Abstract
Two distinct lipoprotein receptors can be expressed in the dog liver. One is the apolipoprotein (apo-) B,E receptor. This receptor binds apo-B-containing low density lipoproteins (LDL), as well as apo-E-containing lipoproteins, such as the cholesterol-induced high density lipoproteins (HDLc). The second hepatic lipoprotein receptor is the apo-E receptor. It binds apo-E HDLc and chylomicron remnants, but not LDL. The present studies were undertaken to determine whether short-term (acute) regulation of the two receptors can occur in response to perturbations in hepatic cholesterol metabolism. The design used three groups of experimental animals: (a) immature dogs (with both hepatic apo-B,E and apo-E receptors expressed), (b) adult dogs (with predominantly the apo-E receptor expressed and little detectable apo-B,E receptor binding activity), and (c) dogs treated with the bile acid sequestrant cholestyramine or those that have undergone biliary diversion (with apo-E receptors and induced apo-B,E receptors).
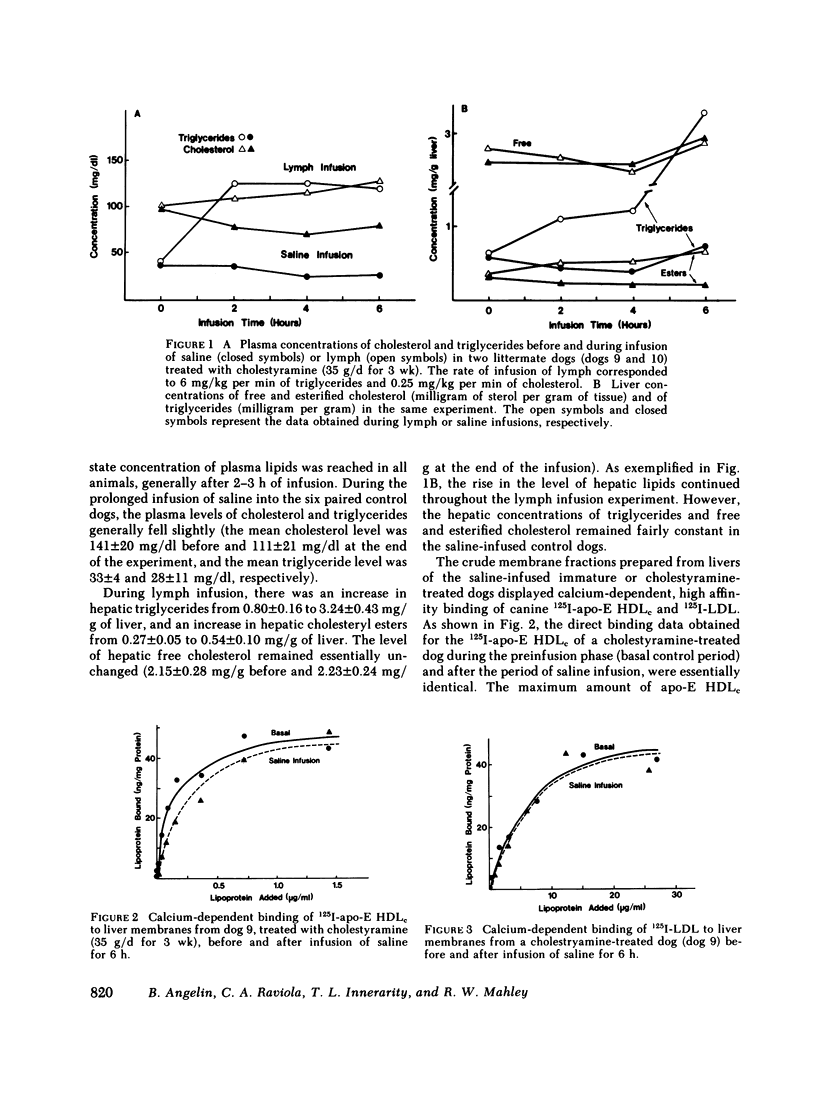
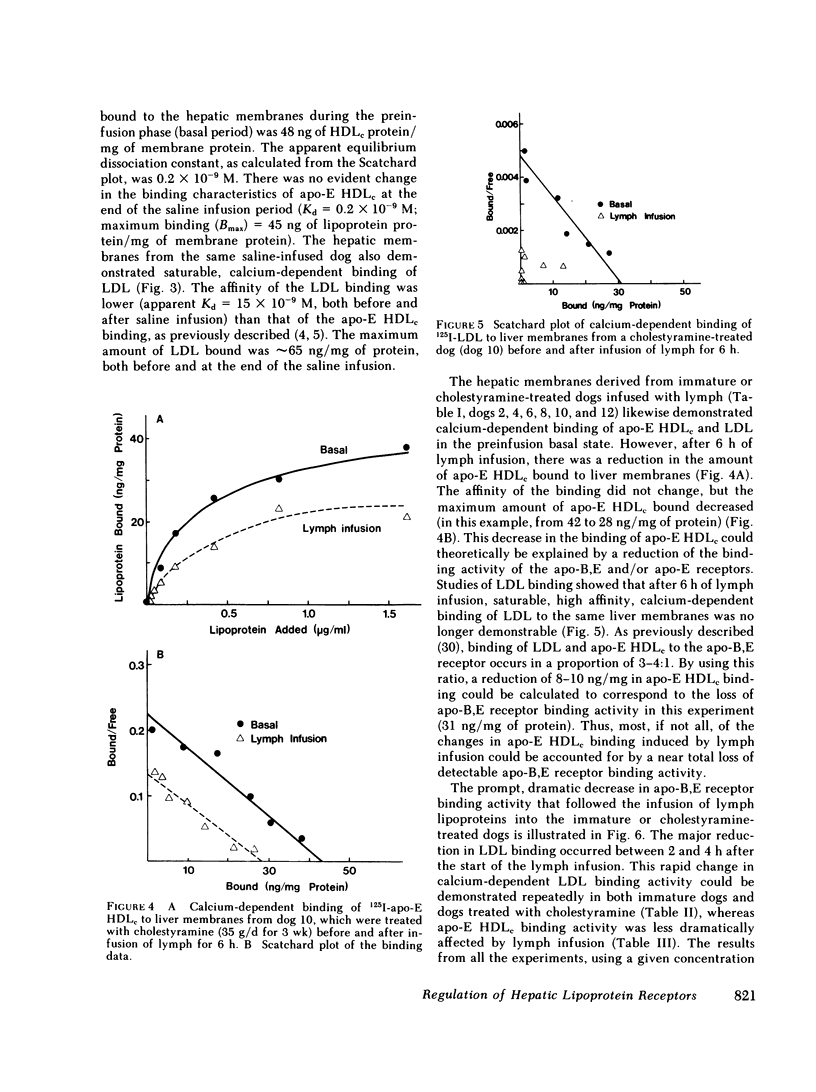
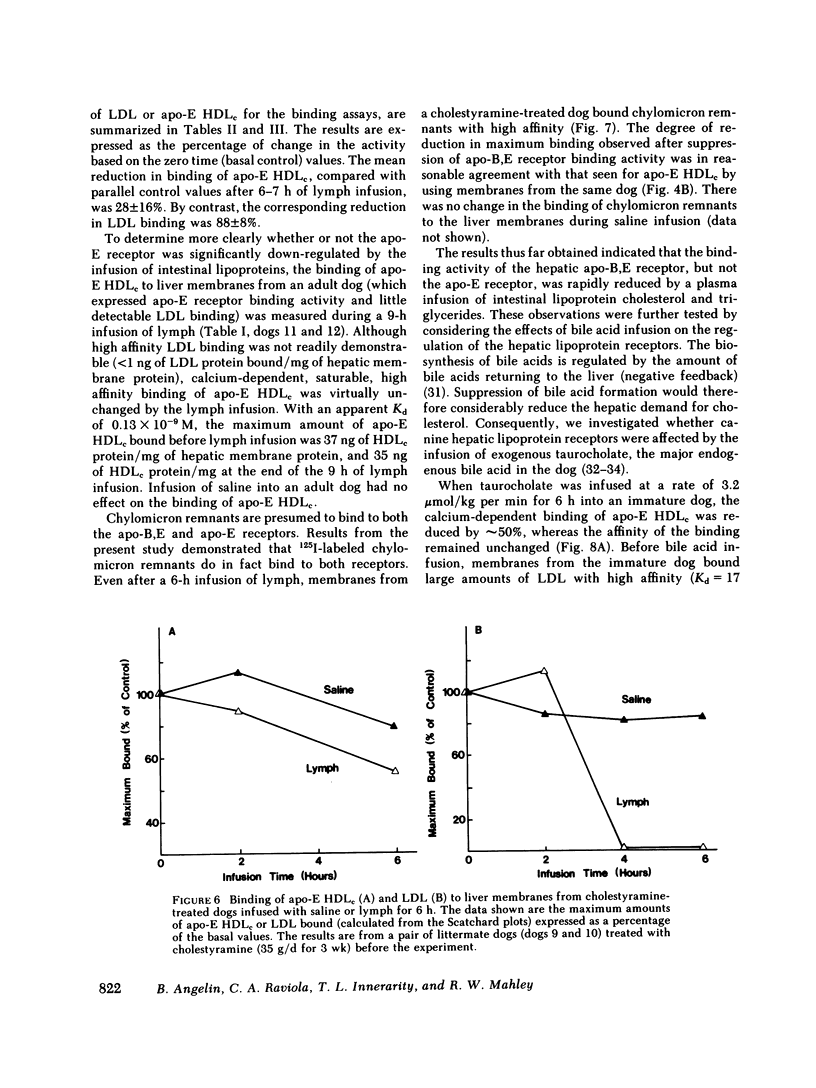
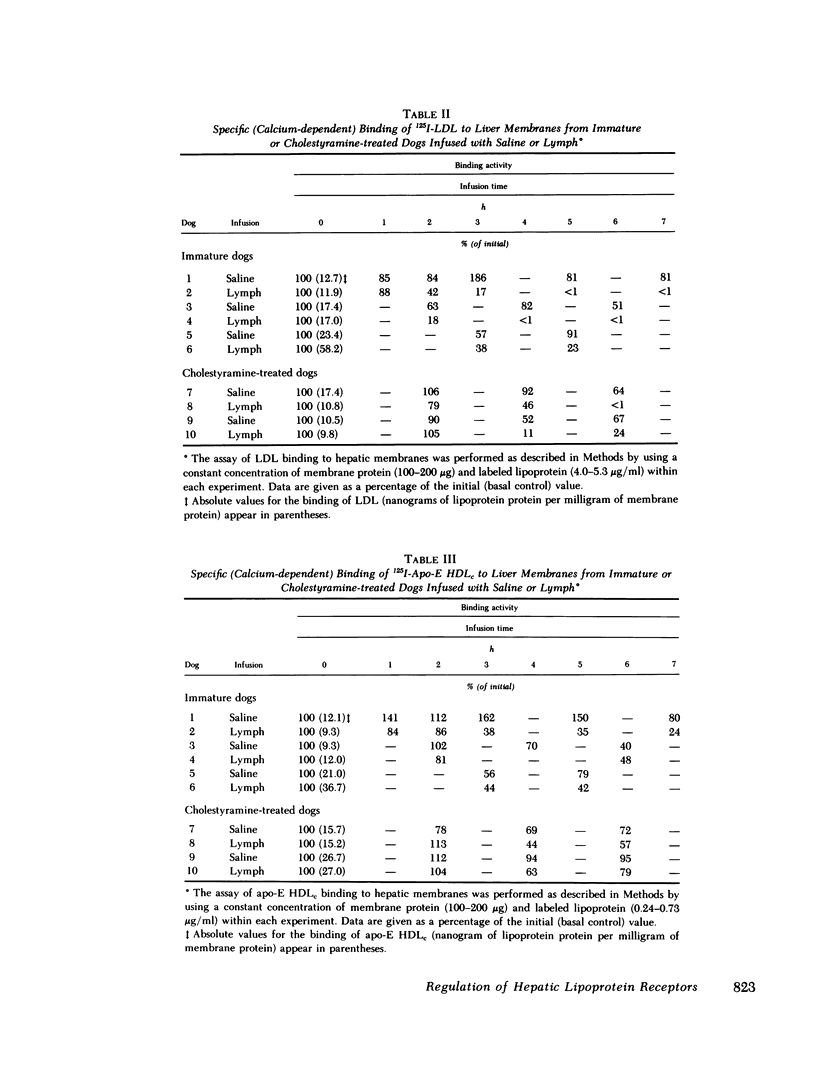
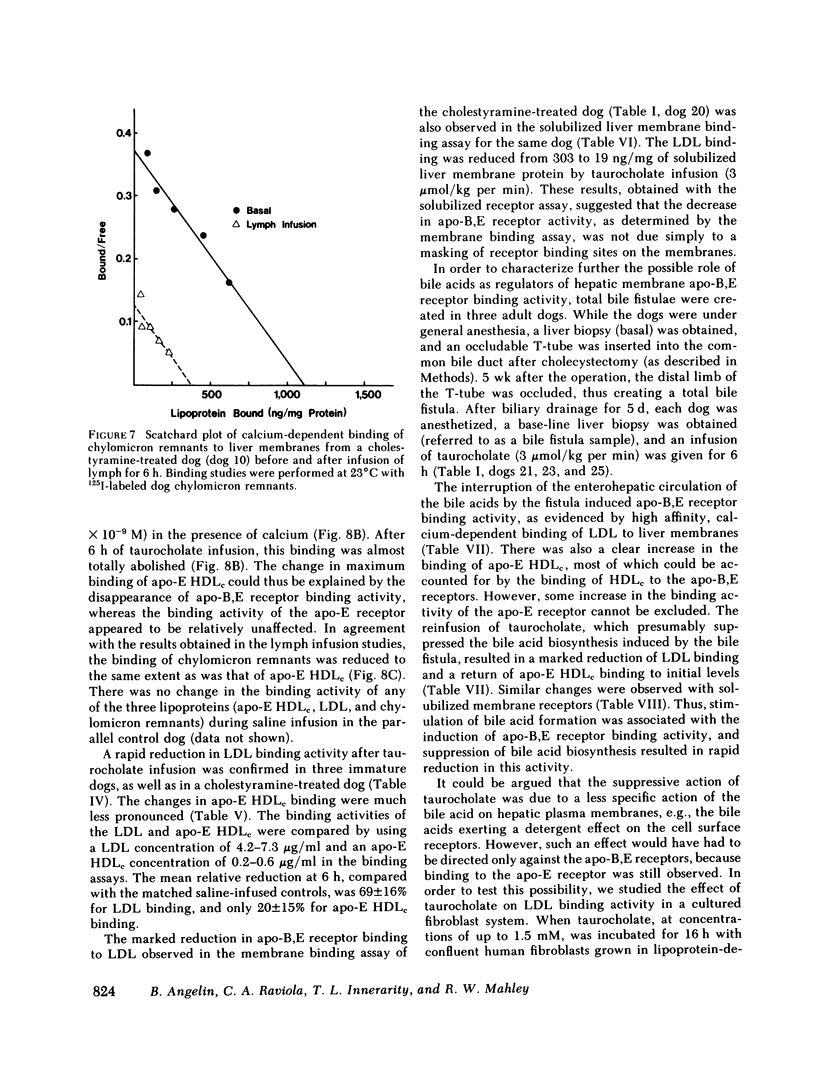
In the first series of experiments, changes in hepatic lipoprotein receptor expression were studied by delivering cholesterol to the liver via intestinal lymph lipoproteins. Dog lymph (5-11 mg of triglycerides/min per kg of body weight, 0.15-0.3 mg of cholesterol/min per kg) or saline were infused intravenously for 6-8 h into matched pairs of dogs. Serial liver biopsies were obtained at intervals of 1-2 h. A progressive loss of specific (calcium-dependent) binding of LDL was seen in hepatic membranes from both immature and cholestyramine-treated dogs. After 4-6 h of lymph infusion, almost no apo-B,E receptor binding could be detected. The decrease in binding of apo-E HDLc to the same membranes was much less pronounced, and could be explained by a loss of binding of HDLc to the apo-B,E receptor; there was little or no effect on apo-E receptor binding.
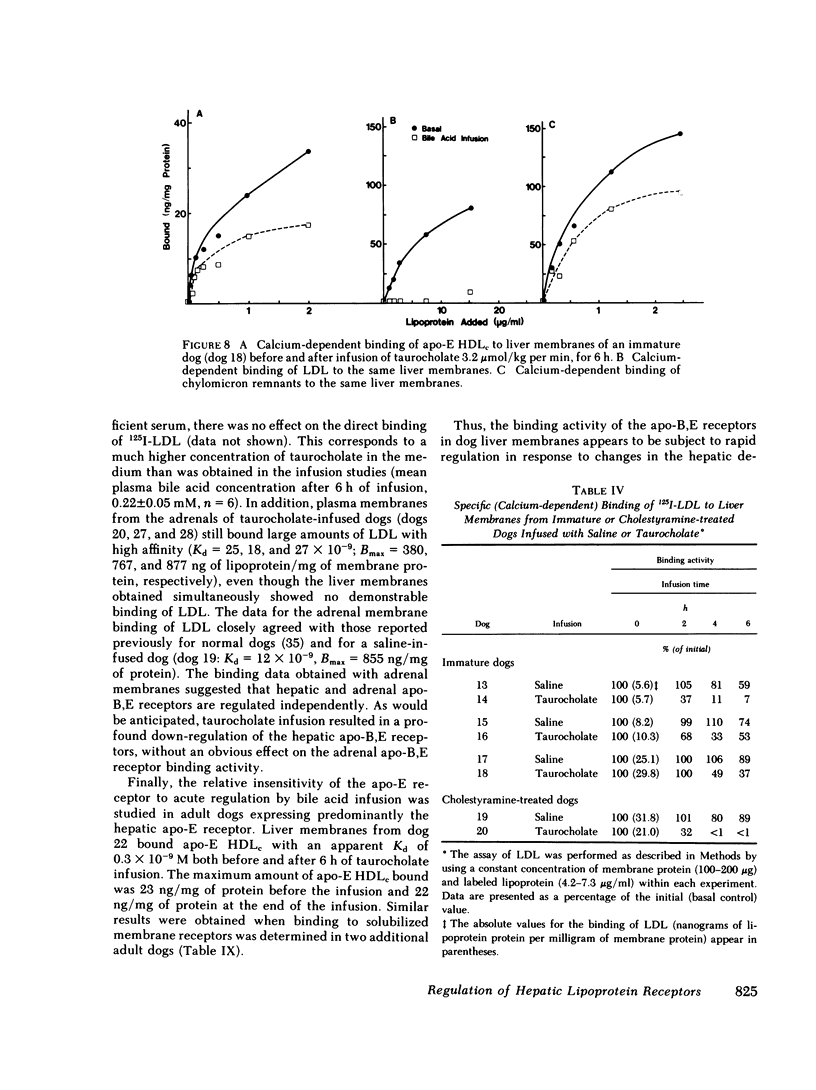
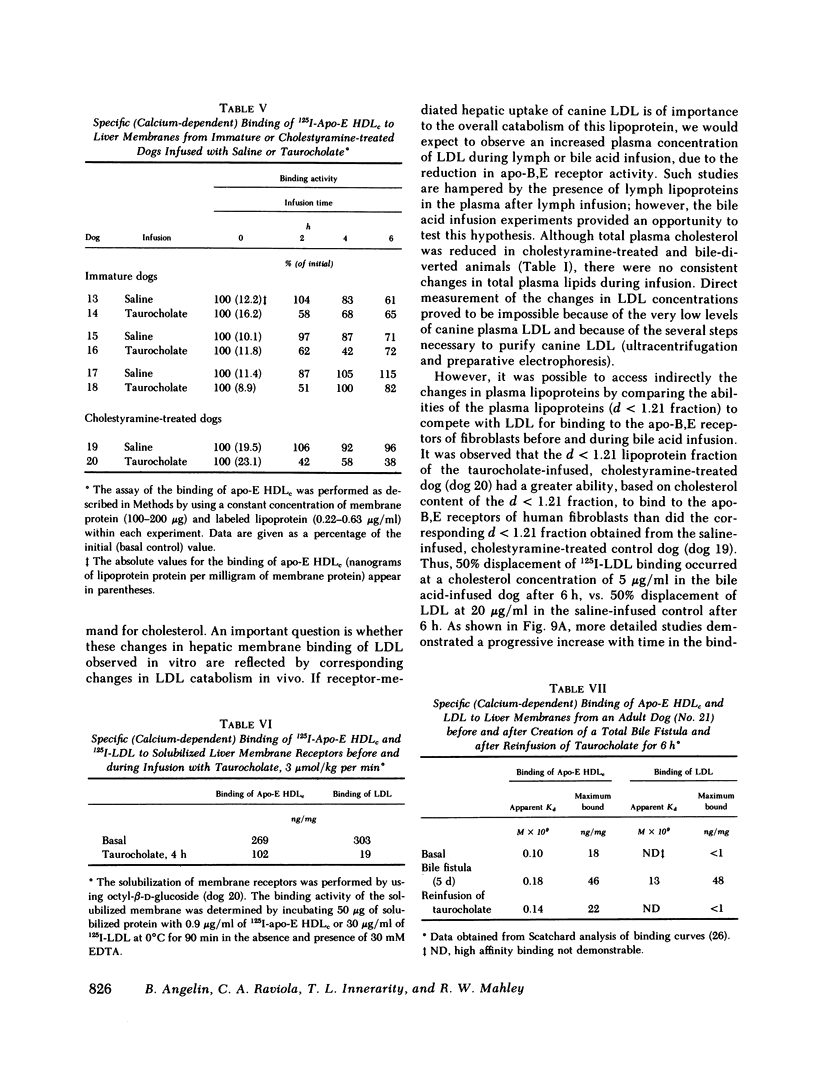
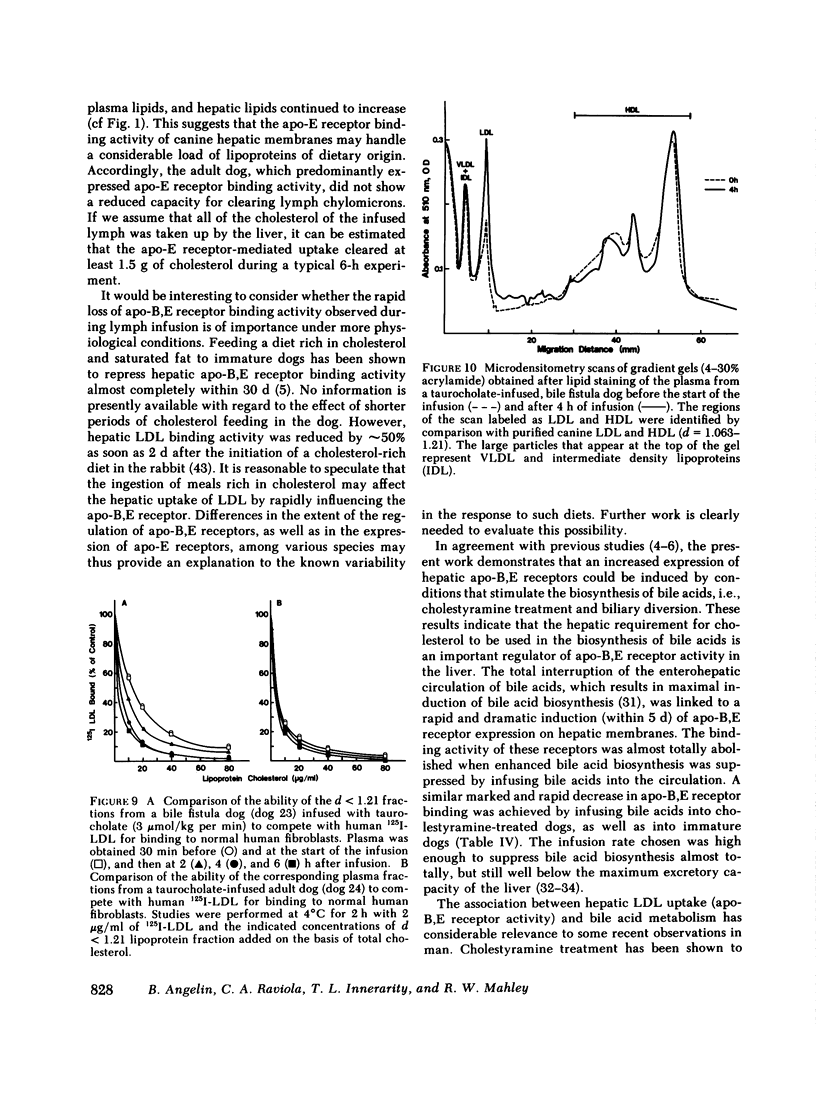
In the second series of experiments, the effects of a diminished hepatic demand for cholesterol on lipoprotein receptor expression were studied by suppressing bile acid synthesis. The bile acid taurocholate (2-3 μmol/kg per min) was infused intravenously over a 6-h interval. This resulted in a progressive loss of LDL binding to liver membranes of immature or cholestyramine-treated dogs. The infusion of taurocholate for 6 h did not significantly alter the expression of the apo-E receptor binding activity, whereas apo-B,E receptor activity was rapidly down-regulated. Preparation of a bile fistula in adult dogs markedly induced the expression of the apo-B,E receptor. In this state, the binding activity of the apo-B,E receptor could be almost totally abolished by reinfusion of taurocholate for 6 h, without profoundly affecting apo-E receptor binding. Evidence from the analysis of plasma lipoprotein patterns and tissue culture reactivity suggested that changes in assayed hepatic lipoprotein receptor activity occurred in concert with changes in plasma lipoproteins.
The results indicate that the two canine hepatic lipoprotein receptors differ in their metabolic regulation. The apo-B,E receptor responds rapidly to changes in hepatic requirements for cholesterol. The apo-E receptor appears to be more refractory to acute regulation. The rapidity of the changes in the activity of the apo-B,E receptor (within 2-4 h) suggests that the binding activity of this receptor may be regulated by factors independent of protein synthesis.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlberg J., Angelin B., Einarsson K. Hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and biliary lipid composition in man: relation to cholesterol gallstone disease and effects of cholic acid and chenodeoxycholic acid treatment. J Lipid Res. 1981 Mar;22(3):410–422. [PubMed] [Google Scholar]
- Albers J. J., Grundy S. M., Cleary P. A., Small D. M., Lachin J. M., Schoenfield L. J. National Cooperative Gallstone Study: the effect of chenodeoxycholic acid on lipoproteins and apolipoproteins. Gastroenterology. 1982 Apr;82(4):638–646. [PubMed] [Google Scholar]
- Angelin B., Einarsson K., Hellström K., Leijd B. Effects of cholestyramine and chenodeoxycholic acid on the metabolism of endogenous triglyceride in hyperlipoproteinemia. J Lipid Res. 1978 Nov;19(8):1017–1024. [PubMed] [Google Scholar]
- Angelin B., Einarsson K., Leijd B. Clofibrate treatment and bile cholesterol saturation: short-term and long-term effects and influence of combination with chenodeoxycholic acid. Eur J Clin Invest. 1981 Jun;11(3):185–189. doi: 10.1111/j.1365-2362.1981.tb01839.x. [DOI] [PubMed] [Google Scholar]
- Attie A. D., Pittman R. C., Steinberg D. Hepatic catabolism of low density lipoprotein: mechanisms and metabolic consequences. Hepatology. 1982 Mar-Apr;2(2):269–281. doi: 10.1002/hep.1840020215. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson R. G., Brown M. S. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981 May;24(2):493–502. doi: 10.1016/0092-8674(81)90340-8. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Brown M. S. Characterization of the low density lipoprotein receptor in membranes prepared from human fibroblasts. J Biol Chem. 1978 Jun 10;253(11):3852–3856. [PubMed] [Google Scholar]
- Beisiegel U., Kita T., Anderson R. G., Schneider W. J., Brown M. S., Goldstein J. L. Immunologic cross-reactivity of the low density lipoprotein receptor from bovine adrenal cortex, human fibroblasts, canine liver and adrenal gland, and rat liver. J Biol Chem. 1981 Apr 25;256(8):4071–4078. [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Regulation of the activity of the low density lipoprotein receptor in human fibroblasts. Cell. 1975 Nov;6(3):307–316. doi: 10.1016/0092-8674(75)90182-8. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Regulation of plasma cholesterol by lipoprotein receptors. Science. 1981 May 8;212(4495):628–635. doi: 10.1126/science.6261329. [DOI] [PubMed] [Google Scholar]
- Cooper A. D., Erickson S. K., Nutik R., Shrewsbury M. A. Characterization of chylomicron remnant binding to rat liver membranes. J Lipid Res. 1982 Jan;23(1):42–52. [PubMed] [Google Scholar]
- Danielsson H., Sjövall J. Bile acid metabolism. Annu Rev Biochem. 1975;44:233–253. doi: 10.1146/annurev.bi.44.070175.001313. [DOI] [PubMed] [Google Scholar]
- Danzinger R. C., Hofmann A. F., Thistle J. L., Schoenfield L. J. Effect of oral chenodeoxycholic acid on bile acid kinetics and biliary lipid composition in women with cholelithiasis. J Clin Invest. 1973 Nov;52(11):2809–2821. doi: 10.1172/JCI107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckelbaum R. J., Lees R. S., Small D. M., Hedberg S. E., Grundy S. M. Failure of complete bile diversion and oral bile acid therapy in the treatment of homozygous familial hypercholesterolemia. N Engl J Med. 1977 Mar 3;296(9):465–470. doi: 10.1056/NEJM197703032960901. [DOI] [PubMed] [Google Scholar]
- Erlinger S. Hepatocellular uptake of taurocholate in the dog. J Clin Invest. 1975 Feb;55(2):419–426. doi: 10.1172/JCI107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florén C. H., Albers J. J., Kudchodkar B. J., Bierman E. L. Receptor-dependent uptake of human chylomicron remnants by cultured skin fibroblasts. J Biol Chem. 1981 Jan 10;256(1):425–433. [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Havel R. J. Familial dysbetalipoproteinemia. New aspects of pathogenesis and diagnosis. Med Clin North Am. 1982 Mar;66(2):441–454. doi: 10.1016/s0025-7125(16)31429-8. [DOI] [PubMed] [Google Scholar]
- Hjelmeland L. M. A nondenaturing zwitterionic detergent for membrane biochemistry: design and synthesis. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6368–6370. doi: 10.1073/pnas.77.11.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D. Y., Innerarity T. L., Mahley R. W. Lipoprotein binding to canine hepatic membranes. Metabolically distinct apo-E and apo-B,E receptors. J Biol Chem. 1981 Jun 10;256(11):5646–5655. [PubMed] [Google Scholar]
- Innerarity T. L., Mahley R. W. Enhanced binding by cultured human fibroblasts of apo-E-containing lipoproteins as compared with low density lipoproteins. Biochemistry. 1978 Apr 18;17(8):1440–1447. doi: 10.1021/bi00601a013. [DOI] [PubMed] [Google Scholar]
- Innerarity T. L., Pitas R. E., Mahley R. W. Binding of arginine-rich (E) apoprotein after recombination with phospholipid vesicles to the low density lipoprotein receptors of fibroblasts. J Biol Chem. 1979 May 25;254(10):4186–4190. [PubMed] [Google Scholar]
- Innerarity T. L., Pitas R. E., Mahley R. W. Receptor binding of cholesterol-induced high-density lipoproteins containing predominantly apoprotein E to cultured fibroblasts with mutations at the low-density lipoprotein receptor locus. Biochemistry. 1980 Sep 2;19(18):4359–4365. doi: 10.1021/bi00559a032. [DOI] [PubMed] [Google Scholar]
- Kallner M. The effect of chenodeoxycholic acid feeding on bile acid kinetics and fecal neutral steroid excretion in patients with hyperlipoproteinemia types II and IV. J Lab Clin Med. 1975 Oct;86(4):595–604. [PubMed] [Google Scholar]
- Kane J. P., Hardman D. A., Paulus H. E. Heterogeneity of apolipoprotein B: isolation of a new species from human chylomicrons. Proc Natl Acad Sci U S A. 1980 May;77(5):2465–2469. doi: 10.1073/pnas.77.5.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen P. T., Basu S. K., Goldstein J. L., Brown M. S. Low density lipoprotein receptors in bovine adrenal cortex. II. Low density lipoprotein binding to membranes prepared from fresh tissue. Endocrinology. 1979 Mar;104(3):610–616. doi: 10.1210/endo-104-3-610. [DOI] [PubMed] [Google Scholar]
- Kovanen P. T., Bilheimer D. W., Goldstein J. L., Jaramillo J. J., Brown M. S. Regulatory role for hepatic low density lipoprotein receptors in vivo in the dog. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1194–1198. doi: 10.1073/pnas.78.2.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanen P. T., Brown M. S., Basu S. K., Bilheimer D. W., Goldstein J. L. Saturation and suppression of hepatic lipoprotein receptors: a mechanism for the hypercholesterolemia of cholesterol-fed rabbits. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1396–1400. doi: 10.1073/pnas.78.3.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss R. M., Burke D. J. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982 Jan;23(1):97–104. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahley R. W., Hui D. Y., Innerarity T. L., Weisgraber K. H. Two independent lipoprotein receptors on hepatic membranes of dog, swine, and man. Apo-B,E and apo-E receptors. J Clin Invest. 1981 Nov;68(5):1197–1206. doi: 10.1172/JCI110365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Weisgraber K. H., Fry D. L. Canine hyperlipoproteinemia and atherosclerosis. Accumulation of lipid by aortic medial cells in vivo and in vitro. Am J Pathol. 1977 Apr;87(1):205–226. [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H. Canine lipoproteins and atherosclerosis. I. Isolation and characterization of plasma lipoproteins from control dogs. Circ Res. 1974 Nov;35(5):713–721. doi: 10.1161/01.res.35.5.713. [DOI] [PubMed] [Google Scholar]
- Melchior G. W., Mahley R. W., Buckhold D. K. Chylomicron metabolism during dietary-induced hypercholesterolemia in dogs. J Lipid Res. 1981 May;22(4):598–609. [PubMed] [Google Scholar]
- O'Máille E. R., Richards T. G., Short A. H. The influence of conjugation of cholic acid on its uptake and secretion: hepatic extraction of taurocholate and cholate in the dog. J Physiol. 1967 Apr;189(2):337–350. doi: 10.1113/jphysiol.1967.sp008172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. F., Albers J. J., Bierman E. L. Rapid regulation of the activity of the low density lipoprotein receptor of cultured human fibroblasts. J Biol Chem. 1980 Jan 25;255(2):475–485. [PubMed] [Google Scholar]
- Pitas R. E., Innerarity T. L., Arnold K. S., Mahley R. W. Rate and equilibrium constants for binding of apo-E HDLc (a cholesterol-induced lipoprotein) and low density lipoproteins to human fibroblasts: evidence for multiple receptor binding of apo-E HDLc. Proc Natl Acad Sci U S A. 1979 May;76(5):2311–2315. doi: 10.1073/pnas.76.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajpal S. G., Kirkpatrick J. R. Creation of a thoracic duct fistula: an improved technique. J Surg Res. 1972 Nov;13(5):260–261. doi: 10.1016/0022-4804(72)90074-1. [DOI] [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Innerarity T. L., Mahley R. W. Structural basis for receptor binding heterogeneity of apolipoprotein E from type III hyperlipoproteinemic subjects. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4696–4700. doi: 10.1073/pnas.79.15.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Mahley R. W. Human apolipoprotein E. The complete amino acid sequence. J Biol Chem. 1982 Apr 25;257(8):4171–4178. [PubMed] [Google Scholar]
- Redgrave T. G., Roberts D. C., West C. E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975 May 12;65(1-2):42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- Schneider W. J., Basu S. K., McPhaul M. J., Goldstein J. L., Brown M. S. Solubilization of the low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5577–5581. doi: 10.1073/pnas.76.11.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. J., Kovanen P. T., Brown M. S., Goldstein J. L., Utermann G., Weber W., Havel R. J., Kotite L., Kane J. P., Innerarity T. L. Familial dysbetalipoproteinemia. Abnormal binding of mutant apoprotein E to low density lipoprotein receptors of human fibroblasts and membranes from liver and adrenal of rats, rabbits, and cows. J Clin Invest. 1981 Oct;68(4):1075–1085. doi: 10.1172/JCI110330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne F., Hanks J., Meyers W., Quarfordt S. Effect of apoproteins on hepatic uptake of triglyceride emulsions in the rat. J Clin Invest. 1980 Mar;65(3):652–658. doi: 10.1172/JCI109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J., Packard C. J., Bicker S., Lawrie T. D., Morgan H. G. Cholestyramine promotes receptor-mediated low-density-lipoprotein catabolism. N Engl J Med. 1980 May 29;302(22):1219–1222. doi: 10.1056/NEJM198005293022202. [DOI] [PubMed] [Google Scholar]
- Sherrill B. C., Innerarity T. L., Mahley R. W. Rapid hepatic clearance of the canine lipoproteins containing only the E apoprotein by a high affinity receptor. Identity with the chylomicron remnant transport process. J Biol Chem. 1980 Mar 10;255(5):1804–1807. [PubMed] [Google Scholar]
- Utermann G., Jaeschke M., Menzel J. Familial hyperlipoproteinemia type III: deficiency of a specific apolipoprotein (apo E-III) in the very-low-density lipoproteins. FEBS Lett. 1975 Aug 15;56(2):352–355. doi: 10.1016/0014-5793(75)81125-2. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982 Mar 10;257(5):2518–2521. [PubMed] [Google Scholar]
- Wheeler H. O., King K. K. Biliary excretion of lecithin and cholesterol in the dog. J Clin Invest. 1972 Jun;51(6):1337–1350. doi: 10.1172/JCI106930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windler E., Chao Y., Havel R. J. Regulation of the hepatic uptake of triglyceride-rich lipoproteins in the rat. Opposing effects of homologous apolipoprotein E and individual C apoproteins. J Biol Chem. 1980 Sep 10;255(17):8303–8307. [PubMed] [Google Scholar]
- Zannis V. I., Just P. W., Breslow J. L. Human apolipoprotein E isoprotein subclasses are genetically determined. Am J Hum Genet. 1981 Jan;33(1):11–24. [PMC free article] [PubMed] [Google Scholar]


