Abstract
Basic lipophilic drugs such as propranolol and lidocaine are strongly bound by α1-acid glycoprotein, also called orosomucoid. Although the liver is known to rapidly clear plasma protein-bound propranolol or lidocaine, it is generally regarded that peripheral tissues, such as brain or heart, are only exposed to the small fraction of drug that is free or dialyzable in vitro. The “free drug” hypothesis is subjected to direct empiric testing in the present studies using human sera and an in vivo rat brain paradigm.
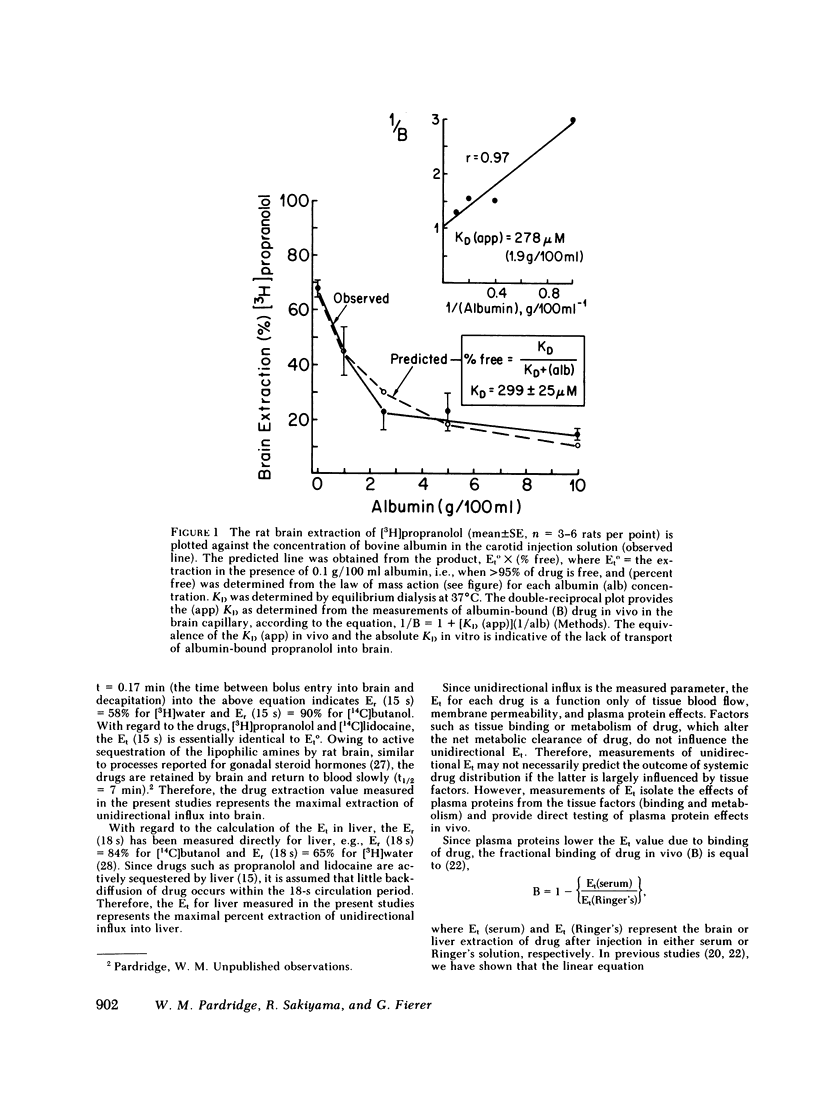
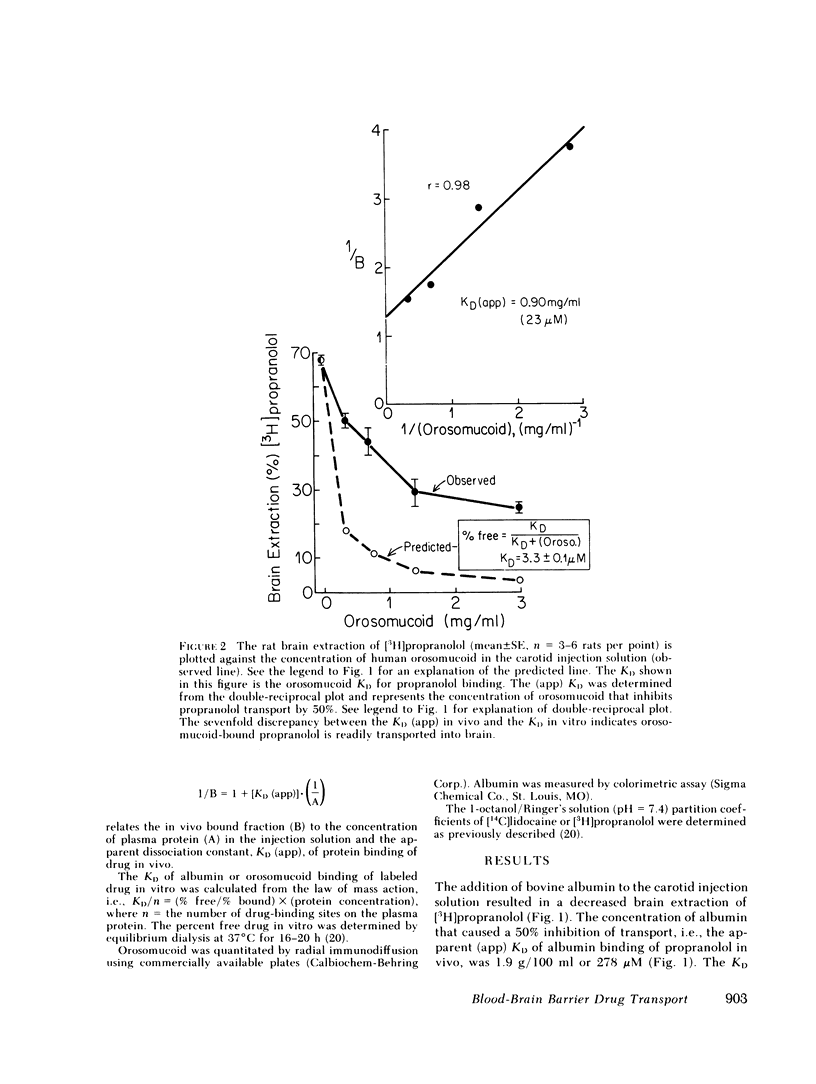
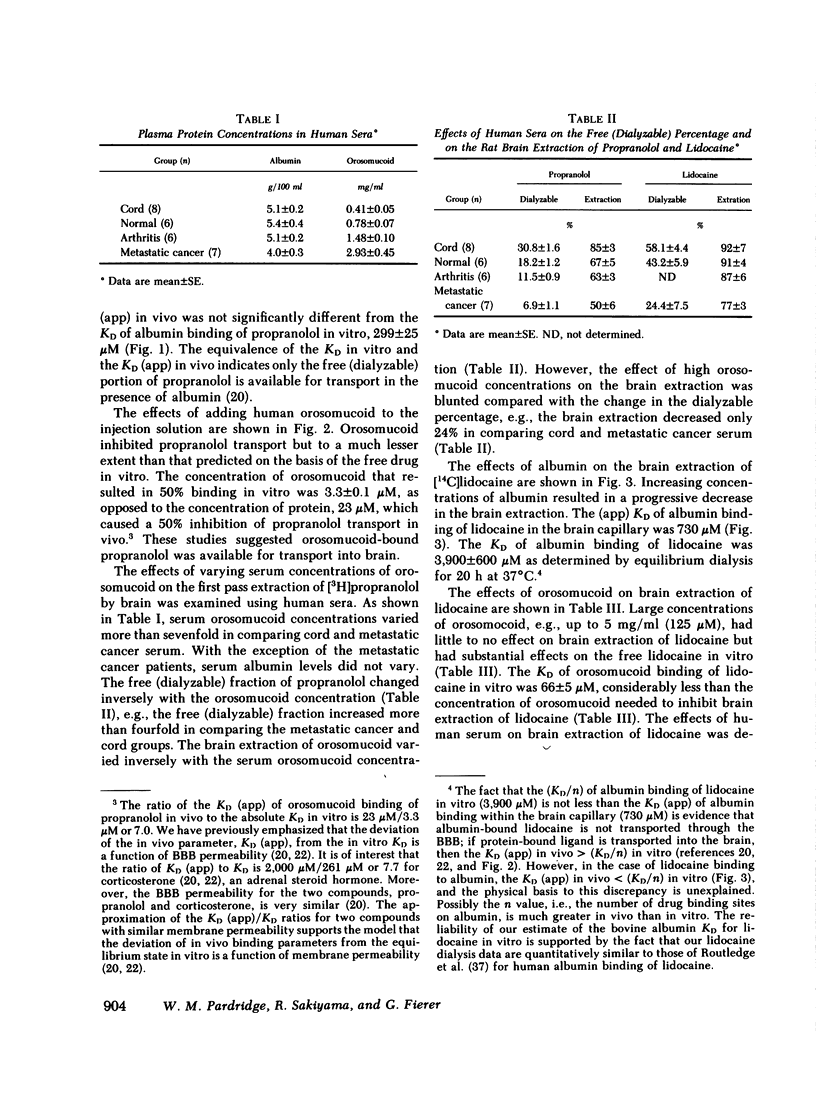
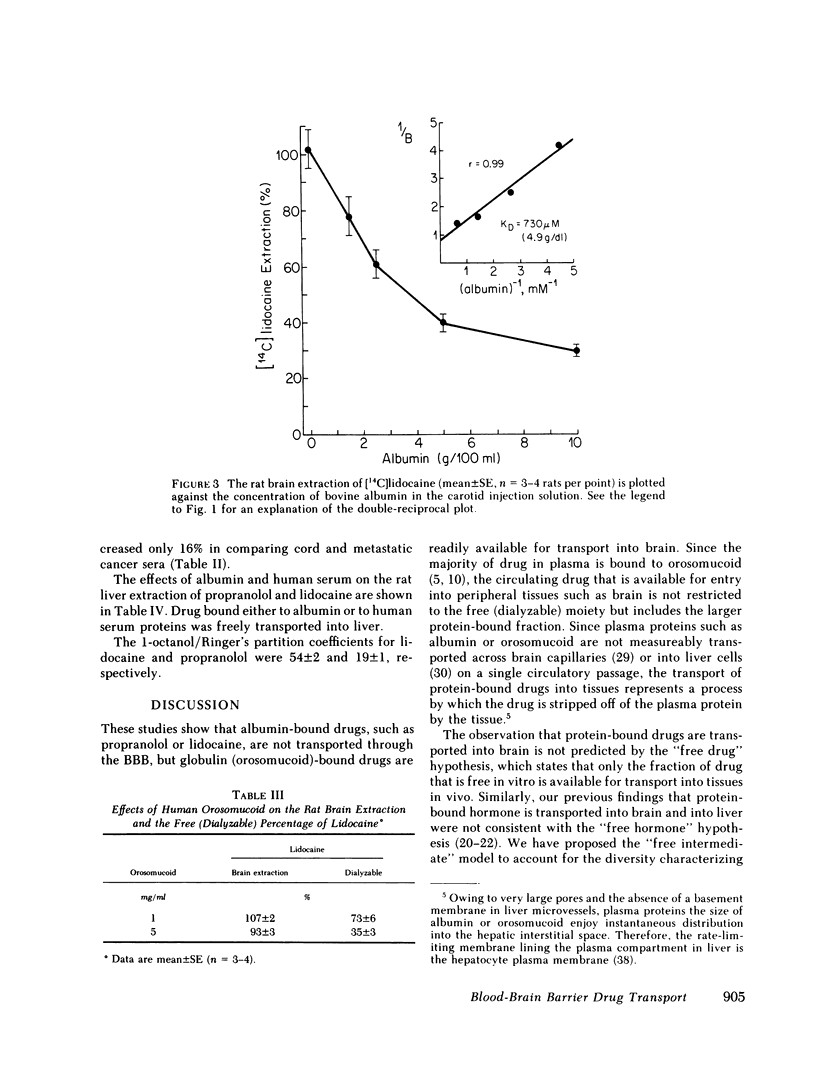
Serum from 27 human subjects (normal individuals, newborns, or patients with either metastatic cancer or rheumatoid arthritis) were found to have up to a sevenfold variation in orosomucoid concentrations. The free propranolol or lidocaine as determined in vitro by equilibrium dialysis at 37°C varied inversely with the orosomucoid concentration. Similarly the rate of transport of propranolol or lidocaine through the blood-brain barrier (BBB) was inversely related to the existing serum concentration of orosomucoid. However, the inhibition of rat brain extraction of drug by orosomucoid in vivo was only about one-fifth of that predicted by free drug measurements in vitro. This large discrepancy suggested orosomucoid-bound drug was readily available for transport into brain in vivo. Studies using purified human orosomucoid in the rat brain extraction assay also showed that orosomucoidbound propranolol or lidocaine is readily transported through the BBB. Conversely, albumin-bound propranolol or lidocaine was not transported through the BBB. The studies using albumin provide evidence that the in vivo rat brain paradigm used in the present investigations is capable of confirming, when possible, predictions made by the “free drug” hypothesis.
These data suggest that the amount of circulating propranolol or lidocaine that is available for transport into a peripheral tissue such as brain is not restricted to the free (dialyzable) moiety but includes the much larger globulin-bound fraction. Therefore, existing pharmacokinetic models should be expanded to account for the transport of protein-bound drugs into peripheral tissues similar to what is known to occur in liver.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Brightman M. W., Klatzo I., Olsson Y., Reese T. S. The blood-brain barrier to proteins under normal and pathological conditions. J Neurol Sci. 1970 Mar;10(3):215–239. doi: 10.1016/0022-510x(70)90151-6. [DOI] [PubMed] [Google Scholar]
- Collinsworth K. A., Kalman S. M., Harrison D. C. The clinical pharmacology of lidocaine as an antiarrhythymic drug. Circulation. 1974 Dec;50(6):1217–1230. doi: 10.1161/01.cir.50.6.1217. [DOI] [PubMed] [Google Scholar]
- Cooper E. H., Stone J. Acute phase reactant proteins in cancer. Adv Cancer Res. 1979;30:1–44. doi: 10.1016/s0065-230x(08)60893-3. [DOI] [PubMed] [Google Scholar]
- Forker E. L., Luxon B. A. Albumin helps mediate removal of taurocholate by rat liver. J Clin Invest. 1981 May;67(5):1517–1522. doi: 10.1172/JCI110182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremstad D., Bergerud K., Haffner J. F., Lunde P. K. Increased plasma binding of quinidine after surgery: a preliminary report. Eur J Clin Pharmacol. 1976;10(6):441–444. doi: 10.1007/BF00563081. [DOI] [PubMed] [Google Scholar]
- Goresky C. A., Rose C. P. Blood-tissue exchange in liver and heart: the influence of heterogeneity of capillary transit times. Fed Proc. 1977 Nov;36(12):2629–2634. [PubMed] [Google Scholar]
- Hull C. J. Pharmacokinetics and pharmacodynamics. Br J Anaesth. 1979 Jul;51(7):579–594. doi: 10.1093/bja/51.7.579. [DOI] [PubMed] [Google Scholar]
- Johnsson G., Regàrdh C. G. Clinical pharmacokinetics of beta-adrenoreceptor blocking drugs. Clin Pharmacokinet. 1976;1(4):233–263. doi: 10.2165/00003088-197601040-00001. [DOI] [PubMed] [Google Scholar]
- Koch-Weser J., Frishman W. H. beta-Adrenoceptor antagonists: new drugs and new indications. N Engl J Med. 1981 Aug 27;305(9):500–506. doi: 10.1056/NEJM198108273050907. [DOI] [PubMed] [Google Scholar]
- Koch-Weser J., Sellers E. M. Binding of drugs to serum albumin (first of two parts). N Engl J Med. 1976 Feb 5;294(6):311–316. doi: 10.1056/NEJM197602052940605. [DOI] [PubMed] [Google Scholar]
- McDevitt D. G., Frisk-Holmberg M., Hollifield J. W., Shand D. G. Plasma binding and the affinity of propranolol for a beta receptor in man. Clin Pharmacol Ther. 1976 Aug;20(2):152–157. doi: 10.1002/cpt1976202152. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H., Braun L. D. [H] Tryptamine and 3H-water as diffusible internal standards for measuring brain extraction of radio-labeled substances following carotid injection. Brain Res. 1976 Aug 20;113(1):219–224. doi: 10.1016/0006-8993(76)90024-x. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H. Measurement of brain uptake of radiolabeled substances using a tritiated water internal standard. Brain Res. 1970 Dec 1;24(2):372–376. doi: 10.1016/0006-8993(70)90123-x. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H., Pardridge W. M., Braun L. D., Crane P. D. Measurement of cerebral glucose utilization using washout after carotid injection in the rat. J Neurochem. 1982 May;38(5):1413–1418. doi: 10.1111/j.1471-4159.1982.tb07920.x. [DOI] [PubMed] [Google Scholar]
- Olesen J., Hougård K., Hertz M. Isoproterenol and propranolol: ability to cross the blood-brain barrier and effects on cerebral circulation in man. Stroke. 1978 Jul-Aug;9(4):344–349. doi: 10.1161/01.str.9.4.344. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Jefferson L. S. Liver uptake of amino acids and carbohydrates during a single circulatory passage. Am J Physiol. 1975 Apr;228(4):1155–1161. doi: 10.1152/ajplegacy.1975.228.4.1155. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Mietus L. J. Effects of progesterone-binding globulin versus a progesterone antiserum on steroid hormone transport through the blood-brain barrier. Endocrinology. 1980 Apr;106(4):1137–1141. doi: 10.1210/endo-106-4-1137. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Mietus L. J. Influx of thyroid hormones into rat liver in vivo. Differential availability of thyroxine and triiodothyronine bound by plasma proteins. J Clin Invest. 1980 Aug;66(2):367–374. doi: 10.1172/JCI109865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Mietus L. J. Transport of protein-bound steroid hormones into liver in vivo. Am J Physiol. 1979 Oct;237(4):E367–E372. doi: 10.1152/ajpendo.1979.237.4.E367. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Mietus L. J. Transport of steroid hormones through the rat blood-brain barrier. Primary role of albumin-bound hormone. J Clin Invest. 1979 Jul;64(1):145–154. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Moeller T. L., Mietus L. J., Oldendorf W. H. Blood-brain barrier transport and brain sequestration of steroid hormones. Am J Physiol. 1980 Jul;239(1):E96–102. doi: 10.1152/ajpendo.1980.239.1.E96. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Transport of protein-bound hormones into tissues in vivo. Endocr Rev. 1981 Winter;2(1):103–123. doi: 10.1210/edrv-2-1-103. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Van Herle A. J., Naruse R. T., Fierer G., Costin A. In vivo quantification of receptor-mediated uptake of asialoglycoproteins by rat liver. J Biol Chem. 1983 Jan 25;258(2):990–994. [PubMed] [Google Scholar]
- Piafsky K. M., Borgá O., Odar-Cederlöf I., Johansson C., Sjöqvist F. Increased plasma protein binding of propranolol and chlorpromazine mediated by disease-induced elevations of plasma alpha1 acid glycoprotein. N Engl J Med. 1978 Dec 28;299(26):1435–1439. doi: 10.1056/NEJM197812282992604. [DOI] [PubMed] [Google Scholar]
- Piafsky K. M., Borgå O. Plasma protein binding of basic drugs. II. Importance of alpha 1-acid glycoprotein for interindividual variation. Clin Pharmacol Ther. 1977 Nov;22(5 Pt 1):545–549. doi: 10.1002/cpt1977225part1545. [DOI] [PubMed] [Google Scholar]
- Romach M. K., Piafsky K. M., Abel J. G., Khouw V., Sellers E. M. Methadone binding to orosomucoid (alpha 1-acid glycoprotein): determinant of free fraction in plasma. Clin Pharmacol Ther. 1981 Feb;29(2):211–217. doi: 10.1038/clpt.1981.34. [DOI] [PubMed] [Google Scholar]
- Routledge P. A., Barchowsky A., Bjornsson T. D., Kitchell B. B., Shand D. G. Lidocaine plasma protein binding. Clin Pharmacol Ther. 1980 Mar;27(3):347–351. doi: 10.1038/clpt.1980.46. [DOI] [PubMed] [Google Scholar]
- Routledge P. A., Shand D. G., Barchowsky A., Wagner G., Stargel W. W. Relationship between alpha 1-acid glycoprotein and lidocaine disposition in myocardial infarction. Clin Pharmacol Ther. 1981 Aug;30(2):154–157. doi: 10.1038/clpt.1981.141. [DOI] [PubMed] [Google Scholar]
- Shand D. G., Cotham R. H., Wilkinson G. R. Perfusion-limited of plasma drug binding on hepatic drug extraction. Life Sci. 1976 Jul 1;19(1):125–130. doi: 10.1016/0024-3205(76)90382-9. [DOI] [PubMed] [Google Scholar]
- Shand D. G., Rangno R. E., Evans G. H. The disposition of propranolol. II. Hepatic elimination in the rat. Pharmacology. 1972;8(4):344–352. doi: 10.1159/000136352. [DOI] [PubMed] [Google Scholar]
- Weisiger R., Gollan J., Ockner R. Receptor for albumin on the liver cell surface may mediate uptake of fatty acids and other albumin-bound substances. Science. 1981 Mar 6;211(4486):1048–1051. doi: 10.1126/science.6258226. [DOI] [PubMed] [Google Scholar]
- Wood M., Wood A. J. Changes in plasma drug binding and alpha 1-acid glycoprotein in mother and newborn infant. Clin Pharmacol Ther. 1981 Apr;29(4):522–526. doi: 10.1038/clpt.1981.73. [DOI] [PubMed] [Google Scholar]


