Abstract
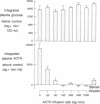
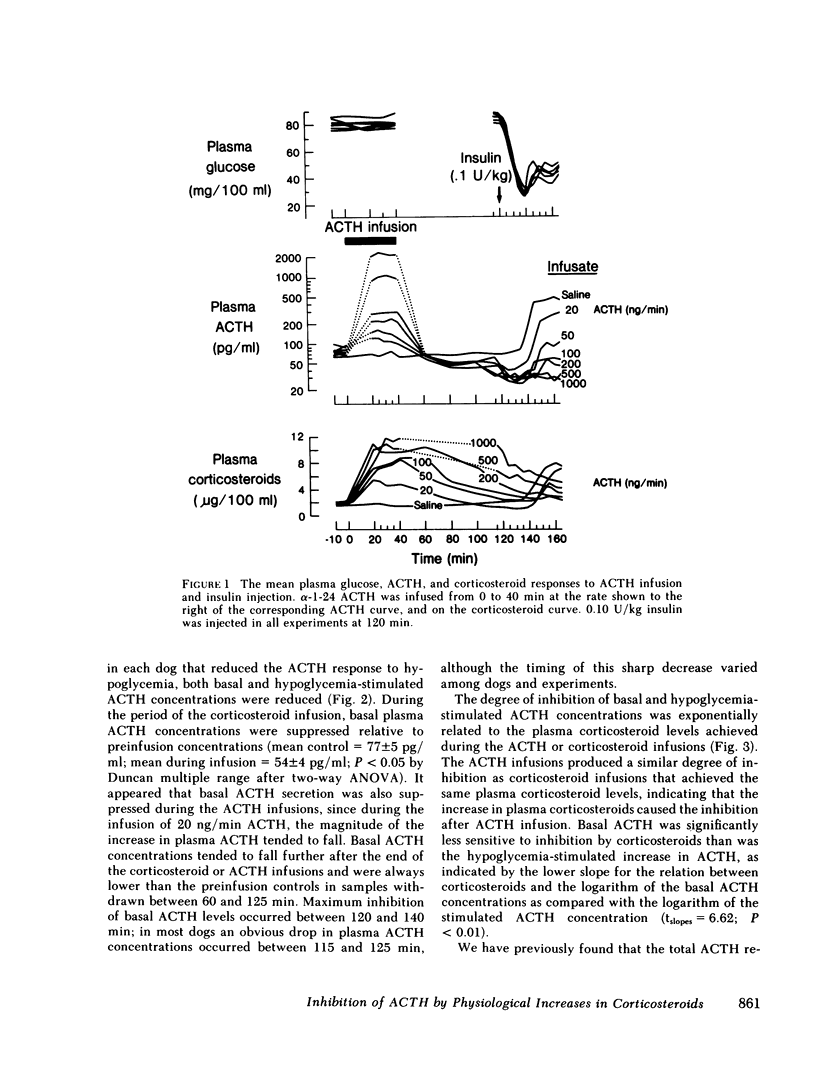
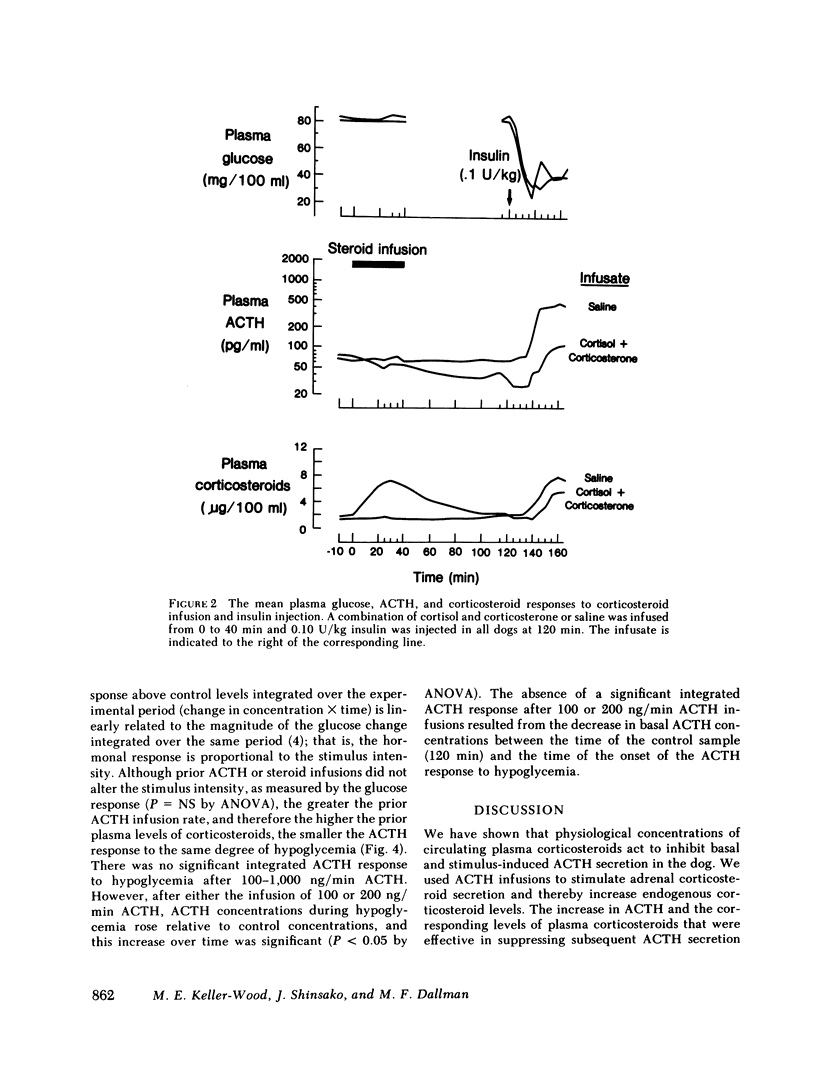
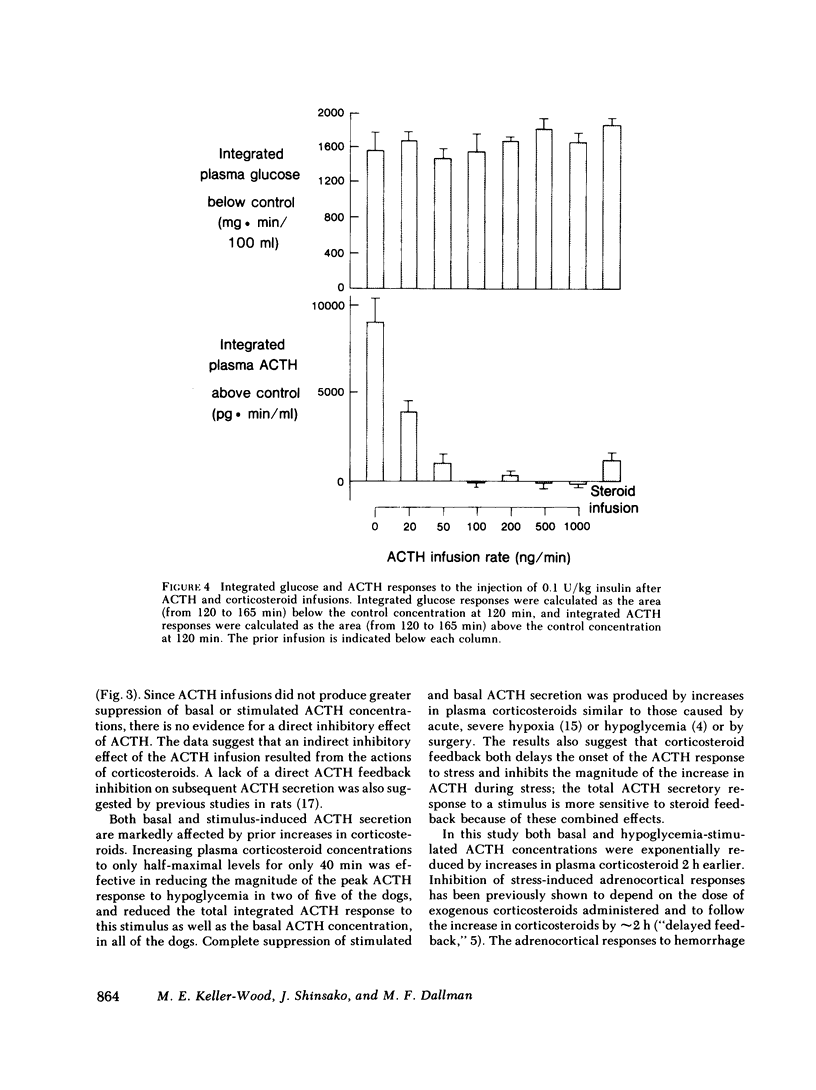
We have tested the effect of physiological increases in plasma corticosteroids in conscious dogs on the levels of basal and hypoglycemia-stimulated adrenocorticotropic hormone (ACTH) 2 h later. Increases in plasma corticosteroids, produced by infusion of alpha-1-24 ACTH or corticosteroids for 40 min, suppressed basal and stimulated ACTH levels. The magnitude of inhibition produced by an increase in plasma corticosteroids induced by the infusion of ACTH was equivalent to the inhibition produced by the same increase in plasma corticosteroids induced by corticosteroid infusion. The infusions did not affect basal plasma glucose concentrations or the decrease in plasma glucose concentrations after administration of 0.1 U insulin/kg. Basal ACTH concentration was less sensitive than hypoglycemia-stimulated ACTH concentration to corticosteroid-induced suppression. Basal and stimulated secretion were significantly inhibited in all dogs after approximately half-maximal increases in plasma corticosteroids; maximum inhibition occurred after maximal increases in plasma corticosteroids. Therefore, physiological increments in plasma corticosteroids, similar to those produced by acute stress, are effective suppressors of subsequent stress-induced ACTH secretion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aron D. C., Tyrrell J. B., Fitzgerald P. A., Findling J. W., Forsham P. H. Cushing's syndrome: problems in diagnosis. Medicine (Baltimore) 1981 Jan;60(1):25–35. doi: 10.1097/00005792-198101000-00003. [DOI] [PubMed] [Google Scholar]
- Beirne J., Jubiz W. Effect of indomethacin on the hypothalamic-pituitary-adrenal axis in man. J Clin Endocrinol Metab. 1978 Oct;47(4):713–716. doi: 10.1210/jcem-47-4-713. [DOI] [PubMed] [Google Scholar]
- Dallman M. F., DeManincor D., Shinsako J. Diminishing corticotrope capacity to release ACTH during sustained stimulation: the twenty-four hours after bilateral adrenalectomy in the rat. Endocrinology. 1974 Jul;95(1):65–73. doi: 10.1210/endo-95-1-65. [DOI] [PubMed] [Google Scholar]
- Dallman M. F., Jones M. T., Vernikos-Danellis J., Ganong W. F. Corticosteroid feedback control of ACTH secretion: rapid effects of bilateral adrenalectomy on plasma ACTH in the rat. Endocrinology. 1972 Oct;91(4):961–968. doi: 10.1210/endo-91-4-961. [DOI] [PubMed] [Google Scholar]
- Dallman M. F., Yates F. E. Dynamic asymmetries in the corticosteroid feedback path and distribution-metabolism-binding elements of the adrenocortical system. Ann N Y Acad Sci. 1969 Apr 21;156(2):696–721. doi: 10.1111/j.1749-6632.1969.tb14008.x. [DOI] [PubMed] [Google Scholar]
- Fehm H. L., Voigt K. H., Kummer G., Lang R., Pfeiffer E. F. Differential and integral corticosteroid feedback effects on ACTH secretion in hypoadrenocorticism. J Clin Invest. 1979 Feb;63(2):247–253. doi: 10.1172/JCI109296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HECHTER O., MACCHI I. A., KORMAN H., FRANK E. D., FRANK H. A. Quantitative variations in the adrenocortical secretion of dogs. Am J Physiol. 1955 Jul;182(1):29–34. doi: 10.1152/ajplegacy.1955.182.1.29. [DOI] [PubMed] [Google Scholar]
- Keller-Wood M. E., Shinsako J., Keil L. C., Dallman M. F. Insulin-induced hypoglycemia in conscious dogs. I. Dose-related pituitary and adrenal responses. Endocrinology. 1981 Sep;109(3):818–824. doi: 10.1210/endo-109-3-818. [DOI] [PubMed] [Google Scholar]
- Murphy B. E. Some studies of the protein-binding of steroids and their application to the routine micro and ultramicro measurement of various steroids in body fluids by competitive protein-binding radioassay. J Clin Endocrinol Metab. 1967 Jul;27(7):973–990. doi: 10.1210/jcem-27-7-973. [DOI] [PubMed] [Google Scholar]
- Raff H., Tzankoff S. P., Fitzgerald R. S. ACTH and cortisol responses to hypoxia in dogs. J Appl Physiol Respir Environ Exerc Physiol. 1981 Nov;51(5):1257–1260. doi: 10.1152/jappl.1981.51.5.1257. [DOI] [PubMed] [Google Scholar]
- Reader S. C., Alaghband-Zadeh J., Daly J. R., Robertson W. R. Negative rate-sensitive feedback effects on adrenocorticotrophin secretion by cortisol in normal subjects. J Endocrinol. 1982 Mar;92(3):443–448. doi: 10.1677/joe.0.0920443. [DOI] [PubMed] [Google Scholar]
- SMELIK P. G. Relation between blood level of corticoids and their inhibiting effect on the hypophyseal stress response. Proc Soc Exp Biol Med. 1963 Jul;113:616–619. doi: 10.3181/00379727-113-28442. [DOI] [PubMed] [Google Scholar]
- Wood C. E., Shinsako J., Keil L. C., Ramsay D. J., Dallman M. F. Apparent dissociation of adrenocorticotropin and corticosteroid responses to 15 ml/kg hemorrhage in conscious dogs. Endocrinology. 1982 Apr;110(4):1416–1421. doi: 10.1210/endo-110-4-1416. [DOI] [PubMed] [Google Scholar]
- Zimmermann E., Critchlow V. Short-latency suppression of pituitary-adrenal function with physiological plasma levels of corticosterone in the female rat. Neuroendocrinology. 1972;9(4):235–243. doi: 10.1159/000122054. [DOI] [PubMed] [Google Scholar]