 engagement activates a number of intracellular signal transduction cascades, and this can result in either increased cell proliferation, survival, migration, differentiation or death, depending on the cellular context (9, 10). Studies using TWEAK-deficient mice, Fn14-deficient mice, and/or TWEAK-neutralizing biologics have revealed that TWEAK/Fn14 signaling after acute tissue injury is critical for efficient wound repair and that chronic, dysregulated Fn14 signaling may play an important role in the pathophysiology of several prominent human diseases, including cancer (9, 10). Both TWEAK and Fn14 expression have been detected in primary tumors and tumor metastases and Fn14 gene expression in particular is frequently elevated in primary tumor tissue compared to matched adjacent normal tissue or normal tissues from non-diseased donors (10–19). Additionally, there are reports indicating that the TWEAK/Fn14 axis may play an important role in regulating various aspects of tumor growth and metastasis, including cancer cell proliferation (15, 16), chemotherapeutic drug sensitivity (20), and migratory/invasive capacity (11, 12, 14, 15, 17, 21–23). In consideration of these findings, a number of TWEAK- or Fn14-targeted agents are presently in pre-clinical development or in clinical trials for cancer therapy (10).
Previously, we showed that Fn14 was overexpressed in NSCLC tumors (17) and that high Fn14 levels were most frequently found in those tumors that exhibited strong p-EGFR (Y-1068) antibody immunostaining, an indicator of constitutive EGFR activation (17). We also reported that the HCC827 NSCLC cell line that harbors an EGFR activating mutation (ΔE746-A750) has relatively high levels of Fn14 protein expression, and that erlotinib treatment of these cells significantly reduced Fn14 levels, indicating that EGFR signaling was in fact inducing Fn14 expression in these cells.
In this study, we investigated which EGFR-triggered downstream intracellular signaling pathways were driving Fn14 expression in HCC827 cells. We found that although EGFR activation of multiple signaling pathways contributes to the overall level of Fn14 expression, the activation of Src, a non-receptor tyrosine kinase that is functionally integrated into various signaling cascades and causally linked to tumor growth and metastasis (24, 25), was a particularly critical signaling event. The importance of the Src signaling node for EGFR-driven Fn14 expression was initially discovered using the Src family kinase (SFK) inhibitor dasatinib and then confirmed using an RNA interference approach. Additionally, we show here that Fn14 expression is significantly higher in an NIH 3T3 cell line engineered to express the constitutively active v-Src oncoprotein in comparison to control NIH 3T3 cells, and that the NIH 3T3/v-Src cells require Fn14 expression for full invasive capacity.
Materials and Methods
Cell culture and treatments
Human NSCLC cell lines A549, H1975, and HCC2279 (American Type Culture Collection (ATCC)) were maintained in RPMI 1640 (Sigma-Aldrich) supplemented with 10% FBS (HyClone), 2 mM L-glutamine and 1% penicillin-streptomycin (both from CellGro). HCC827 cells (ATCC) were maintained in RPMI 1640 supplemented with 5% FBS, 2 mM L-glutamine and 1% penicillin-streptomycin. The four cell lines listed above were validated by short-tandem repeat (STR) DNA fingerprinting using the Promega PowerPlex 16HS System at the University of Arizona Genetics Core Facility in October 2014. NIH 3T3 cells (ATCC) were maintained in DMEM (Cellgro) supplemented with 10% FBS, 2 mM L-glutamine, 0.15% sodium bicarbonate (CellGro) and 1% penicillin-streptomycin. The NIH 3T3/v-Src cell line was provided by Dr. Steven Zhan (University of Maryland School of Medicine, Baltimore, MD, USA) and maintained in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 0.15% sodium bicarbonate and 1% penicillin-streptomycin. All cells were maintained at 37°C in 5% CO2.
Cells were serum-starved overnight in media containing 0.5% FBS unless otherwise indicated and then treated with either DMSO vehicle (Sigma-Aldrich) or the indicated concentrations of erlotinib (BioVision), MK-2206 (BioVision), EGF (R & D Systems), dasatinib (Cell Signaling Technology), U0126 (Cell Signaling Technology), BAY 11-7082 (EMD Millipore), or STAT3 inhibitor VIII (5,15-DPP; Santa Cruz Biotechnology).
Western blot analysis
Cells were harvested by scraping and lysed in 20 mM HEPES, 150 mM NaCl, 1.5 mM MgCl2, 10% glycerol, and 1% Triton X-100 supplemented with a protease inhibitor cocktail (Sigma-Aldrich) and two phosphatase inhibitor cocktails (Calbiochem). The protein concentration of each lysate was determined by BCA protein assay (Pierce Protein Biology). Equal amounts of protein were subjected to SDS-PAGE (Life Technologies) and electrotransferred to PVDF membranes (Millipore). Membranes were blocked in either 5% bovine serum albumin (BSA) in Tris-Buffered Saline with 0.05% Tween (TBST) or 5% non-fat dry milk (NFDM) in TBST buffer and then sequentially incubated with the appropriate primary antibody and horseradish peroxidase (HRP)-conjugated secondary antibody (Cell Signaling Technology). The membranes were washed in TBST and then immunoreactive proteins were detected using the Amersham Enhanced Chemiluminescence Plus kit (GE Healthcare) according to the manufacturer’s instructions. The following primary antibodies were used: Fn14, p-EGFR (Y-1068), p-EGFR (Y-845), EGFR, pan p-Src (Y-416), Src, Lyn, Lck, GAPDH, p-ERK (T-202/Y-204), ERK, p-JNK (T-183/Y-185), JNK, p-Akt (S-473), Akt, p-STAT3 (Y-705), STAT3, p100/p52 (all from Cell Signaling Technology), and tubulin (Sigma-Aldrich). Densitometric analysis was conducted using Image J software and all Fn14 or Src expression values were normalized to GAPDH or tubulin values.
RNA isolation and quantitative real-time RT-PCR assays
Total cellular RNA was extracted using the RNeasy kit (Qiagen) according to the manufacturer’s instructions and 1 μg of RNA was converted to cDNA using the ProtoScript AMV LongAmp Taq RT-PCR kit (New England Biolabs). Fn14, GAPDH and ribosomal protein L13a mRNA levels were quantified as previously described (21).
Small interfering RNA transfections
Cells were plated and allowed to attach for 5 hours and then transfected with transfection reagent alone (no siRNA), luciferase siRNA, Src siRNAs #7 or #10 targeted to the human Src transcript, or Fn14 siRNAs #1 and #4 targeted to the murine Fn14 transcript at a final concentration of 20 nM using RNAiMax transfection reagent (Life Technologies) according to the manufacturer’s instructions. All siRNAs were purchased from Qiagen. Cells were harvested at 48 (Fn14 siRNA) or 72 (Src siRNA) hours post-transfection, lysed and Western blot analysis conducted as described above.
Cell invasion assays
Cells were harvested, resuspended in media containing 0.5% serum and plated in triplicate in Boyden chambers precoated with growth factor-reduced Matrigel (BD Biosciences). The chambers were then placed in 24-well plates (Corning) with growth media containing 10% FBS as a chemoattractant. Cells were allowed to invade for 20 hours and then fixed and stained as previously described (17). Cells from five randomly chosen fields were counted at 20X magnification under a light microscope and summed to calculate total number of cells invaded.
Statistics
Real-time RT-PCR and cell invasion assay results are presented as mean ± SEM and the two-sample Student’s t-test was used to determine statistical significance. P-values <0.05 were considered significant.
Results and Discussion
Dasatinib is a potent inhibitor of EGFR-driven Fn14 expression in HCC827 cells
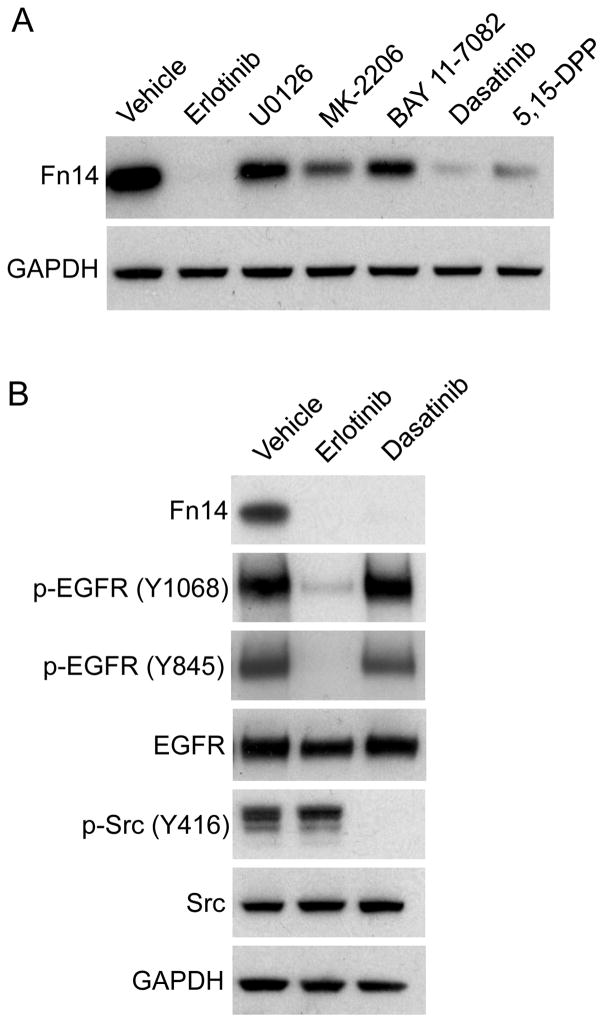
We previously showed that HCC827 cells, which contain an EGFR activating mutation, express relatively high levels of Fn14 (17). Treatment of these cells with the EGFR TKI erlotinib resulted in complete Fn14 down-regulation (17). EGFR activation triggers the stimulation of various interrelated signaling cascades, including the Ras/Raf/MEK/ERK and PI3K/Akt pathways, which are generally associated with cell proliferation and survival (7, 8). EGFR activation also stimulates the STAT (8, 26) and NF-κB (27) pathways, which trigger the activation of latent, cytoplasmic transcriptional regulators that modulate gene expression. Additionally, both ligand-activated, wild-type EGFR (28) and the gain-of-function EGFR mutants that are expressed in a subset of NSCLC tumors (29) can physically associate with c-Src, leading to Y-416 autophosphorylation, kinase activation, and downstream cellular responses (28–31). We investigated whether one or more of these signaling pathways were critical for EGFR-driven Fn14 expression by treating HCC827 cells with either erlotinib (EGFR inhibitor; a positive control for complete Fn14 down-regulation (17)), U0126 (MEK inhibitor), MK-2206 (Akt inhibitor), BAY-11-7082 (IKK inhibitor), dasatinib (Src inhibitor), or 5,15-DPP (STAT3 inhibitor) for 8 hours. Cell lysates were prepared and Western blot analysis was performed. All of the downstream pathway pharmacological inhibitors decreased Fn14 levels, but dasatinib had the most potent inhibitory effect under our experimental conditions (i.e., drug doses and treatment time) (Fig. 1A).
Figure 1. Effect of erlotinib or signaling pathway inhibitor treatment on EGFR-driven Fn14 expression in HCC827 cells.
(A) HCC827 cells were serum-starved overnight and then treated with either vehicle (DMSO), erlotinib (1 μM), U0126 (1 μM), MK-2206 (1 μM), BAY 11-7082 (10 μM), dasatinib (30 nM), or 5,15-DPP (20 μM) for 8 hours. Cells were harvested and Fn14 and GAPDH levels were analyzed by Western blotting. (B) HCC827 cells were treated with vehicle, erlotinib or dasatinib as described in (A). Cells were harvested and Fn14, p-EGFR, EGFR, p-Src, Src and GAPDH levels were analyzed by Western blotting.
Although dasatinib is largely selective for BCR-ABL and SFK members at low doses, it can inhibit many other tyrosine kinases (32–34), including EGFR (35), at higher doses. We chose our dasatinib concentration (30 nM) in consideration of a prior report using HCC827 cells indicating that this dosage should effectively inhibit Src signaling but not EGFR signaling (35). To confirm that this was indeed the case, HCC827 cells were treated with erlotinib or dasatinib for 8 hours, cell lysates were prepared, and Western blot analysis was conducted. Src activation was assessed using a pan p-Src antibody that was raised against a synthetic phosphopeptide corresponding to residues surrounding Y-416 of human Src. This antibody may cross-react with other SFK members when phosphorylated at an equivalent site. EGFR activation was assessed using an antibody that detects phosphorylation at Y-1068, one of the major EGFR autophosphorylation sites. We also examined the phosphorylation status of EGFR residue Y-845. Phosphorylation on this tyrosine can be mediated by Src (28, 29, 36), Brk/PTK6 (37), and in some cases, by EGFR itself (38). Constitutive EGFR and Src phosphorylation was noted in HCC827 cells, consistent with previous reports (17, 30, 31, 35, 39) (Fig. 1B). Cells treated with erlotinib for 8 hours had reduced p-EGFR levels (Y-1068, Y-845) but not reduced p-Src levels, consistent with a prior report (40). However, when cells were treated with erlotinib for 24 hours, p-Src levels were reduced, indicating a linkage between EGFR kinase activity and Src phosphorylation (Supplementary Fig. S1). Cells treated with dasatinib had significantly reduced p-Src levels, but p-EGFR (Y-1068, Y-845) levels were unaffected (Fig. 1B). Taken together, these results indicate that at the drug dosages and time point used for this experiment, (i) EGFR tyrosine kinase activity is responsible for both Y-1068 and Y-845 phosphorylation in HCC827 cells, and (ii) the dasatinib-mediated reduction in Fn14 expression in HCC827 cells is not due to inhibition of EGFR tyrosine kinase activity.
Dasatinib treatment of HCC827 cells decreases both Fn14 protein and mRNA expression levels
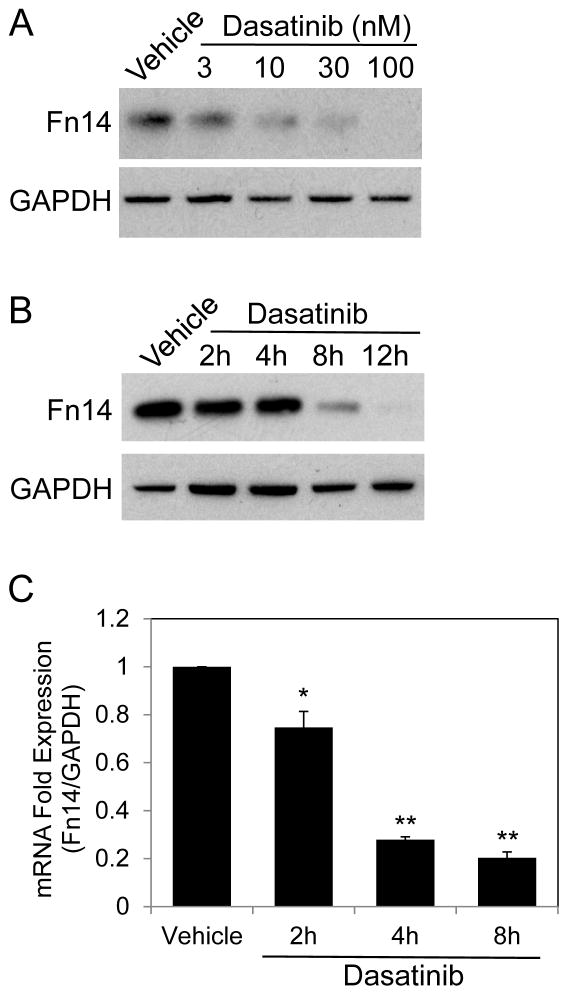
We next examined the effect of dasatinib on Fn14 protein expression in more detail by conducting dose-response and time-course experiments. First, HCC827 cells were treated with varying concentrations of dasatinib for 8 hours, cell lysates were prepared, and Western blot analysis was performed. Fn14 levels decreased in a dose-dependent manner, with the greatest effect detected using 100 nM dasatinib (Fig. 2A). Next, we treated HCC827 cells with 30 nM dasatinib for increasing amounts of time. Dasatinib also inhibited Fn14 levels in a time-dependent manner, with the greatest effect seen at 12 hours post-drug addition (Fig. 2B).
Figure 2. Effect of dasatinib treatment on Fn14 protein and mRNA levels in HCC827 cells.
(A) HCC827 cells were serum-starved overnight and then treated with either vehicle or the indicated concentrations of dasatinib for 8 hours. Cells were harvested and Fn14 and GAPDH levels were analyzed by Western blotting. (B) Cells were either vehicle treated or treated with dasatinib (30 nM) for the indicated time periods. Western blot analysis was conducted as described in (A). (C) Cells were serum-starved overnight and then treated with either vehicle or dasatinib (30 nM) for the indicated times. Cells were harvested, RNA was isolated, and Fn14 and GAPDH mRNA levels were measured using quantitative real-time PCR. Fn14 mRNA expression levels were normalized to GAPDH expression levels and presented as fold expression relative to vehicle control. The values shown are mean ± SEM of triplicate wells. Significance was measured by Student’s t-test. * p < 0.05, ** p < 0.001
We then determined whether dasatinib treatment of HCC827 cells reduced Fn14 mRNA levels. For this analysis, cells were treated with 30 nM dasatinib for 2, 4, or 8 hours, cells were harvested, RNA was isolated, and Fn14 and GAPDH mRNA levels were quantitated by real-time RT-PCR. We found that Fn14 mRNA levels decreased within 2 hours of dasatinib treatment, with maximal reduction noted at 8 hours, the last time point examined (Fig. 2C). This finding indicates that the dasatinib-mediated reduction in Fn14 protein levels in HCC827 cells is likely due, at least in part, to a decrease in Fn14 mRNA levels.
Dasatinib treatment of EGFR-mutant HCC2279 cells, EGFR-mutant H1975 cells, and EGF-stimulated, EGFR wild-type A549 cells also decreases Fn14 protein levels
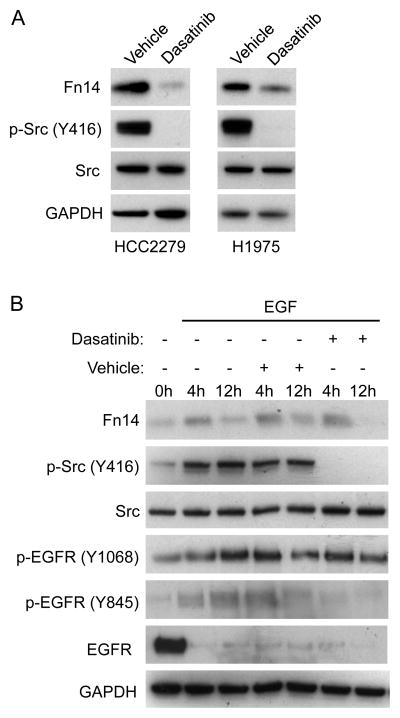
To assess whether the inhibitory effect of dasatinib on Fn14 gene expression occurred in other NSCLC cell lines that harbor an activating EGFR mutation, we treated HCC2279 and H1975 cells, which are known to express constitutively active EGFR (17) and Src (30, 35), with 30 nM dasatinib for 8 hours. We found that dasatinib was also a potent inhibitor of Src activation and Fn14 protein expression in these two cell lines (Fig. 3A).
Figure 3. Effect of dasatinib treatment on Fn14 protein levels in EGFR-mutant HCC2279 and H1975 cells and EGF-stimulated, EGFR wild-type A549 cells.
(A) HCC2279 and H1975 cells were serum-starved overnight and then either treated with vehicle or dasatinib (30 nM) for 8 hours. Cells were harvested and Fn14, p-Src, Src, and GAPDH levels were analyzed by Western blotting. (B) A549 cells were serum-starved overnight and then either left untreated or treated with EGF (50 ng/mL) alone, EGF and drug vehicle, or EGF and dasatinib (30 nM) for the indicated time periods. Cells were harvested and Fn14, p-Src, Src, p-EGFR, EGFR, and GAPDH levels were analyzed by Western blotting.
We reported previously that EGF stimulation of serum-starved A549 NSCLC cells transiently increases Fn14 protein levels (17). These cells do not contain an EGFR activating mutation and do not exhibit constitutive Src signaling (17, 35). Therefore, we selected these cells for testing whether EGF-driven Fn14 expression could also be mediated by Src activity. Cells were either left untreated or treated with EGF in the absence or presence of vehicle (DMSO) or dasatinib for 4 or 12 hours. Cell lysates were prepared and Western blot analysis was conducted. We found that EGF treatment of A549 cells stimulated Fn14 expression by ~2.5-fold and ~1.4-fold at the 4 and 12 hour time-points, respectively (Fig. 3B). EGF also increased p-Src and p-EGFR (Y-1068, Y-845) levels. EGF exposure reduced total EGFR levels, consistent with previous studies showing that EGF:EGFR engagement promotes EGFR internalization, ubiquitination, and degradation via the lysosomal pathway (41, 42). The EGF-mediated p-Src and p-EGFR (Y-845) increase, but not the p-EGFR (Y-1068) increase, was inhibited by dasatinib co-treatment. Dasatinib treatment had no significant effect on EGF-stimulated Fn14 expression at the 4 hour time-point, but completely inhibited Fn14 expression at the 12 hour time-point, indicating that Src activation following EGF:EGFR engagement also contributes to Fn14 gene regulation.
Fn14 protein levels are decreased in Src-depleted HCC827 cells
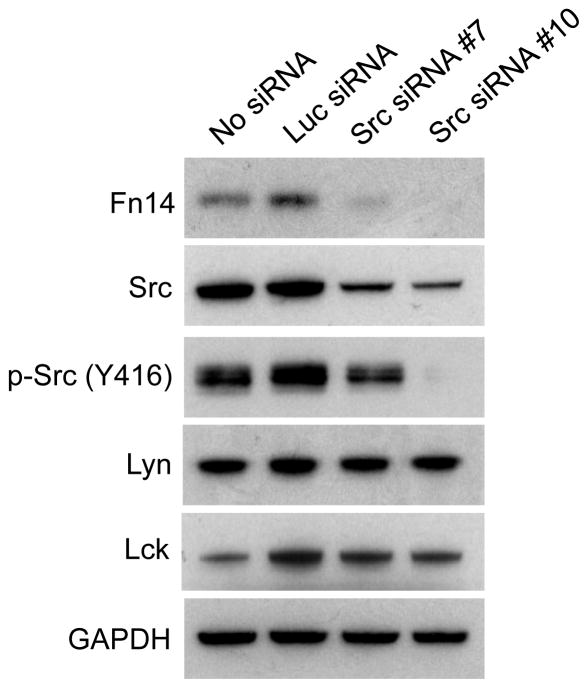
As mentioned above, dasatinib likely inhibits all SFK members at the dosage we have employed here and could potentially reduce the activity of other structurally similar tyrosine kinases as well (32, 34). A prior study examining global tyrosine kinase activation in cancer cell lines found that HCC827 cells express tyrosine phosphorylated Src, Lyn and Lck but not Blk, Fgr, Fyn, nor Hck (39). We hypothesized that Src may be the main SFK member contributing to EGFR-driven Fn14 expression in HCC827 cells because it has been previously demonstrated to both be physically associated with EGFR in NSCLC cells with activating EGFR mutations (29) and constitutively active in these cells (39). To test this hypothesis, cells were either left untransfected or transfected with luciferase (Luc) siRNA or two different Src siRNAs. Cells were harvested 72 hours later and Western blot analysis was conducted. Src expression was increased slightly in the Luc siRNA-transfected cells (by ~2%) but it was reduced by ~48% and ~65% in the Src siRNA #7- or Src siRNA #10-transfected cells, respectively (as compared to the untransfected cells) (Fig. 4). The amount of intracellular p-Src protein was also reduced following Src siRNA addition. Neither Luc siRNA nor Src siRNA treatment had an effect on expression of the related SFKs Lyn or Lck. We found that Luc siRNA treatment increased Fn14 protein levels (by ~24%) whereas Src siRNA #7 or Src siRNA #10 treatment reduced Fn14 protein levels by ~72% and ~93%, respectively, as compared to the untransfected cells, indicating that full Src function is required for EGFR-driven Fn14 expression.
Figure 4. Effect of Src depletion on Fn14 protein levels in HCC827 cells.
HCC827 cells were either left untransfected or transfected with the indicated siRNAs (20 nM). Cells were harvested 72 hours post-transfection and Fn14, Src, p-Src, Lyn, Lck, and GAPDH levels were analyzed by Western blotting.
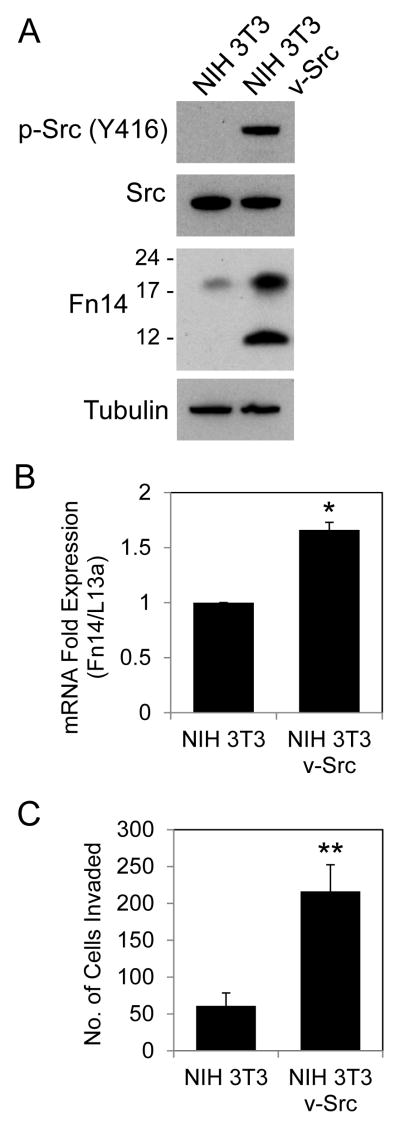
Constitutive Src activity in engineered NIH 3T3 cells increases both Fn14 expression and invasive activity
In order to test whether constitutive Src signaling could upregulate Fn14 expression in other cell lines besides EGFR-activated NSCLC cells, we compared Fn14 levels in control and v-Src-transformed NIH 3T3 cells by Western blot analysis. The NIH 3T3/v-Src cells exhibited constitutive Src phosphorylation (Fig. 5A), as expected, and numerous other signaling pathways were also activated in these cells, including the ERK, JNK, Akt, and STAT3 pathways (Supplementary Fig. S2), consistent with previous reports (43, 44). Furthermore, v-Src expression in NIH 3T3 cells stimulated NF-κB2 (p100/p52) processing, which is indicative of alternative NF-κB pathway activation (Supplementary Fig. S2). We found that the NIH 3T3/v-Src cells expressed significantly more Fn14 protein than the control NIH 3T3 cells (~4.5-fold increase) (Fig. 5A), which supports our NSCLC cell data indicating that Fn14 is a Src-inducible gene. We should note here that in some cell lines, including these NIH 3T3/v-Src cells, two Fn14 isoforms are detected by Western blot analysis. The higher molecular weight form is full-length Fn14, while the lower molecular weight form is believed to be a either an Fn14 mRNA splice variant product or a proteolytically-cleaved isoform of full-length Fn14 missing most of the extracellular domain (45). To determine whether v-Src expression also increased Fn14 mRNA levels, control and v-Src expressing NIH 3T3 cells were harvested, RNA was isolated, and Fn14 and ribosomal protein L13a mRNA levels were quantitated by real-time RT-PCR. We found that Fn14 mRNA levels were increased in the NIH 3T3/v-Src cells (Fig. 5B).
Figure 5. Effect of constitutive Src activity in NIH 3T3 cells on Fn14 expression and invasive capacity.
(A) NIH 3T3 and NIH 3T3/v-Src cells were harvested and p-Src, Src, Fn14 and tubulin levels were analyzed by Western blotting. The positions of molecular size markers are shown on the left (in kDa) for the Fn14 blot. (B) NIH 3T3 and NIH 3T3/v-Src cells were harvested, RNA was isolated, and Fn14 and ribosomal protein L13a mRNA levels were measured using quantitative real-time PCR. Fn14 mRNA expression levels were normalized to L13a expression levels and presented as fold expression relative to control NIH 3T3 cells. The values shown are mean ± SEM of triplicate wells. Significance was measured by Student’s t-test. * p < 0.05. (C) NIH 3T3 and NIH 3T3/v-Src cells (1 × 105) were resuspended in medium containing 0.5% serum and plated on Boyden chambers coated with Matrigel. Medium containing 10% serum was placed in the lower wells as a chemoattractant. After 20 hours of incubation, the number of cells that had invaded through the Matrigel was determined. Values shown are the mean ± SEM of triplicate (NIH 3T3) or quadruplicate (NIH 3T3/v-Src) chambers. Significance was measured by Student’s t-test. ** p < 0.01.
Src family kinases, via their effects on gene expression, cytoskeletal reorganization and cellular adhesion, are major intracellular mediators of tumor cell migration and invasion (24, 25, 46, 47). Therefore, we next compared the basal invasive activity of control NIH 3T3 cells and the NIH 3T3/v-Src cells using modified Boyden chambers coated with basement membrane extract (Matrigel). We found that the NIH 3T3/v-Src cells invaded ~3.5-fold more than the control NIH 3T3 cells (Fig. 5C), consistent with an earlier report (43).
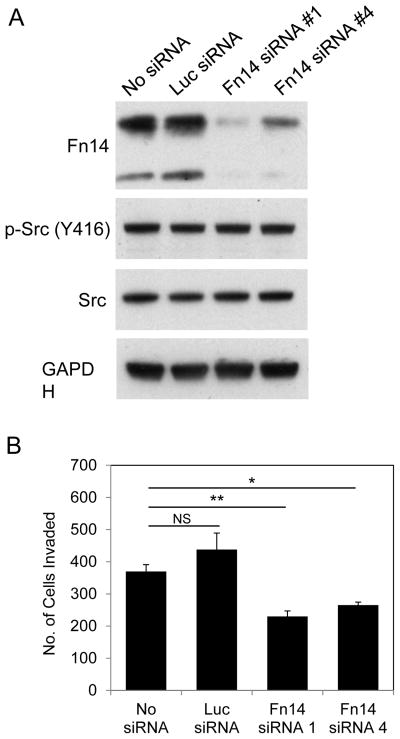
NIH 3T3/v-Src cell invasive activity is decreased by Fn14 depletion
Several studies have demonstrated that experimental manipulation of Fn14 levels in cancer cells using either an RNA interference or ectopic Fn14 overexpression strategy can modulate the basal invasive activity of these cells (11, 12, 14, 15, 17, 21). However, there are no reports indicating that Fn14 can act as a downstream effector of Src-driven invasiveness. To test if this was the case, we used the Fn14-positive, highly invasive NIH 3T3/v-Src cells described above and an RNA interference approach to investigate if Fn14 expression was important for Src-driven cell invasion. Cells were either left untransfected or transfected with Luc siRNA or two different Fn14 siRNAs, harvested 48 hours later, and Western blot analysis was conducted. Fn14 expression was increased in the Luc siRNA-transfected cells by ~10%, but reduced in the Fn14 siRNA #1- or #4-transfected cells by ~88% and ~57%, respectively (as compared to the untransfected cells) (Fig. 6A). Neither Luc siRNA nor Fn14 siRNA treatment had an effect on p-Src or Src expression levels. A portion of the same cells harvested for Western blot analysis was plated into Matrigel invasion chambers to determine the effect of Fn14 depletion on NIH 3T3/v-Src cell invasive activity. There was no statistically significant difference in the invasive capacity of the untransfected cells as compared with the Luc siRNA-transfected cells (Fig. 6B). However, Fn14 depletion using siRNA #1 or #4 reduced the invasive capacities of the NIH 3T3/v-Src cells by ~40% and ~27%, respectively (as compared to the untransfected cells), demonstrating that Fn14 expression is required for maximal Src-mediated cellular invasiveness.
Figure 6. Effect of Fn14 depletion on NIH 3T3/v-Src cell invasive capacity.
(A) NIH 3T3/v-Src cells were either left untransfected or transfected with the indicated siRNAs (20 nM). Cells were harvested 48 hours post-transfection, some of the cells were lysed, and Fn14, p-Src, Src, and GAPDH levels were analyzed by Western blotting. (B) The remaining NIH 3T3 v-Src cells from the four treatment groups (1 × 105) were resuspended in medium containing 0.5% serum and plated in Boyden chambers coated with Matrigel. Medium containing 10% serum was placed in the well below the chambers as a chemoattractant. After 20 hours of incubation, the number of cells that had invaded through the Matrigel was determined. Values shown are the mean ± SEM of triplicate chambers. NS = not significant, * p < 0.05, ** p < 0.01.
In summary, we report here that EGFR-driven Fn14 expression in NSCLC cells, resulting from either ligand (EGF) engagement or the presence of an EGFR activating mutation, is mediated in part by activation of the Src tyrosine kinase. The ability of Src to induce Fn14 gene expression was also observed in v-Src-transformed NIH 3T3 fibroblasts, so this effect may occur in most cell lines that exhibit either transient Src activation in response to growth factor exposure or persistent Src activation as a consequence of activating mutations in upstream Src regulators (e.g., receptor tyrosine kinases). It appears from our quantitative RT-PCR data that Src activation may be increasing Fn14 protein levels, at least in part, by regulating Fn14 gene transcription, but the molecular basis for this effect is currently unknown. Src targets multiple substrates (24, 25, 46–48) and many of these play key roles in intracellular signaling pathways that influence transcription factor activity (24, 25, 46). Since (i) both HCC827 and NIH 3T3/v-Src cells exhibit constitutive STAT3 Y-705 phosphorylation (44, 49) (Supplementary Fig. S2), (ii) it has been reported that Fn14 is a STAT3-inducible gene (50), and (iii) a STAT3 inhibitor reduced Fn14 levels in HCC827 cells by ~77% (Fig. 1A), STAT3 could be involved in Src-induced Fn14 gene expression. However, this is unlikely to be the case in HCC827 cells because prior studies have shown that dasatinib treatment of these cells does not reduce STAT3 phosphorylation (activation) (35, 49). Another potential intermediary signaling node that could link an increase in Src activity to an increase in Fn14 gene transcription is the NF-κB pathway. Specifically, (i) Src has been shown to modulate NF-κB signaling in various cell types (51, 52) (Supplementary Fig. S2), (ii) NF-κB binding to the Fn14 promoter has been reported (14), and (iii) an NF-κB inhibitor reduced Fn14 levels in HCC827 cells by ~37%. (Fig. 1A). Future studies will examine the potential roles of the STAT and NF-κB transcription factor family members in Src-mediated Fn14 expression.
Elevated Src kinase activity has been reported in a wide range of cancer types, including breast (53), ovarian (54), melanoma (55), and NSCLC (30, 31), and Fn14 is also frequently upregulated in these same cancers (11–13, 17–19). Thus, our findings suggest that the Src signaling pathway could be an important driver of Fn14 overexpression in tumor tissue. Accordingly, Fn14 down-regulation could contribute to the efficacy of Src inhibitors noted in some cancer patients enrolled in clinical trials (25).
We also report here that v-Src-driven NIH 3T3 cell invasiveness through a Matrigel barrier is mediated, at least in part, by the level of Fn14 expression in these cells. Although numerous downstream effectors of Src-stimulated invasion have been identified, many of these proteins are Src substrates (e.g., FAK, CAS, cortactin) linked to intracellular pathways that impact cell adhesion and motility (46–48). However, since Fn14 has no tyrosine residues, it cannot be a Src substrate and therefore its pro-invasive activity appears to be primarily modulated by its expression level. We have shown that elevated Fn14 levels in HEK293 cells can trigger NF-κB pathway activation, and that this can occur without ligand (TWEAK) engagement (45); therefore, Fn14 pro-invasive activity may also not require TWEAK:Fn14 cell surface interaction. The molecular basis for Fn14 modulation of NIH 3T3/v-Src cell invasiveness is not known, but in glioblastoma cells there is evidence that TWEAK/Fn14 pathway-triggered invasion is mediated by the NF-κB signaling pathway (14), the SFK member Lyn (22), and the Rho GTPase family members Rac1 and Cdc42 (14, 23).
In conclusion, our novel findings showing that (i) Src, a non-receptor tyrosine kinase constitutively activated in many types of human cancer, can up-regulate Fn14 gene expression, and (ii) Src-driven invasion is dependent in part on Fn14 expression, supports the notion that Fn14-targeted imaging and therapeutic agents could have broad utility for many cancer patients and that Fn14 inhibition could attenuate tumor cell metastatic spread.
Supplementary Material
Implications.
These results indicate that oncogenic Src may contribute to Fn14 overexpression in solid tumors and that Src-mediated cell invasion could potentially be inhibited with Fn14-targeted therapeutics.
Acknowledgments
We thank Dr. Steve Zhan (University of Maryland School of Medicine) for the NIH3T3/v-Src cell line and Rebeca Galisteo and Cheryl Armstrong for technical support.
Grant Support
This work was supported in part by NIH grants R01 CA130967 (J.A.W.) and R01 CA130940 (N.L.T.). Emily Cheng was supported in part by NIH training grant T32-HL007698.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stinchcombe TE, Socinski MA. Current treatments for advanced stage non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:233–41. doi: 10.1513/pats.200809-110LC. [DOI] [PubMed] [Google Scholar]
- 4.Li T, Kung HJ, Mack PC, Gandara DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol. 2013;31:1039–49. doi: 10.1200/JCO.2012.45.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:201–05. doi: 10.1513/pats.200809-107LC. [DOI] [PubMed] [Google Scholar]
- 6.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small cell lung cancer. Nat Rev Cancer. 2010;10:760–74. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegelin MD, Borczuk AC. Epidermal growth factor receptor mutations in lung adenocarcinoma. Lab Invest. 2014;94:129–37. doi: 10.1038/labinvest.2013.147. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida T, Zhang G, Haura EB. Targeting epidermal growth factor receptor: central signaling kinase in lung cancer. Biochem Pharmacol. 2010;80:613–23. doi: 10.1016/j.bcp.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Burkly LC. TWEAK/Fn14 axis: The current paradigm of tissue injury-inducible function in the midst of complexities. Semin Immunol. 2014;26:229–36. doi: 10.1016/j.smim.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Cheng E, Armstrong CL, Galisteo R, Winkles JA. TWEAK/Fn14 axis-targeted therapeutics: moving basic science discoveries to the clinic. Front Immunol. 2013;4:473. doi: 10.3389/fimmu.2013.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willis AL, Tran NL, Chatigny JM, Charlton N, Vu H, Brown SA, et al. The fibroblast growth factor-inducible 14 receptor is highly expressed in HER2-positive breast tumors and regulates breast cancer cell invasive capacity. Mol Cancer Res. 2008;6:725–34. doi: 10.1158/1541-7786.MCR-08-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitsett TG, Fortin Ensign SP, Dhruv HD, Inge LJ, Kurywchak P, Wolf KK, et al. Fn14 expression correlates with MET in NSCLC and promotes MET-driven cell invasion. Clin Exp Metastasis. 2014;31:613–23. doi: 10.1007/s10585-014-9653-6. [DOI] [PubMed] [Google Scholar]
- 13.Michaelson JS, Cho S, Browning B, Zheng TS, Lincecum JM, Wang MZ, et al. TWEAK induces mammary epithelial branching morphogenesis. Oncogene. 2005;24:2613–24. doi: 10.1038/sj.onc.1208208. [DOI] [PubMed] [Google Scholar]
- 14.Tran NL, McDonough WS, Savitch BA, Fortin SP, Winkles JA, Symons M, et al. Increased fibroblast growth factor-inducible 14 expression levels promote glioma cell invasion via Rac1 and nuclear factor-kappaB and correlate with poor patient outcome. Cancer Res. 2006;66:9535–42. doi: 10.1158/0008-5472.CAN-06-0418. [DOI] [PubMed] [Google Scholar]
- 15.Huang M, Narita S, Tsuchiya N, Ma Z, Numakura K, Obara T, et al. Overexpression of Fn14 promotes androgen-independent prostate cancer progression through MMP-9 and correlates with poor treatment outcome. Carcinogenesis. 2011;32:1589–96. doi: 10.1093/carcin/bgr182. [DOI] [PubMed] [Google Scholar]
- 16.Kwon OH, Park SJ, Kang TW, Kim M, Kim JH, Noh SM, et al. Elevated fibroblast growth factor-inducible 14 expression promotes gastric cancer growth via nuclear factor-kappaB and is associated with poor patient outcome. Cancer Lett. 2012;314:73–81. doi: 10.1016/j.canlet.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Whitsett TG, Cheng E, Inge L, Asrani K, Jameson NM, Hostetter G, et al. Elevated expression of Fn14 in non-small cell lung cancer correlates with activated EGFR and promotes tumor cell migration and invasion. Am J Pathol. 2012;181:111–20. doi: 10.1016/j.ajpath.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H, Ekmekcioglu S, Marks JW, Mohamedali KA, Asrani K, Phillips KK, et al. The TWEAK receptor Fn14 is a therapeutic target in melanoma: immunotoxins targeting Fn14 receptor for malignant melanoma treatment. J Invest Dermatol. 2013;133:1052–62. doi: 10.1038/jid.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu L, Dai L, Cao C, Zhu J, Ding C, Xu HB, et al. Functional expression of TWEAK and the receptor Fn14 in human malignant ovarian tumors: possible implication for ovarian tumor intervention. PLoS One. 2013;8:e57436. doi: 10.1371/journal.pone.0057436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitsett TG, Mathews IT, Cardone MH, Lena RJ, Pierceall WE, Bittner M, et al. Mcl-1 mediates TWEAK/Fn14-induced non-small cell lung cancer survival and therapeutic response. Mol Cancer Res. 2014;12:550–59. doi: 10.1158/1541-7786.MCR-13-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asrani K, Keri RA, Galisteo R, Brown SA, Morgan SJ, Ghosh A, et al. The HER2- and heregulin-beta1 (HRG)-inducible TNFR superfamily member Fn14 promotes HRG-driven breast cancer cell migration, invasion, and MMP9 expression. Mol Cancer Res. 2013;11:393–04. doi: 10.1158/1541-7786.MCR-12-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhruv HD, Whitsett TG, Jameson NM, Patel F, Winkles JA, Berens ME, et al. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) promotes glioblastoma cell chemotaxis via Lyn activation. Carcinogenesis. 2014;35:218–26. doi: 10.1093/carcin/bgt289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortin SP, Ennis MJ, Schumacher CA, Zylstra-Diegel CR, Williams BO, Ross JT, et al. Cdc42 and the guanine nucleotide exchange factors Ect2 and Trio mediate Fn14-induced migration and invasion of glioblastoma cells. Mol Cancer Res. 2012;10:958–68. doi: 10.1158/1541-7786.MCR-11-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeatman TJ. A renaissance for Src. Nat Rev Cancer. 2004;4:470–80. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci. 2012;33:122–28. doi: 10.1016/j.tips.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez JV, Greulich H, Sellers WR, Meyerson M, Frank DA. Signal transducer and activator of transcription 3 is required for the oncogenic effects of non-small cell lung cancer-associated mutations of the epidermal growth factor receptor. Cancer Res. 2006;66:3162–68. doi: 10.1158/0008-5472.CAN-05-3757. [DOI] [PubMed] [Google Scholar]
- 27.De S, Dermawan JK, Stark GR. EGF receptor uses SOS1 to drive constitutive activation of NF-κB in cancer cells. Proc Natl Acad Sci USA. 2014;111:11721–26. doi: 10.1073/pnas.1412390111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA. 1999;96:1415–20. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung BM, Dimri M, George M, Reddi AL, Chen G, Band V, et al. The role of cooperativity with Src in oncogenic transformation mediated by non-small cell lung cancer-associated EGF receptor mutants. Oncogene. 2009;28:1821–32. doi: 10.1038/onc.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Kalyankrishna S, Wislez M, Thilaganathan N, Saigal B, Wei W, et al. Src-family kinases are activated in non-small cell lung cancer and promote the survival of epidermal growth factor receptor-dependent cell lines. Am J Pathol. 2007;170:366–76. doi: 10.2353/ajpath.2007.060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung EL, Tam IY, Tin VP, Chua DT, Sihoe AD, Cheng LC, et al. Src promotes survival and invasion of lung cancers with epidermal growth factor receptor abnormalities and is a potential candidate for molecular-targeted therapy. Mol Cancer Res. 2009;7:923–32. doi: 10.1158/1541-7786.MCR-09-0003. [DOI] [PubMed] [Google Scholar]
- 32.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–61. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 33.Kantarjian H, Jabbour E, Grimley J, Kirkpatrick P. Dasatinib. Nat Rev Drug Discov. 2006;5:717–18. doi: 10.1038/nrd2135. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Rix U, Fang B, Bai Y, Edwards A, Colinge J, et al. A chemical and phosphoproteomic characterization of dasatinib action in lung cancer. Nat Chem Biol. 2010;6:291–99. doi: 10.1038/nchembio.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song L, Morris M, Bagui T, Lee FY, Jove R, Haura EB. Dasatinib (BMS-354825) selectively induces apoptosis in lung cancer cells dependent on epidermal growth factor receptor signaling for survival. Cancer Res. 2006;66:5542–48. doi: 10.1158/0008-5472.CAN-05-4620. [DOI] [PubMed] [Google Scholar]
- 36.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–43. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Lu Y, Liang K, Hsu JM, Albarracin C, Mills GB, et al. Brk/PTK6 sustains activated EGFR signaling through inhibiting EGFR degradation and transactivating EGFR. Oncogene. 2012;31:4372–83. doi: 10.1038/onc.2011.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song H, Huang L, Zhang M, Wang X, Song S, Yang L. Transphosphorylation of EGFR at Y845 plays an important role in its autophosphorylation and kinase activity. Oncol Rep. 2014;31:2393–98. doi: 10.3892/or.2014.3102. [DOI] [PubMed] [Google Scholar]
- 39.Du J, Bernasconi P, Clauser KR, Mani DR, Finn SP, Beroukhim R, et al. Bead-based profiling of tyrosine kinase phosphorylation identifies Src as a potential target for glioblastoma therapy. Nat Biotechnol. 2009;27:77–83. doi: 10.1038/nbt.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Looyenga BD, Hutchings D, Cherni I, Kingsley C, Weiss GJ, Mackeigan JP. STAT3 is activated by JAK2 independent of key oncogenic driver mutations in non-small cell lung carcinoma. PLoS One. 2012;7:e30820. doi: 10.1371/journal.pone.0030820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alwan HAJ, van Zoelen EJJ, van Leeuwen JEM. Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteosome-dependent EGFR de-ubiquitination. J Biol Chem. 2003;278:35781–90. doi: 10.1074/jbc.M301326200. [DOI] [PubMed] [Google Scholar]
- 42.Tomas A, Futter CE, Eden ER. EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol. 2014;24:26–34. doi: 10.1016/j.tcb.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauck CR, Hsia DA, Puente XS, Cheresh DA, Schlaepfer DD. FRNK blocks v-Src-stimulated invasion and experimental metastases without effects on cell motility or growth. EMBO J. 2002;21:6289–02. doi: 10.1093/emboj/cdf631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao X, Tay A, Guy GR, Tan YH. Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol. 1996;16:1595–1603. doi: 10.1128/mcb.16.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown SA, Cheng E, Williams MS, Winkles JA. TWEAK-independent Fn14 self-association and NF-κB activation is mediated by the C-terminal region of the Fn14 cytoplasmic domain. PLoS One. 2013;8:e65248. doi: 10.1371/journal.pone.0065248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guarino M. Src signaling in cancer invasion. J Cell Physiol. 2010;223:14–26. doi: 10.1002/jcp.22011. [DOI] [PubMed] [Google Scholar]
- 47.Boateng LR, Huttenlocher A. Spatiotemporal regulation of Src and its substrates at invadosomes. Eur J Cell Biol. 2012;91:878–88. doi: 10.1016/j.ejcb.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrando IM, Chaerkady R, Zhong J, Molina H, Jacob HK, Herbst-Robinson K, et al. Identification of targets of c-Src tyrosine kinase by chemical complementation and phosphoproteomics. Mol Cell Proteomics. 2012;11:355–69. doi: 10.1074/mcp.M111.015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song L, Rawal B, Nemeth JA, Haura EB. JAK1 activates STAT3 activity in non-small-cell lung cancer cells and IL-6 neutralizing antibodies can suppress JAK1-STAT3 signaling. Mol Cancer Ther. 2011;10:481–94. doi: 10.1158/1535-7163.MCT-10-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dauer DJ, Ferraro B, Song L, Yu B, Mora L, Buettner R, et al. STAT3 regulates genes common to both wound healing and cancer. Oncogene. 2005;24:3397–08. doi: 10.1038/sj.onc.1208469. [DOI] [PubMed] [Google Scholar]
- 51.Funakoshi-Tago M, Tago K, Andoh K, Sonoda Y, Tominaga S, Kasahara T. Functional role of c-Src in IL-1-induced NF-κB activation: c-Src is a component of the IKK complex. J Biochem. 2005;137:189–97. doi: 10.1093/jb/mvi018. [DOI] [PubMed] [Google Scholar]
- 52.Bijli KM, Minhajuddin M, Fazal F, O'Reilly MA, Platanias LC, Rahman A. c-Src interacts with and phosphorylates RelA/p65 to promote thrombin-induced ICAM-1 expression in endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L396–04. doi: 10.1152/ajplung.00163.2006. [DOI] [PubMed] [Google Scholar]
- 53.Anbalagan M, Moroz K, Ali A, Carrier L, Glodowski S, Rowan BG. Subcellular localization of total and activated Src kinase in African American and Caucasian breast cancer. PLoS One. 2012;7:e33017. doi: 10.1371/journal.pone.0033017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiener JR, Windham TC, Estrella VC, Parikh NU, Thall PF, Deavers MT, et al. Activated Src protein tyrosine kinase is overexpressed in late-stage human ovarian cancers. Gynecol Oncol. 2003;88:73–79. doi: 10.1006/gyno.2002.6851. [DOI] [PubMed] [Google Scholar]
- 55.Homsi J, Cubitt CL, Zhang S, Munster PN, Yu H, Sullivan DM, et al. Src activation in melanoma and Src inhibitors as therapeutic agents in melanoma. Melanoma Res. 2009;19:167–75. doi: 10.1097/CMR.0b013e328304974c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.