Abstract
T lymphocytes from control subjects were separated into subsets using monoclonal antibodies of the OKT series and complement lysis and analyzed for ecto-5′-nucleotidase activity both by quantitative radiochemical assay and a histochemical stain. T cells from 15 control subjects contained 54±4% OKT4+ (helper/inducer) cells and 32±3% OKT8+ (cytotoxic/suppressor) cells. Total T cell ecto-5′-nucleotidase activity was 10.9±2.1 nmol/h per 106 cells with 25±7% positive by histochemical stain. Ecto-5′-nucleotidase activity in OKT4-enriched populations was 5.43±1.8 nmol/h per 106 cells with 14±2% positive by histochemical stain; that in OKT8-enriched populations was 17.1±5.9 nmol/h per 106 cells with 35±8% positive by histochemical stain.
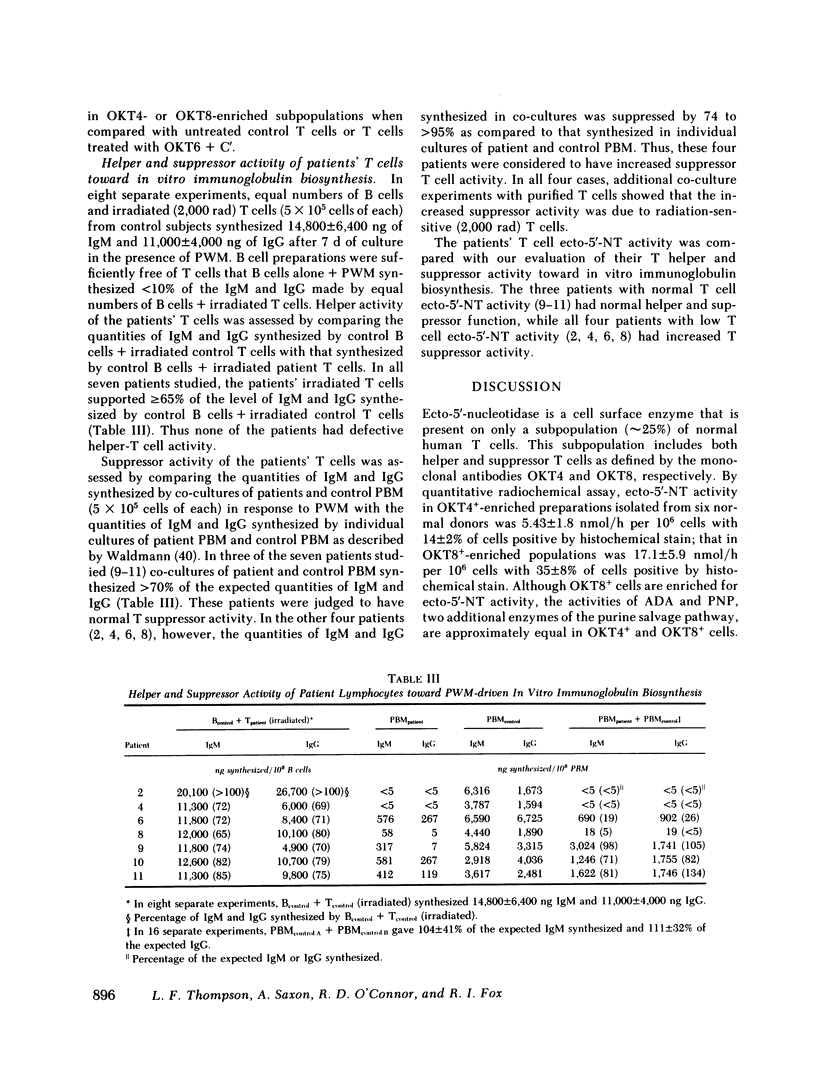
Two of four patients with congenital agammaglobulinemia and four of seven patients with common variable immunodeficiency had decreased proportions of OKT4+ T cells with corresponding increases in the proportions of OKT8+ T cells (OKT4/OKT8 = 0.60 to 1.0 as compared with 1.7±0.2 for control subjects). All four patients with congenital agammaglobulinemia, and three of seven patients with common variable immunodeficiency also had low T cell ecto-5′-nucleotidase activity (<5.5 nmol/h per 106 cells). Ecto-5′-nucleotidase activity in OKT4- enriched populations isolated from four patients with low total T cell activity was 2.85±0.90 nmol/h per 106 cells with 10±4% positive by histochemical stain; that in OKT8-enriched populations was 6.82±1.7 nmol/h per 106 cells with 7.5±3% positive by histochemical stain. Thus, the number of ecto-5′-nucleotidase positive cells is decreased, especially in the OKT8+ subpopulation, and the low total T cell ecto-5′-nucleotidase activity seen in these patients is due to fewer positive cells rather than to substantially less activity per cell.
Our data indicate that ecto-5′-nucleotidase activity defines two subpopulations of T lymphocytes (ecto-5′-nucleotidase positive and negative), the proportions of which are markedly altered in many patients with hypogammaglobulinemia. In preliminary studies with seven patients, increased numbers of ecto-5′-nucleotidase negative T cells appeared to correlate with increased suppressor T cell activity toward in vitro immunoglobulin synthesis. Therefore, ecto-5′-nucleotidase may be a useful cell surface marker in the study of imbalances of regulatory T cell subsets in patients with antibody synthesis disorders.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boss G. R., Thompson L. F., O'Connor R. D., Ziering R. W., Seegmiller J. E. Ecto-5'-nucleotidase deficiency: association with adenosine deaminase deficiency and nonassociation with deoxyadenosine toxicity. Clin Immunol Immunopathol. 1981 Apr;19(1):1–7. doi: 10.1016/0090-1229(81)90042-8. [DOI] [PubMed] [Google Scholar]
- Boss G. R., Thompson L. F., Spiegelberg H. L., Pichler W. J., Seegmiller J. E. Age-dependency of lymphocyte ecto-5'-nucleotidase activity. J Immunol. 1980 Aug;125(2):679–682. [PubMed] [Google Scholar]
- Boss G. R., Thompson L. F., Spiegelberg H. L., Waldmann T. A., O'Connor R. D., Hamburger R. N., Seegmiller J. E. Lymphocyte ecto-5'-nucleotidase activity as a marker of B-cell maturation. Trans Assoc Am Physicians. 1979;92:309–315. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carson D. A., Goldblum R., Seegmiller J. E. Quantitative immunoassay of adenosine deaminase in combined immunodeficiency disease. J Immunol. 1977 Jan;118(1):270–273. [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Gudas L. J., Ammann A. J., Staal G. E., Martin D. W., Jr Deoxyguanosine triphosphate as a possible toxic metabolite in the immunodeficiency associated with purine nucleoside phosphorylase deficiency. J Clin Invest. 1978 May;61(5):1405–1409. doi: 10.1172/JCI109058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Hirschhorn R., Horowitz S. D., Rubinstein A., Polmar S. H., Hong R., Martin D. W., Jr Deoxyadenosine triphosphate as a potentially toxic metabolite in adenosine deaminase deficiency. Proc Natl Acad Sci U S A. 1978 Jan;75(1):472–476. doi: 10.1073/pnas.75.1.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Lee J. W., Dosch H. M., Gelfand E. W. The expression of deoxyguanosine toxicity in T lymphocytes at different stages of maturation. J Immunol. 1980 Oct;125(4):1578–1582. [PubMed] [Google Scholar]
- Coleman M. S., Donofrio J., Hutton J. J., Hahn L., Daoud A., Lampkin B., Dyminski J. Identification and quantitation of adenine deoxynucleotides in erythrocytes of a patient with adenosine deaminase deficiency and severe combined immunodeficiency. J Biol Chem. 1978 Mar 10;253(5):1619–1626. [PubMed] [Google Scholar]
- Durandy A., Fischer A., Griscelli C. Dysfunctions of pokeweed mitogen-stimulated T and B lymphocyte responses induced by gammaglobulin therapy. J Clin Invest. 1981 Mar;67(3):867–877. doi: 10.1172/JCI110104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards N. L., Cassidy J. T., Fox I. H. Lymphocyte 5'-nucleotidase deficiency in hypogammaglobulinemia: clinical characteristics. Clin Immunol Immunopathol. 1980 Sep;17(1):76–88. doi: 10.1016/0090-1229(80)90075-6. [DOI] [PubMed] [Google Scholar]
- Edwards N. L., Gelfand E. W., Burk L., Dosch H. M., Fox I. H. Distribution of 5'-nucleotidase in human lymphoid tissues. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3474–3476. doi: 10.1073/pnas.76.7.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards N. L., Magilavy D. B., Cassidy J. T., Fox I. H. Lymphocyte ecto-5'-nucleotidase deficiency in agammaglobulinemia. Science. 1978 Aug 18;201(4356):628–630. doi: 10.1126/science.27864. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Thompson L. F., Huddlestone J. R. T gamma cells express T lymphocyte-associated antigens. J Immunol. 1981 May;126(5):2062–2063. [PubMed] [Google Scholar]
- Giblett E. R., Ammann A. J., Wara D. W., Sandman R., Diamond L. K. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet. 1975 May 3;1(7914):1010–1013. doi: 10.1016/s0140-6736(75)91950-9. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Molina A., Spiegelberg H. L. A subpopulation of normal human peripheral B lymphcytes that bind IgE. J Clin Invest. 1977 Apr;59(4):616–624. doi: 10.1172/JCI108679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Zannis V. I., Clift S. M., Ammann A. J., Staal G. E., Martin D. W., Jr Characterization of mutant subunits of human purine nucleoside phosphorylase. J Biol Chem. 1978 Dec 25;253(24):8916–8924. [PubMed] [Google Scholar]
- Hershfield M. S., Kredich N. M., Ownby D. R., Ownby H., Buckley R. In vivo inactivation of erythrocyte S-adenosylhomocysteine hydrolase by 2'-deoxyadenosine in adenosine deaminase-deficient patients. J Clin Invest. 1979 Apr;63(4):807–811. doi: 10.1172/JCI109367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Martiniuk F., Rosen F. S. Adenosine deaminase activity in normal tissues and tissues from a child with severe combined immunodeficiency and adenosine deaminase deficiency. Clin Immunol Immunopathol. 1978 Mar;9(3):287–292. doi: 10.1016/0090-1229(78)90100-9. [DOI] [PubMed] [Google Scholar]
- Izui S., Eisenberg R. A., Dixon F. J. Subclass-restricted IgG polyclonal antibody production in mice injected with lipid A-rich lipopolysaccharides. J Exp Med. 1981 Feb 1;153(2):324–338. doi: 10.1084/jem.153.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janossy G., Tidman N., Papageorgiou E. S., Kung P. C., Goldstein G. Distribution of t lymphocyte subsets in the human bone marrow and thymus: an analysis with monoclonal antibodies. J Immunol. 1981 Apr;126(4):1608–1613. [PubMed] [Google Scholar]
- Johnson S. M., North M. E., Asherson G. L., Allsop J., Watts R. W., Webster A. D. Lymphocyte purine 5'-nucleotidase edficiency in primary hypogammaglobulinaemia. Lancet. 1977 Jan 22;1(8004):168–170. doi: 10.1016/s0140-6736(77)91765-2. [DOI] [PubMed] [Google Scholar]
- Kamoun M., Martin P. J., Hansen J. A., Brown M. A., Siadak A. W., Nowinski R. C. Identification of a human T lymphocyte surface protein associated with the E-rosette receptor. J Exp Med. 1981 Jan 1;153(1):207–212. doi: 10.1084/jem.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay H. D., Horwitz D. A. Evidence by reactivity with hybridoma antibodies for a probable myeloid origin of peripheral blood cells active in natural cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Clin Invest. 1980 Oct;66(4):847–851. doi: 10.1172/JCI109923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loken M. R., Herzenber L. A. Analysis of cell populations with a fluorescence-activated cell sorter. Ann N Y Acad Sci. 1975 Jun 30;254:163–171. doi: 10.1111/j.1749-6632.1975.tb29166.x. [DOI] [PubMed] [Google Scholar]
- Matamoros N., Horwitz D. A., Newton C., Asherson G. L., Webster A. D. Histochemical studies for 5'-nucleotidase and alpha-naphthyl (non-specific) esterase in lymphocytes from patients with primary immunoglobulin deficiencies. Clin Exp Immunol. 1979 Apr;36(1):102–106. [PMC free article] [PubMed] [Google Scholar]
- Mitchell B. S., Mejias E., Daddona P. E., Kelley W. N. Purinogenic immunodeficiency diseases: selective toxicity of deoxyribonucleosides for T cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5011–5014. doi: 10.1073/pnas.75.10.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta L., Ferrarini M., Mingari M. C., Moretta A., Webb S. R. Subpopulations of human T cells identified by receptors for immunoglobulins and mitogen responsiveness. J Immunol. 1976 Dec;117(6):2171–2174. [PubMed] [Google Scholar]
- Moretta L., Mingari M. C., Sekaly P. R., Moretta A., Chapuis B., Cerottini J. C. Surface markers of cloned human T cells with various cytolytic activities. J Exp Med. 1981 Aug 1;154(2):569–574. doi: 10.1084/jem.154.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler W. J., Broder S. In vitro functions of human T cells expressing Fc-IgG or Fc-IgM receptors. Immunol Rev. 1981;56:163–197. doi: 10.1111/j.1600-065x.1981.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Recker D. P., Edwards N. L., Fox I. H. Histochemical evaluation of lymphocytes in hypogammaglobulinemia. Decreased number of 5'-nucleotidase-positive cells. J Lab Clin Med. 1980 Feb;95(2):175–179. [PubMed] [Google Scholar]
- Reinherz E. L., Cooper M. D., Schlossman S. F., Rosen F. S. Abnormalities of T cell maturation and regulation in human beings with immunodeficiency disorders. J Clin Invest. 1981 Sep;68(3):699–705. doi: 10.1172/JCI110305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Geha R., Wohl M. E., Morimoto C., Rosen F. S., Schlossman S. F. Immunodeficiency associated with loss of T4+ inducer T-cell function. N Engl J Med. 1981 Apr 2;304(14):811–816. doi: 10.1056/NEJM198104023041403. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Moretta L., Roper M., Breard J. M., Mingari M. C., Cooper M. D., Schlossman S. F. Human T lymphocyte subpopulations defined by Fc receptors and monoclonal antibodies. A comparison. J Exp Med. 1980 Apr 1;151(4):969–974. doi: 10.1084/jem.151.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe M., de Gast C. G., Platts-Mills T. A., Asherson G. L., Webster A. D., Johnson S. M. 5'-nucleotidase of B and T lymphocytes isolated from human peripheral blood. Clin Exp Immunol. 1979 Apr;36(1):97–101. [PMC free article] [PubMed] [Google Scholar]
- Silber R., Conklyn M., Grusky G., Zucker-Franklin D. Human lymphocytes: 5'-nucleotidase-positive and -negative subpopulations. J Clin Invest. 1975 Nov;56(5):1324–1327. doi: 10.1172/JCI108209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds H. A., Sahota A., Potter C. F., Cameron J. S. Purine metabolism and immunodeficiency: urinary purine excretion as a diagnostic screening test in adenosine deaminase and purine nucleoside phosphorylase deficiency. Clin Sci Mol Med. 1978 May;54(5):579–584. doi: 10.1042/cs0540579. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L., Dainer P. M. Fc receptors for IgG, IgM and IgE on human leukaemic lymphocytes. Clin Exp Immunol. 1979 Feb;35(2):286–295. [PMC free article] [PubMed] [Google Scholar]
- Thomas Y., Rogozinski L., Irigoyen O. H., Shen H. H., Talle M. A., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. V. Suppressor cells within the activated OKT4+ population belong to a distinct subset. J Immunol. 1982 Mar;128(3):1386–1390. [PubMed] [Google Scholar]
- Thomas Y., Sosman J., Irigoyen O., Friedman S. M., Kung P. C., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. I. Collaborative T-T interactions in the immunoregulation of B cell differentiation. J Immunol. 1980 Dec;125(6):2402–2408. [PubMed] [Google Scholar]
- Thompson L. F., Boss G. R., Spiegelberg H. L., Bianchino A., Seegmiller J. E. Ecto-5'-nucleotidase activity in lymphoblastoid cell lines derived from heterozygotes for congenital X-linked agammaglobulinemia. J Immunol. 1980 Jul;125(1):190–193. [PubMed] [Google Scholar]
- Thompson L. F., Boss G. R., Spiegelberg H. L., Jansen I. V., O'Connor R. D., Waldmann T. A., Hamburger R. N., Seegmiller J. E. Ecto-5'-nucleotidase activity in T and B lymphocytes from normal subjects and patients with congenital X-linked agammaglobulinemia. J Immunol. 1979 Dec;123(6):2475–2478. [PubMed] [Google Scholar]
- Ullman B., Gudas L. J., Cohen A., Martin D. W., Jr Deoxyadenosine metabolism and cytotoxicity in cultured mouse T lymphoma cells: a model for immunodeficiency disease. Cell. 1978 Jun;14(2):365–375. doi: 10.1016/0092-8674(78)90122-8. [DOI] [PubMed] [Google Scholar]
- Van der Weyden M. B., Buckley R. H., Kelley W. N. Molecular form of adenosine deaminase in severe combined immunodeficiency. Biochem Biophys Res Commun. 1974 Apr 8;57(3):590–595. doi: 10.1016/0006-291x(74)90587-7. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A., Broder S. Suppressor cells in the regulation of the immune response. Prog Clin Immunol. 1977;3:155–199. [PubMed] [Google Scholar]
- Webster A. D., Rowe M., Johnson S. M., Asherson G. L., Harkness A. Ecto 5'-nucleotidase deficiency in primary hypogammaglobulinaemia. Ciba Found Symp. 1978;(68):135–151. doi: 10.1002/9780470720516.ch9. [DOI] [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]
- Yachie A., Miyawaki T., Nagaoki T., Yokoi T., Mukai M., Uwadana N., Taniguchi N. Regulation of B cell differentiation by T cell subsets defined with monoclonal OKT4 and OKT8 antibodies in human cord blood. J Immunol. 1981 Oct;127(4):1314–1317. [PubMed] [Google Scholar]
- van Heukelom L. H., Staal G. E., Stoop J. W., Zegers B. J. An abnormal form of purine nucleoside phosphorylase in a family with a child with severe defective T-cell-and normal B-cell immunity. Clin Chim Acta. 1976 Oct 1;72(1):117–124. doi: 10.1016/0009-8981(76)90042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


