Abstract
Replacement of lost and/or dysfunctional astrocytes via multipotent neural stem cell (NSC) and lineage-restricted neural progenitor cell (NPC) transplantation is a promising therapeutic approach for traumatic spinal cord injury (SCI). Cell transplantation in general offers the potential to replace central nervous system (CNS) cell types, achieve remyelination, deliver missing gene products, promote and guide axonal growth, modulate the host immune response, deliver neuroprotective factors, and provide a cellular substrate for bridging the lesion site, amongst other possible benefits. A host of cell types that differ in their developmental stage, CNS region and species of derivation, as well as in their phenotypic potential, have been tested in a variety of SCI animal models. Historically in the SCI field, most pre-clinical NSC and NPC transplantation studies have focused on neuronal and oligodendrocyte replacement. However, much less attention has been geared towards targeting astroglial dysfunction in the inured spinal cord, despite the integral roles played by astrocytes in both normal CNS function and in the diseased nervous system. Despite the relative lack of studies, cell transplantation-based targeting of astrocytes dates back to some of the earliest transplant studies in SCI animal models. In this review, we will describe the history of work involving cell transplantation for targeting astrocytes in models of SCI. We will also touch on the current state of affairs in the field, as well as on important future directions as we move forward in trying to develop this approach into a viable strategy for SCI patients. Practical issues such as timing of delivery, route of transplantation and immunesuppression needs are beyond the scope of this review.
Keywords: Astrocyte, glial progenitor, cell replacement, transplantation, spinal cord injury
Spinal cord injury (SCI)
SCI is a debilitating and heterogeneous set of conditions resulting from direct damage to spinal cord parenchyma and associated spinal nerves. The National Spinal Cord Injury Statistical Center reports that there are approximately 12,000 new cases each year in the United States alone, most of which result from preventable causes such as motor vehicle accidents, falls, sports or violence. Dysfunctions in the musculoskeletal, respiratory, urinary and gastrointestinal systems (to name a few) are common outcomes of SCI. The heterogeneity of the disease results from differences in the location, type and severity of trauma, as well as on the consequent types and degree of functional impairment.
Despite this disease heterogeneity, all forms of SCI are associated with phenotypic changes in populations of spinal cord astrocytes. These changes include: (1) acquiring disease-specific protective functions such as glial scar formation to prevent secondary expansion of the lesion, (2) loss of crucial homeostatic functions such as the glutamate transporter system that are key to normal CNS physiology, and (3) gain of toxic functions such as the generation of pro-inflammatory signaling molecules that contribute to degeneration, neuronal hyperexcitability and other detrimental effects. The response of astrocytes is not an all-or-none phenomenon; there is diversity in these changes that vary with the type and severity of trauma and with proximity to the lesion. Furthermore, even within a population of astrocytes within the spinal cord, the response of individual astrocytes to the same injury can vary, likely reflecting the normal heterogeneity amongst astrocytes that is now becoming increasingly appreciated.
Importance of astrocytes in the CNS
Astrocytes are supportive glial cells in the CNS that serve a variety of important functions. These cells are classically defined by their characteristic stellate morphology and expression of specific intermediate filaments such as glial fibrillary acidic protein (GFAP). While astrocytes have been traditionally viewed as a homogenous cell population, recent studies have illustrated that it is in fact a diverse set of cells that shares certain common characteristics. During development, astrocytes arise from different progenitor sources, including radial glia in the cortex, glial progenitors in the postnatal ventricular zone and bi-potential glial-restricted precursors in the spinal cord. This may provide a developmental explanation for astrocyte diversity (Wang and Bordey, 2008), but likely only represents a single mechanism underlying astrocyte heterogeneity. In the adult CNS, astrocytes can outnumber their neuronal counterparts, potentially by several fold in some regions. They tile the entire CNS in a contiguous, non-overlapping and well-organized manner. Importantly, astrocytes serve a number of essential functions in the healthy CNS and undergo characteristic changes at the molecular, cellular and functional levels during pathological states.
Normal functions served by astrocytes
While astrocytes do not generate action potentials, they play several critical roles that are absolutely necessary for the normal functioning of the CNS (Sofroniew and Vinters, 2010). Astrocytes are a major source of extracellular matrix (ECM) proteins and cell adhesion molecules in the CNS. In this way, they regulate axon outgrowth during development and after injury. Laminin, N-cadherin, neural cell adhesion molecules (NCAM) and fibronectin are some of these growth-promoting molecules (Liesi et al., 1983; Liesi and Silver, 1988; Neugebauer et al., 1988; Price and Hynes, 1985; Tomaselli et al., 1988). On the other hand, inhibitory proteoglycans serve as negative guidance cues for axon growth, and their expression by astrocytes is increased following SCI (Gonzalez Mde et al., 1993; Snow et al., 1990). Matrix metalloproteases synthesized and secreted by astrocytes serve to remodel the ECM, which plays a role in axon growth and plasticity (Wells et al., 1996). Astrocytes also regulate neuronal maturation and survival by releasing growth factors such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and fibroblast growth factor (FGF) (Rudge et al., 1992). During development, synapse generation coincides with the differentiation of astrocytes. Recent studies have elucidated the mechanisms by which astrocytes regulate synaptogenesis, including the release of cholesterol and thrombospondins that promote synapse formation (Christopherson et al., 2005; Mauch et al., 2001).
Astrocytes play a key role in maintaining homeostasis in the brain and spinal cord. They regulate access to the CNS by controlling both the formation of blood vessels (angiogenesis) and the formation and maintenance of the blood-brain barrier (BBB). The BBB is formed by astrocytes extending their processes, known as “end feet,” onto blood vessels, as well as by special tight junction-forming endothelial cells. Additionally, astrocytes regulate transporters expressed by and the tight junctions formed between endothelial cells. This barrier prevents toxin entry into CNS parenchyma and plays a homeostatic role, ensuring that the brain is supplied with appropriate levels of energy substrates (Haseloff et al., 2005; Hawkins and Davis, 2005; Kacem et al., 1998). For example, astrocytes take glucose from the blood and provide it to neurons (Morgello et al., 1995). Additionally, glycogen, a glucose polymer that serves as a short-term energy reserve for use during high neuronal activity, is stored mainly in astrocytes (Cataldo and Broadwell, 1986; Wender et al., 2000).
Astrocytes maintain homeostasis of small molecules key to neuronal and oligodendrocyte function. Potassium ion (K+) homeostasis is critical to neuronal excitability and synaptic transmission as excessive extracellular K+ causes depolarization of neurons, seizures and hyperexcitability. Astrocytes mediate K+ buffering by taking up extracellular K+ from regions with high K+, transporting it via gap-junctions that couple neighboring astrocytes, and then releasing this K+ at extracellular sites with low K+ levels (Ballanyi et al., 1987; Djukic et al., 2007; Holthoff and Witte, 2000; Kuffler et al., 1966). Astrocytes also centrally regulate extracellular levels of neurotransmitters such as GABA and glutamate at synaptic and extrasynaptic sites, which is important in maintaining normal synaptic communication. In addition, accumulation of excess extracellular glutamate in the absence of this uptake system can cause excitotoxicity (Kinney and Spain, 2002; Rothstein et al., 1996; Schousboe et al., 1992). Astrocytes also serve to metabolically re-supply neurons with these neurotransmitters for synaptic signaling. For example, glutamate is converted in astrocytes by glutamine synthetase to the glutamate precursor, glutamine, which is then shuttled back to neurons where it is converted to glutamate for subsequent synaptic release (Westergaard et al., 1995).
Astrocytes play several neuroprotective roles. Astrocytes express proteins such as metallothioneins, which enable neuroprotection from heavy metals and reactive oxygen species (Aschner, 1997; Struzynska et al., 2001). Astrocytes also serve as an arm of the immune system within the CNS. They phagocytose cells and act as antigen-presenting cells (Dong and Benveniste, 2001), and they also secrete chemokines and cytokines that regulate the immune system (Farina et al., 2007). Astrocyte also extend processes that envelope nodes of Ranvier in myelinated axons. These perinodal astrocytes contribute, for example, towards maintaining the high density of voltage-sensitive sodium channels in the axonal membrane in this region. In addition, there is growing appreciation for the roles played by astrocytes in additional processes in the adult CNS such as neurogenesis, control of cerebral blow, and normal synaptic communication and plasticity via the release of gliotransmitters, to name a few.
Reactive astrogliosis and glial scar formation
Following SCI, astrocytes undergo a complex and heterogeneous set of morphological, gene expression and functional changes that depend on factors such as severity, location and timing relative to the insult (Sofroniew, 2009). In response to CNS trauma, many different types of molecules released by cell types in the CNS including neurons, microglia, oligodendrocyte lineage cells, endothelial cells, leukocytes and other astrocytes serve as triggers for reactive astrogliosis. Interleukins, ciliary neurotrophic factor (CNTF), lipopolysaccharides, glutamate, ATP, nitric oxide, amyloid beta and NH4+ are some examples of such molecular triggers. These signaling mechanisms elicit changes in astrocytes that lead to a gradated response termed as reactive astrogliosis (Sofroniew, 2009). Extreme forms of this process can even lead to astrocyte proliferation and glial scar formation (Buffo et al., 2008; Gadea et al., 2008).
Scar-forming reactive astrocytes play certain neuroprotective functions. In transgenic mice in which astrocytes were specifically induced to express herpes simplex virus thymidine kinase (HSV-TK), treatment with the antiviral drug ganciclovir selectively ablated only dividing astrocytes. Consequent loss of glial scar forming reactive astrocytes led to increased spread and persistence of immune cell invasion, BBB repair failure, increased tissue damage, neuronal loss and demyelination, and impaired functional recovery following SCI (Bush et al., 1999; Faulkner et al., 2004).
Depending on the signaling trigger, reactive astrocytes have the capacity to produce a variety of molecules that possess pro- or anti-inflammatory properties. Furthermore, their interaction with microglia can exert both pro- and anti-inflammatory effects in microglia (Eddleston and Mucke, 1993; John et al., 2003; Min et al., 2006). Importantly, reactive astrogliosis induced by trauma, stroke, neurodegenerative disease or other CNS maladies can disrupt the normal functioning of astrocytes. This loss of astrocyte function can in turn result in toxic downstream events, which exacerbate effects of the disease. Furthermore, there can be a gain of certain toxic properties such as an increase in cytokine or VEGF-A production that leads to BBB breakdown and increased inflammation (Argaw et al., 2009). Additionally, production of reactive oxygen species may increase to cytotoxic levels, and compromised glutamate reuptake leads to excitotoxic neurodegeneration and seizures (Hamby et al., 2006; Swanson et al., 2004; Takano et al., 2005). Cytotoxicity and seizures can also be caused by edema created by hyperactivity of the brain’s chief aquaporin, AQP4, which is primarily found in astrocytes (Zador et al., 2009).
These are just a few examples of disease-relevant changes occurring in reactive astrocytes following SCI. These findings demonstrate that in response to CNS injury, astrocytes undergo both beneficial and harmful alterations that are not uniform across all astrocytes and that vary with the type and degree of insult.
Identifying novel astrocyte-specific therapeutic targets
An understanding of the molecular mechanisms of astrogliosis has provided us with therapeutic astrocyte-related targets. In the following section, we discuss a few example studies that sought to develop therapeutic strategies for SCI by targeting molecules produced by reactive astrocytes. Importantly, this knowledge can be used to harness cell transplantation in a mechanistically-informed manner.
Parawixin1, a compound obtained from spider venom, enhanced the activity of the astrocyte glutamate transporter EAAT2/GLT1, reduced glutamate-mediated excitotoxicity and increased the survival of retinal neurons (Fontana et al., 2003; Fontana et al., 2007; Hamby and Sofroniew, 2010). Similarly, a number of additional pharmacological compounds have been shown to boost astrocyte GLT1 expression in the diseased CNS. Addressing the central role played by astrocytes in regulating neurotransmitter homeostasis may be a useful approach with transplantation.
Nitric oxide synthetase (NOS-2), an enzyme responsible for production of nitric oxide (NO), is induced following injury or inflammation by a variety of stimuli such as IL1- LPS and TNFα in astrocytes, as well as in microglia. A variety of in vitro studies have established that NO generated by NOS-2 can contribute to cell death through depletion of cellular energy sources by causing DNA strand breaks and via inhibiting mitochondrial respiration. These data and other evidence suggest that astrocyte-specific NOS-2 may be an important therapeutic target for treating neurological diseases (Hamby et al., 2008; Liberatore et al., 1999).
Gluthathione (GSH) synthesized by astrocytes contributes to neuroprotection against oxidative stress. GSH synthesis is regulated by cytokine signaling mechanisms that mediate astrogliosis, including the signal transducer and activator of transcription 3 (Stat3) pathway in astrocytes (Chen et al., 2001; Sarafian et al., 2010). In transgenic mice with selective deletion of Stat3, reactive astrocytes showed limited migration, resulting in widespread infiltration of inflammatory cells, neural damage and demyelination, and more severe motor deficits following contusion SCI. These and other experiments suggest that Stat3 is a key regulator of reactive astrocytes in the healing process after SCI, providing a potential target for intervention (Okada et al., 2006).
Cell types used for transplantation
Over the past few decades, a number of laboratories have focused on using transplantation for targeting astrocyte pathogenesis in SCI. The source of cell types used has evolved with our increased understanding of NSC and NPC biology. The earliest studies used early postnatal astrocytes or fetal tissue grafts that included differentiated astrocytes and/or glial progenitor cells. Investigation then progressed to the use of various classes of isolated NSCs and lineage-restricted glial progenitors derived from either the developing or adult CNS and from various sub-regions of the nervous system. With improved technology for harvesting and maintaining NSC and NPC lines from the human nervous system, studies then began to test these more clinically-relevant human cell types. The appreciation that pluripotent stem cells can serve as a powerful source for obtaining large numbers of uniform cells for clinical translation then led the way to testing of embryonic stem (ES) cell-derived cell types. Most recently, the excitement of using induced pluripotent stem (iPS) cells as an autologous source, while avoiding some of the ethical concerns associated with ES cell derivation, represents the newest direction in the arsenal for targeting astrocytes in SCI using transplantation.
Neonatal rodent astrocytes
In early studies by George Smith and Jerry Silver, transplantation of rodent neonatal astrocytes was tested as a therapeutic strategy following CNS insults. Even before transplanting astrocytes, the plasticity response induced by endogenous astrocytes to injury was assessed. When the cerebral midline was lesioned, severed callosal axons formed neuromas. Transplantation of a nitrocellulose bridge into P8 (postnatal day 8) or younger pups with this injury produced encouraging results. There was no tissue necrosis and within 24 hours, glial cells migrated over and integrated into the graft, providing a substrate on which injured axons could then extend. However, the effectiveness and speed of this process was dependent upon the age of the animal. When this procedure was carried out at P14 or later, animals exhibited extensive tissue degeneration. Additionally, the implant was covered by a scar-like mixture of fibroblasts and astrocytes that failed to promote axon extension. Interestingly, glial cells from younger pups were capable of promoting axon outgrowth in this model and retained this ability when transplanted into the lesions of older rodents. These important studies demonstrated an age dependent decrease in the ability of astrocytes to promote axon growth and laid the foundation for the idea of therapeutically recreating a “young glial environment” for increasing plasticity and recovery (Smith et al., 1986).
Further in vitro studies by the same investigators indicated that astrocyte maturation correlated with changes in their intrinsic properties and in the extent and molecular basis of neurite outgrowth they support. The rate and extent of neurite outgrowth from embryonic rat cortical and chick retinal neurons were consistently greater over the surface of immature than mature astrocytes. Furthermore, antibodies to NCAM and G4/L1 significantly reduced neurite outgrowth on immature but not mature astrocytes, while antibodies to the integrin B1 receptor reduced outgrowth on immature, and to a lesser extent, mature astrocytes (Smith et al., 1990).
Additional differences in the motile properties of astrocytes were documented. Immature and mature astrocytes were labeled and separately transplanted into the adult rat brain. While both cell types survived transplantation, their post-transplant trajectories differed. Mature astrocytes were more likely to be phagocytosed by the host immune system. Most critically, in contrast to mature astrocytes that largely remained near the transplant site, immature astrocytes migrated more rapidly and extensively and interacted with blood vessels. In addition, these astrocyte-associated blood vessels were less permeable. Collectively, these findings suggest that the ability of immature astrocytes to distribute throughout the injured CNS and become associated with blood vessels may represent some of the important factors underlying their ability to suppress scar formation and promote neuronal growth in the adult brain (Smith and Miller, 1991).
In an independent study, adult rats were subjected to a dorsal hemisection at the thoracic spinal cord, followed by transplantation of cultured neonatal cortical astrocytes in a collagen matrix into the lesion area. Transplants remained in the injury/implant site, associated with ingrowing axon fibers and promoted growth of neuron populations such as corticospinal tract axons. Locomotor assays including the Basso, Beattie and Bresnahan (BBB) and walkway crossing tests revealed that astrocyte transplant treated rats exhibited some motor recovery (Joosten et al., 2004).
These studies provide proof of principle that immature astrocytes are endowed with certain beneficial properties in the context of CNS injury compared to mature astrocyte populations. However, it will be difficult to develop therapeutic strategies for patients based directly on these studies due to the difficulty and ethical concerns associated with harvesting primary astrocytes from human tissue. Fetal and adult CNS derived multipotent NSCs and lineage-restricted glial progenitors represent more viable sources for generating sufficient numbers and homogenous populations of transplantable cells of the astrocyte lineage.
Rodent multipotent neural stem cells (NSCs)
Early NSC/NPC studies for SCI utilized multipotent NSC transplantation. For example, work from the Whittemore lab employed NSCs isolated from E14 rat cortex. These cells had round cell bodies and thin processes and expressed characteristic NSC/NPC markers such as nestin. When cultured in vitro, these cells retained their multipotent capacity to differentiate into neurons, oligodendrocytes and astrocytes depending on the treatment protocol. Following transplantation as undifferentiated cells into uninjured spinal cord, the cells displayed robust survival and some rostral-caudal migration. Within the first week post-injection, the majority of transplant-derived cells remained as undifferentiated nestin-positive cells. At longer time points up to two months following engraftment, only a small percentage of undifferentiated NSCs remained, while a significant proportion of transplanted cells differentiated into GFAP-positive astrocytes. NSCs were also able to differentiate into oligodendrocytes and neurons, but in lower numbers than astrocytes. When transplanted into the lesioned spinal cord, these cells again differentiated predominantly into astrocytes, but neuronal differentiation was not detected. These findings revealed that unless pre-differentiation is carried out prior to transplantation, the phenotypic fate of NSC grafts in SCI is restricted to glial lineages, particularly astrocytes (Cao et al., 2001). These findings demonstrate that the injured spinal cord does not support neuronal differentiation of NSCs, suggesting that more differentiated lineage-restricted neuronal progenitors will be needed for neuron replacement. These and other studies also show that NSCs can be used to successfully achieve astrocyte differentiation in the injured spinal cord; however, these multipotent cells also generate oligodendrocytes (which may not be desirable if only targeting astrocyte replacement) and have a greater propensity to remain in an undifferentiated state in vivo than more lineage-committed NPCs.
Attempts have been made to address the propensity of NSC transplants to differentiate into astrocytes in the injured spinal cord. For example, forced Ngn-2 expression in transplanted NSCs attenuated astrocyte differentiation following transplantation into injured spinal cord, resulting in a 25-fold reduction in astrocyte differentiation and instead promoted differentiation into oligodendroglial and some neuronal lineages. Additionally, these Ngn-2 NSCs gave rise to cells expressing myelin-associated proteins such as MBP, which may explain the preservation of white matter at injury sites treated with Ngn-2 NSCs. Motor function tests administered 9 weeks after transplantation showed that Ngn-2-NSC treated rats exhibited improved performance compared to rats that received naïve unmodified NSCs, which in turn demonstrated improved function compared to control rats that were only administered the vehicle. Cerebral blood oxygen level-dependent (BOLD) signal testing was used to assess contralateral primary somatosensory cortex in response to hindpaw stimulation. While injured rats that were either treated with vehicle or control NSCs showed no improvement, rats with Ngn-2 NSC treatment exhibited partial recovery of this circuitry.
Additional observations made in this Ngn-2 NSC study also serve as a cautionary tale for using NSC transplants. Injured rats exhibited greater sensitivity to cold stimulation of the forepaws, a form of SCI-induced neuropathic pain. This sensitivity was increased in injured rats with control NSC transplants, but was identical to vehicle control when treated with Ngn-2 NSCs. This result could be explained by the correlation between thermal hypersensitivity and greater numbers of transplant-derived GFAP-positive astrocytes. Examination of naïve NSC transplants showed that grafted cells were associated with an increase in calcitonin gene related peptide (CGRP) expressing nociceptive fibers in lamina III of the dorsal horn, suggesting that NSC-derived astrocytes could be inducing abnormal plasticity of primary afferent nociceptive neuronal populations (Hofstetter et al., 2005). Given the neuronal growth-inducing properties exhibited by immature astrocyte transplants, it is possible that transplanted NSC-derived astrocytes possess great therapeutic potential for promoting host neuronal plasticity following SCI, but that care must be taken to avoid unwanted reorganization that could lead to outcomes such as neuropathic pain, spasticity and other detrimental effects.
A novel approach to implanting neural stem cells into injured spinal cord was explored by Ted Teng and Evan Snyder (Teng et al., 2002). In this study, a polymer scaffold was designed to resemble the gray-white matter architecture of the spinal cord. The outer portion of the scaffold was modeled on white matter, with long, axially-oriented pores for axon guidance, fluid transport and inhibition of glial scar formation, and the inner portion of the scaffold was seeded with multipotent murine NSCs prior to implantation. Implantation of this synthetic, degradable, multi-component scaffold into a rat thoracic hemisection model led to significant recovery of hindlimb motor function. Histological analysis suggested that functional benefits were at least partly due to increased tissue sparing. Immunohistochemical findings supported the notion that axonal re-growth may have also been a component contributing to therapeutic benefit. Interestingly, animals implanted with scaffold alone (without NSC seeding) showed greater functional recovery and tissue sparing compared to the cells-alone and lesion-only control groups. This work nicely illustrates the importance of using multi-faceted approaches for cell transplantation-based treatment of SCI.
Rodent glial progenitor cells
While NSCs provide the benefit of being able to give rise to both neurons and glia, the use of lineage-restricted NPCs is likely a more desirable strategy as they allow for targeted generation of only a specific cell type of interest following transplantation. With respect to targeting astrocytes, a number of studies in the SCI field have employed the glial-restricted precursor (GRP) derived from the fetal CNS, which gives rise to only oligodendrocytes and two astrocyte populations (Rao et al., 1998).
In an early study, the fate of transplanted GRPs harvested from E13.5 spinal cord of hPLAP (human placental alkaline phosphatase) transgenic reporter rats was assessed in the intact and injured spinal cord. Cells exhibited robust migration along white matter tracts in both the intact and injured conditions. In terms of phenotypic properties, transplanted cells only differentiated into glial lineages, with predominantly astrocyte generation. This study provided evidence that GRP transplants can serve as useful therapeutic tools for targeting astrocyte replacement in the injured spinal cord (Han et al., 2004).
In a subsequent study, GRPs were transplanted into a thoracic contusion SCI site. Time course analysis showed that transplanted cells survived in the environment of the injury site for long periods following injection. At six weeks post-injury, while the majority of transplant-derived cells remained in the injury site, some cells migrated beyond the transplant area. These GRPs differentiated primarily into GFAP-positive astrocytes, but some cells also expressed markers of oligodendrocytes (CC1), myelin producing cells (MBP) or immature NPCs (nestin). GRP transplants decreased scar formation and reduced expression of growth-inhibiting proteoglycans. While these transplants did not induce any long-distance regeneration of either raphespinal or corticospinal tract (CST) axons, they did exert beneficial effects by promoting a growth cone-like morphology in CST axons. Additionally, some CST fibers were found to extend into the transplant site, suggesting that GRP-derived astrocyte support some degree of neuronal re-growth (Hill et al., 2004).
A study by the group of Stephen Davies showed that transplantation of in vitro pre-differentiated astrocytes derived from GRPs leads to significantly improved outcomes compared to transplantation of undifferentiated GRPs. In contrast to GRP-derived astrocytes (GDAs), undifferentiated GRPs neither repressed scar formation nor promoted recovery in locomotor function. BMP-4 (bone morphogenetic protein-4) mediated in vitro pre-differentiation of GRPs produced GDAs that antigenically resembled type-1 GFAP+/A2B5− astrocytes. When transplanted into a stab-wound lesion of the rat spinal cord dorsal white matter, these GDAs promoted axonal regrowth. GDA transplants also prevented both scar formation and atrophy of axotomized neurons and integrated and aligned well with host astrocytes at the periphery of the injury site. In addition, transplantation of these BMP-4 GDAs did not promote allodynia or other neuropathic pain related effects. Similar results were obtained after transplantation into a separate rat SCI model that eliminated the possibility of axon sparing. Newly growing axons from co-transplanted GFP-labeled DRG neurons failed to cross GRP-transplanted injury sites or lesions injected with medium. In contrast, significant numbers of GFP+ axons (from transplanted neurons) extended into the center of GDA-transplanted injuries, with some axons even growing beyond the lesion site into host tissue. (Davies et al., 2006).
GRPs can also be differentiated into GFAP+/A2B5+ GDAs by exposure to a different astrogenic signaling molecule, CNTF. These cells differ from BMP-4 GDAs in the expression of several transcription factors and cell surface molecules such as chondroitin sulphate proteoglycans (CSPGs), neuron-glial antigen 2 (CSPG4) and phosphocans. In one study, these CNTF GDAs survived transplantation and were able to span the injury site. However, they failed to suppress host astrogliosis and did not align with endogenous astrocytes at the periphery of transplant site, leading to misaligned astrocyte processes and were comparable to the injury periphery in untreated and GRP-transplanted injuries. Most critically, CNTF GDAs failed to promote the growth of endogenous ascending dorsal column axons across the dorsal column transection injury. In agreement with this result, axons from DRG neuron transplants failed to cross CNTF GDA transplants. Additionally, CNTF GDAs promoted thermal hyperalgesia and mechanical allodynia to an even greater degree than undifferentiated GRP transplants. Finally, locomotor testing showed that injured rats treated with CNTF GDAs did not exhibit any functional recovery (Davies et al., 2008). These findings suggest that heterogeneity exists in the sub-populations of astrocytes that can be generated from glial progenitors and that this heterogeneity may result in differential therapeutic effects in the injured spinal cord. These data also suggest that selection of the appropriate astrocyte sub-type(s) may be a necessary approach for avoiding unwanted side effects such as neuropathic pain that were observed in these studies and in the previously mentioned work with Ngn-2 overexpressing NSCs.
In contrast to the Davies experiments, work from the lab of Itzhak Fischer carried out under similar experimental conditions found that both BMP-4 GDAs and CNTF GDAs were able to promote axon growth to a similar degree. BMP-4 produced a heterogeneous population of GFAP+/A2B5− astrocytes in which some cells had a stellate morphology. On the contrary, CNTF GRPs maintained some immature markers, suggesting that they were not completely mature. Comparisons of these two GDA subtypes in SCI showed that cells from both transplants migrated along white matter tracts and equally promoted axon regrowth. The reason for this inconsistency between studies from the Davies and Fischer labs remains unclear. Nevertheless, these findings at least raise the possibility that not all astrocyte subtypes may perform equally well in the injured spinal cord. Future studies are needed to clarify this important issue (Haas et al., 2012).
Studies have begun to mechanistically tease out the factors responsible for the growth-promoting properties of astrocyte transplants in SCI. A recent report from the Proschel lab demonstrated that Periostin, a molecule secreted by BMP-4 GDAs, is one key component that promotes axon regeneration. Periostin is enriched in GDAs, and shRNA mediated knockdown of periostin in GDAs reduces their ability to promote axonal growth in vitro. Furthermore, transplantation of periostin-deficient BMP-4 GDAs resulted in compromised axon regeneration in vivo in a dorsal column transection model of SCI. These interesting findings illustrate one mechanism underlying the therapeutic effects of BMP-4 GDAs (Shih et al., 2014).
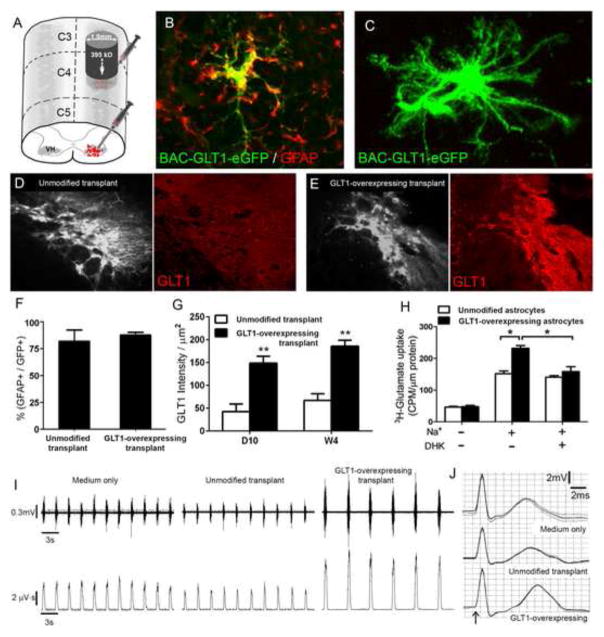
The previous studies employing astrocyte and glial progenitor transplantation in SCI models mostly targeted the axonal growth promoting properties of astrocytes. However, these studies did not target the normal homeostatic functions of astrocytes in the CNS. To begin to utilize astrocyte replacement in a mechanistically-targeted fashion based on their crucial functions in the intact nervous system, we have used GRP transplantation to restore extracellular glutamate uptake in the injured spinal cord. Astrocytes are responsible for the vast majority of glutamate uptake throughout the CNS via expression of the plasma membrane glutamate transporter GLT1, thereby playing a central role in maintaining normal synaptic communication and preventing glutamate-mediated excitotoxicity. Following SCI, astrocyte GLT1 expression and function are severely compromised, which contributes to excitotoxicity-induced cell death and consequent functional loss during the delayed secondary injury phase. In a unilateral cervical contusion model of SCI, we injected rodent GRP-derived astrocytes engineered to overexpress GLT1 into the cervical ventral horn (Figure 1A) as a therapeutic strategy for achieving transplantation-based delivery of astrocytes, reconstituting GLT1 function, preventing excitotoxicity, and consequently protecting respiratory phrenic motor neurons and preserving diaphragm function. Unmodified transplants robustly survived but expressed relatively low levels of GLT1 in the injured cervical spinal cord at both 10 days and 4 weeks post-transplantation (Figure 1D, G), suggesting that the injured host spinal cord exerts similar effects on GLT1 expression by these cells as on endogenous astrocytes. Excitingly, GLT1 overexpressing cells (engineered using an AAV-Gfa2-GLT1 vector in vitro prior to injection) expressed persistently high levels of GLT1 protein following transplantation into the injured spinal cord (Figure 1C, E), including at early time points post-injection (Figure 1G). Overexpression also enhanced actual GLT1-mediated glutamate uptake compared to control cells (Figure 1H), demonstrating the functional effects of this manipulation. In addition, these overexpressing astrocyte transplants significantly promoted survival of phrenic motor neurons, preservation of diaphragm neuromuscular junction innervation, and protection of diaphragmatic respiratory function (Figure 1I–J). GLT1 overexpression did not change the in vivo properties of the cells (compared to control transplants) such as proliferation and efficient astrocyte differentiation (Figure 1F), suggesting that therapeutic benefit was a product of increased GLT1 levels and not some other changes in transplanted cell function. Our findings demonstrate the therapeutic value of targeting specific mature astrocyte functions using cell transplantation in the injured spinal cord. Given the long list of important astrocyte functions, our approach represents just one example of using this powerful approach in SCI.
Figure 1. Astrocyte replacement following cervical spinal cord injury preserves diaphragm function via expression of the glutamate transporter, GLT1.
Rats received unilateral cervical contusion SCI at the C4 level. Immediately following injury, rodent GRP-derived astrocytes were injected into the cervical ventral horn to target the location of respiratory phrenic motor neurons (A). Transplants robustly survived, and nearly all transplant-derived cells expressed the astrocyte-specific marker, GFAP (B, F). Control unmodified transplants expressed relatively low levels of GLT1 in the injured cervical spinal cord at both 10 days and 4 weeks post-injection (D, G), while GLT1 overexpressing cells expressed persistently high levels of GLT1 protein following transplantation (E, G). These GLT1-expressing cells also displayed similar mature astrocyte morphology as control cells (C). Overexpression also enhanced actual sodium-dependent GLT1-mediated glutamate uptake compared to control cells which was blocked by the GLT1 inhibitor, DHK (H). In addition, overexpressing astrocyte transplants significantly protected diaphragmatic respiratory function compared to medium-only control and unmodified control transplants, as assessed by spontaneous EMG (F) and compound muscle action potential (G) recordings from the ipsilateral hemi-diaphragm.
Human glial progenitor cells
The described studies involving rodent-derived glial progenitors provide an understanding of GRP properties and proof-of-principle of their therapeutic potential. Subsequent work has extended this approach to transplantation of analogous classes of human glial progenitors into SCI.
Anatomical and functional effects of transplanting human GRPs (hGRPs) and astrocytes derived from hGRPs (hGDAs) into the contused spinal cord have been assessed. hGRPs were prepared from fetal human brain tissue via both negative and positive selection based on cell surface marker expression to remove neuronal progenitors and retain GRPs, respectively. Similar to rodent GRP studies, hGDAs were prepared in vitro prior to transplantation by treatment with BMP-4. Athymic rats were used as recipients to avoid the need for immunosuppression and zenograft rejection. These animals received moderate contusion at T10 and were transplanted 9 days after injury with medium control, hGRPs or hGDAs. At 8 weeks after transplantation, both hGRPs and hGDAs showed robust survival and extensive migration within host tissue. Total numbers of transplant-derived cells showed 2–3 fold increases in both groups compared to numbers of initially injected cells (but with few proliferating cells remaining at 8 weeks), suggesting graft expansion at earlier time points post-injection followed by efficient differentiation. Cells differentiated predominantly into astrocytes, and few cells remained in a progenitor state by this time point. The majority of transplant-derived cells near the injury site differentiated into GFAP-positive astrocytes, with the remaining cells being mostly of the oligodendrocyte lineage. At greater distances from the lesion site, the proportion of oligodendrocyte lineage cells increased, possibly due to the persistence and migratory nature of oliogodendrocyte progenitors derived from transplanted hGRPs. In both hGRP and hGDA transplantation groups, cyst and scar formation at the injury site were reduced compared to medium injection control. Microglia/macrophages were present at and around the lesion area, and axons grew along spared tissue with no differences among groups. No significant improvement in motor function was observed with BBB or grid walk testing in any experimental group. Importantly, tactile hypersensitivity was not observed, and the hGDA group showed sensory function returning towards the pre-injury level. These findings suggest that although the improved lesion environment did not lead to robust functional recovery, their permissive properties and lack of induced neuropathic pain phenotype make human glial progenitors promising candidates for therapy after SCI (Jin et al., 2011).
As an extension of their rodent GRP work, the Fischer group also generated hGDAs by treatment with CNTF or BMP-4. Morphologically, BMP-4 treated cells had shorter, more branched processes, while cells produced via CNTF treatment exhibited longer processes. Examination of marker expression showed that BMP-4 treatment produced a more mature astrocyte phenotype. Despite these differences, undifferentiated GRPs and GDAs generated from BMP-4 or CNTF survived and promoted axonal regrowth following transplantation into a cervical dorsal column SCI model, with no discernible in vivo differences amongst the cell types. In summary, these particular results suggest that glial progenitors and glial progenitor-derived astrocytes possess robust neuronal growth promoting properties, regardless of differentiation state or astrocyte sub-type (Haas and Fischer, 2013).
In contrast to these results from Itzhak Fischer’s group, the Davies and Proschel labs again reported differential effects amongst transplants of human GRPs and hGDA subtypes on axonal growth, neuroprotection and functional recovery in a dorsolateral funiculotomy injury of the cervical spinal cord. Similar to their results with transplantation of rodent GRP and GDAs, they observed selective efficacy with BMP-4 (Davies et al., 2011).
Human pluripotent stem cell-derived glial progenitors and astrocytes
A number of studies have utilized pluripotent embryonic stem (ES) cells as a source for deriving large numbers of homogenous CNS cell types for transplantation into the injured spinal cord (cite some examples). The relatively recent discovery of induced pluripotent stem (iPS) cells has generated great excitement as they represent a clinically-relevant source of pluripotent cells generated from adult somatic cell types, avoiding ethical issues of ES cell derivation. This technology also allows for homogeneous derivation of mature cell types in large quantities, potentially in an autologous fashion from the eventual transplant recipient. Despite the promise of this approach, the iPS cell field is in its infancy with respect to evaluating in vivo graft integration and therapeutic usefulness in relevant SCI models, particularly in the context of astrocytes.
The laboratory of Su-Chun Zhang reported on a protocol in which they were capable of producing large quantities of enriched astrocytes in vitro from human ES and iPS cells by first generating populations of neuroepithelial cells and region-specific NPCs. Regionally specific subtypes of astrocytes were generated using this protocol, including those with spinal cord or ventral forebrain phenotypes (Krencik et al., 2011). A shortcoming of this study was that six months of culturing was needed to generate these astrocytes. Shorter duration protocols have been developed by other laboratories for generating astrocytes from iPS cells (Emdad et al., 2012; Juopperi et al., 2012); however, relatively immature astrocytes were mostly produced.
Astrocyte maturation is thought to take place in two phases: (1) an embryonic phase in which astrocytes are generated from radial glia or NPCs; (2) second phase that takes place during early postnatal weeks in which astrocytes acquire more mature, quiescent phenotypes. The sequence of astrocyte lineage marker expression has been characterized during these phases, which led to the development of protocols for generating astrocytes with mature or reactive phenotypes from pluripotent stem cells in vitro (Roybon et al., 2013). When GFAP-expressing immature astrocytes derived from mouse ES cells were briefly treated with fibroblast growth factor 1 (FGF1) or FGF 2, the cells showed robust up-regulation in the expression of differentiated astrocyte markers such as glutamate transporters, as well as GFAP down-regulation. Similar results were observed with human-derived ES and iPS cells. While FGF triggered biochemical and functional astrocyte maturation, TNFα triggered a reactive astrocyte phenotype. These distinct populations of pluripotent stem cell-derived astrocytes can be used both to model human pathological processes and for transplantation in SCI and other nervous system diseases.
Another recent report from Su-Chun Zhang’s lab provides a protocol for deriving homogenous populations of astrocyte subtypes from human pluripotent stem cells, including ES and iPS cells, within a reduced time frame of approximately 90 days. In this protocol, human pluripotent stem cell-derived neuroepithelial cells were differentiated into astrocyte progenitor subtypes in the presence of mitogens, followed by further astrocyte maturation via mitogen removal and addition of CNTF (Krencik and Zhang, 2011). These types of approaches represent important practical steps towards harnessing pluripotent cell-derived astrocytes to both understand and treat CNS disease.
The laboratory of Nicholas Maragakis transplanted astrocyte progenitors derived from human iPS cells into the rodent spinal cord. Two iPS cell lines and one human ES cell line were subjected to an extended in vitro astrocyte differentiation protocol. After 100 days of differentiation, cells expressed markers of immature astrocytes such as CD44 and S1003. At this stage, cells were transplanted into rat cervical spinal cord. Although graft survival was limited (<5%), the surviving cells did remain integrated for at least 12 weeks post-injection. More than 90% of transplant-derived cells expressed GFAP and the astrocyte water channel aquaporin 4 (AQP4), indicative of astrocyte differentiation. No or very little differentiation into oligodendrocytes or neurons was observed. Further analysis using nanostring technology revealed in vivo expression of astrocyte lineage genes, including GFAP, AQP4, Connexin43, MLC1 and the glutamate transporters, EAAT1 and EAAT2, consistent with immunohistochemical analysis. Comparison of gene expression profiles before and after transplantation indicated up-regulation of mature astrocyte-specific genes in vivo. These results show that human iPS cell-derived transplants not only exhibit long-term survival and integration into the adult spinal cord, but also mature into differentiated astrocytes (Haidet-Phillips et al., 2014).
Before iPS cell-derived glial progenitors and astrocytes can be used for clinical applications, it is important to evaluate their in vivo safety in terms of tumor formation potential and targeted differentiation. Two recent reports from the Okano lab conducted such analyses on both murine and human iPS cells. In the first study, iPS cell lines generated from mouse fibroblasts were classified as either “safe” or “unsafe” according to teratoma-forming activity of iPS cell-derived neurospheres following transplantation into mouse brain. Therapeutic potential was then evaluated following transplantation into contused spinal cord. Transplantation of “safe” iPS cell-derived secondary neurospheres (SNSs) resulted in successful differentiation into neurons, astrocytes and oligodendrocytes, with no signs of tumor formation. Furthermore, cells participated in myelin formation, supported axon growth and led to functional recovery. In contrast, transplantation of “unsafe” iPS-SNSs resulted in tumor formation and a sudden deterioration in motor function that followed initial recovery. These findings suggest that pre-evaluated sources of iPS cell-derived NSCs and NPCs will be important for conducting transplantation therapy in patient populations. In a second study, iPS cells were generated from adult human dermal fibroblasts, followed by transplantation of iPS cell-derived neurospheres into contused spinal cord. Similar to their work with murine iPS cells, these human transplants differentiated into all mature CNS lineages. Graft-derived neurons formed synapses with the host neurons. Furthermore, transplanted cells promoted angiogenesis and axon regrowth in the lesion. These anatomical changes were accompanied by recovery of motor function, as assessed by behavioral testing and electrophysiological recordings. Importantly, no tumor formation was observed in the spinal cords of transplanted mice (Nori et al., 2011; Tsuji et al., 2010). These observations suggest that human iPS cell-derived NSCs and NPCs are a promising source for cell transplantation therapies following SCI. Future work will be needed to characterize the potential of specifically targeting astrocytes with iPS cells.
As an extension of our work targeting astrocyte GLT1 using rodent GRPs, we have taken a similar approach with human iPS cells. We derived pluripotent iPS cells from non-diseased human donors, generated glial progenitors and then differentiating these cells into astrocytes prior to transplantation (Figure 2A–C). Similar to rodent GRPs and GDAs, human iPS cell-derived transplants survive for long periods of time in the injured cervical spinal (Figure 2D–F), do not form tumors or show uncontrolled proliferation, differentiate into only GFAP-positive astrocytes (Figure 2D), and do not localize to ectopic locations or differentiate into unexpected lineages. In addition, these cells can be engineered to overexpress GLT1, resulting in robust and persistent transporter expression in the injured spinal cord (Figure 2E–F). Our data show that clinically-relevant populations of human iPS cells can be used as a safe source of transplants for targeted replacement of astrocytes in SCI.
Figure 2. Human iPS cell-derived astrocyte transplants safely integrate into the injured spinal cord.
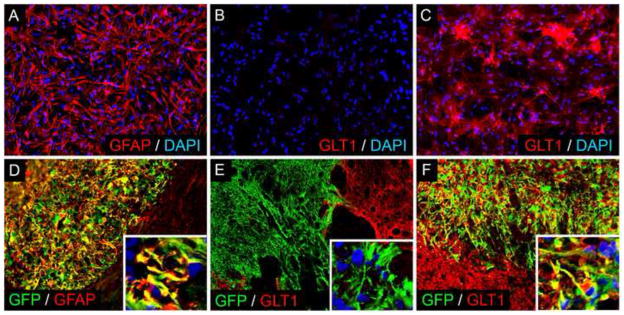
Pluripotent iPS cells derived from non-diseased human donors were used to generate glial progenitors and subsequently GFAP-positive astrocytes (A) in vitro. In culture, these astrocytes normally do not express high levels of GLT1 (B). Viral overexpression results in significant GLT1 protein expression (C). Human iPS cell-derived astrocytes were then transplanted into a cervical contusion SCI model. Transplanted cells survive for at least 6 weeks in the injured cervical spinal of immunesuppressed rats (D–F), do not form tumors or show uncontrolled proliferation, differentiate into only GFAP-positive astrocytes (D), and do not localize to ectopic locations or differentiate into unexpected lineages. Similar to results in vitro, engineering these cells to overexpress GLT1 results in robust and persistent transporter expression in the injured spinal cord (F) compared to control unmodified transplants (E).
Conclusions
Until recently, astrocytes were studied in SCI mainly in the context of scar formation and its inhibitory effects on axonal regeneration. Rapid progress in understanding both astrocyte heterogeneity and the specific changes that occur during transformation into reactive astrocytes has underscored the importance of studying astrocyte function in the intact CNS and in diseased conditions. The studies described in this review, as well as other work not detailed here, demonstrate the potential of and growing appreciation for using cell transplantation to therapeutically target astrocytes in SCI and other CNS disorders. As the field moves forward with this approach, there are a number of important issues that need to be addressed. We need to acquire a better understanding of normal astrocyte heterogeneity in the intact CNS, as well as in diseased conditions. Furthermore, we need to develop protocols for deriving astrocyte progenitor and mature astrocyte subtypes for transplantation, and we need to better elucidate the contribution of these subtypes to therapeutic efficacy of transplantation strategies in SCI. While the axon growth-promoting property of astrocyte lineage transplants is undoubtedly a powerful tool, we also need to target astrocyte-specific functions (e.g. neurotransmitter uptake, regulation of synaptogenesis, control of cerebral blood flow and BBB function, etc.) on a mechanistic level to fully harness the potential of transplantation. In addition, we need to address important practical considerations for clinically translating this approach. As one example detailed in this review, the derivation from pluripotent sources such as ES and iPS cells needs to be optimized for assuring efficient, homogenous and safe generation of astrocyte lineage cells.
HIGHLIGHTS.
Cell transplantation is a promising therapeutic strategy for SCI.
Astrocytes play crucial roles in both normal and diseased CNS, including SCI.
Transplant-based targeting of astrocytes in SCI has not been extensively explored.
Transplant-based targeting of astrocytes represents a powerful tool for SCI treatment.
A number of important issues must be addressed to facilitate clinical translation.
Acknowledgments
This work was supported by the Craig H. Neilsen Foundation (#190140) and the NINDS (grant #1R01NS079702).
ABBREVIATIONS
- NSC
multipotent neural stem cell
- NPC
lineage-restricted neural progenitor cell
- SCI
traumatic spinal cord injury
- GFAP
glial fibrillary acidic protein
- BBB
blood-brain barrier
- ES cell
embryonic stem cell
- iPS cell
induced pluripotent stem cell
- GRP
glial-restricted precursor
- GDA
GRP-derived astrocyte
- hGRP
human glial-restricted precursor (GRP)
- BMP-4
bone morphogenetic protein 4
- GLT1
glutamate transporter 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATUE CITED
- Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106:1977–82. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M. Astrocyte metallothioneins (MTs) and their neuroprotective role. Ann N Y Acad Sci. 1997;825:334–47. doi: 10.1111/j.1749-6632.1997.tb48445.x. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Grafe P, ten Bruggencate G. Ion activities and potassium uptake mechanisms of glial cells in guinea-pig olfactory cortex slices. J Physiol. 1987;382:159–74. doi: 10.1113/jphysiol.1987.sp016361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105:3581–6. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Cao QL, Zhang YP, Howard RM, Walters WM, Tsoulfas P, Whittemore SR. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167:48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Broadwell RD. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions. II. Choroid plexus and ependymal epithelia, endothelia and pericytes. J Neurocytol. 1986;15:511–24. doi: 10.1007/BF01611733. [DOI] [PubMed] [Google Scholar]
- Chen Y, Vartiainen NE, Ying W, Chan PH, Koistinaho J, Swanson RA. Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J Neurochem. 2001;77:1601–10. doi: 10.1046/j.1471-4159.2001.00374.x. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–33. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Davies JE, Huang C, Proschel C, Noble M, Mayer-Proschel M, Davies SJ. Astrocytes derived from glial-restricted precursors promote spinal cord repair. J Biol. 2006;5:7. doi: 10.1186/jbiol35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JE, Proschel C, Zhang N, Noble M, Mayer-Proschel M, Davies SJ. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J Biol. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SJ, Shih CH, Noble M, Mayer-Proschel M, Davies JE, Proschel C. Transplantation of specific human astrocytes promotes functional recovery after spinal cord injury. PLoS One. 2011;6:e17328. doi: 10.1371/journal.pone.0017328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27:11354–65. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–90. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Mucke L. Molecular profile of reactive astrocytes--implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdad L, D’Souza SL, Kothari HP, Qadeer ZA, Germano IM. Efficient differentiation of human embryonic and induced pluripotent stem cells into functional astrocytes. Stem Cells Dev. 2012;21:404–10. doi: 10.1089/scd.2010.0560. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–45. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–55. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana AC, Guizzo R, de Oliveira Beleboni R, Meirelles ESAR, Coimbra NC, Amara SG, dos Santos WF, Coutinho-Netto J. Purification of a neuroprotective component of Parawixia bistriata spider venom that enhances glutamate uptake. Br J Pharmacol. 2003;139:1297–309. doi: 10.1038/sj.bjp.0705352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana AC, de Oliveira Beleboni R, Wojewodzic MW, Ferreira Dos Santos W, Coutinho-Netto J, Grutle NJ, Watts SD, Danbolt NC, Amara SG. Enhancing glutamate transport: mechanism of action of Parawixin1, a neuroprotective compound from Parawixia bistriata spider venom. Mol Pharmacol. 2007;72:1228–37. doi: 10.1124/mol.107.037127. [DOI] [PubMed] [Google Scholar]
- Gadea A, Schinelli S, Gallo V. Endothelin-1 regulates astrocyte proliferation and reactive gliosis via a JNK/c-Jun signaling pathway. J Neurosci. 2008;28:2394–408. doi: 10.1523/JNEUROSCI.5652-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gonzalez ML, Malemud CJ, Silver J. Role of astroglial extracellular matrix in the formation of rat olfactory bulb glomeruli. Exp Neurol. 1993;123:91–105. doi: 10.1006/exnr.1993.1143. [DOI] [PubMed] [Google Scholar]
- Haas C, Neuhuber B, Yamagami T, Rao M, Fischer I. Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration. Exp Neurol. 2012;233:717–32. doi: 10.1016/j.expneurol.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C, Fischer I. Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. J Neurotrauma. 2013;30:1035–52. doi: 10.1089/neu.2013.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Roybon L, Gross SK, Tuteja A, Donnelly CJ, Richard JP, Ko M, Sherman A, Eggan K, Henderson CE, Maragakis NJ. Gene profiling of human induced pluripotent stem cell-derived astrocyte progenitors following spinal cord engraftment. Stem Cells Transl Med. 2014;3:575–85. doi: 10.5966/sctm.2013-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby ME, Hewett JA, Hewett SJ. TGF-beta1 potentiates astrocytic nitric oxide production by expanding the population of astrocytes that express NOS-2. Glia. 2006;54:566–77. doi: 10.1002/glia.20411. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Hewett JA, Hewett SJ. TGF-beta1 reduces the heterogeneity of astrocytic cyclooxygenase-2 and nitric oxide synthase-2 gene expression in a stimulus-independent manner. Prostaglandins Other Lipid Mediat. 2008;85:115–24. doi: 10.1016/j.prostaglandins.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby ME, Sofroniew MV. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics. 2010;7:494–506. doi: 10.1016/j.nurt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SS, Liu Y, Tyler-Polsz C, Rao MS, Fischer I. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation, and preferential migration in white matter. Glia. 2004;45:1–16. doi: 10.1002/glia.10282. [DOI] [PubMed] [Google Scholar]
- Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factor(s) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25:25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hill CE, Proschel C, Noble M, Mayer-Proschel M, Gensel JC, Beattie MS, Bresnahan JC. Acute transplantation of glial-restricted precursor cells into spinal cord contusion injuries: survival, differentiation, and effects on lesion environment and axonal regeneration. Exp Neurol. 2004;190:289–310. doi: 10.1016/j.expneurol.2004.05.043. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisen J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–53. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- Holthoff K, Witte OW. Directed spatial potassium redistribution in rat neocortex. Glia. 2000;29:288–92. doi: 10.1002/(sici)1098-1136(20000201)29:3<288::aid-glia10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Jin Y, Neuhuber B, Singh A, Bouyer J, Lepore A, Bonner J, Himes T, Campanelli JT, Fischer I. Transplantation of human glial restricted progenitors and derived astrocytes into a contusion model of spinal cord injury. J Neurotrauma. 2011;28:579–94. doi: 10.1089/neu.2010.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Lee SC, Brosnan CF. Cytokines: powerful regulators of glial cell activation. Neuroscientist. 2003;9:10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- Joosten EA, Veldhuis WB, Hamers FP. Collagen containing neonatal astrocytes stimulates regrowth of injured fibers and promotes modest locomotor recovery after spinal cord injury. J Neurosci Res. 2004;77:127–42. doi: 10.1002/jnr.20088. [DOI] [PubMed] [Google Scholar]
- Juopperi TA, Kim WR, Chiang CH, Yu H, Margolis RL, Ross CA, Ming GL, Song H. Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington’s disease patient cells. Mol Brain. 2012;5:17. doi: 10.1186/1756-6606-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23:1–10. [PubMed] [Google Scholar]
- Kinney GA, Spain WJ. Synaptically evoked GABA transporter currents in neocortical glia. J Neurophysiol. 2002;88:2899–908. doi: 10.1152/jn.00037.2002. [DOI] [PubMed] [Google Scholar]
- Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–34. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Zhang SC. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat Protoc. 2011;6:1710–7. doi: 10.1038/nprot.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG, Orkand RK. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:768–87. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–9. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Liesi P, Dahl D, Vaheri A. Laminin is produced by early rat astrocytes in primary culture. J Cell Biol. 1983;96:920–4. doi: 10.1083/jcb.96.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P, Silver J. Is astrocyte laminin involved in axon guidance in the mammalian CNS? Dev Biol. 1988;130:774–85. doi: 10.1016/0012-1606(88)90366-1. [DOI] [PubMed] [Google Scholar]
- Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–7. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- Min KJ, Yang MS, Kim SU, Jou I, Joe EH. Astrocytes induce hemeoxygenase-1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation. J Neurosci. 2006;26:1880–7. doi: 10.1523/JNEUROSCI.3696-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgello S, Uson RR, Schwartz EJ, Haber RS. The human blood-brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia. 1995;14:43–54. doi: 10.1002/glia.440140107. [DOI] [PubMed] [Google Scholar]
- Neugebauer KM, Tomaselli KJ, Lilien J, Reichardt LF. N-cadherin, NCAM, and integrins promote retinal neurite outgrowth on astrocytes in vitro. J Cell Biol. 1988;107:1177–87. doi: 10.1083/jcb.107.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, Ikeda E, Toyama Y, Yamanaka S, Nakamura M, Okano H. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A. 2011;108:16825–30. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829–34. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- Price J, Hynes RO. Astrocytes in culture synthesize and secrete a variant form of fibronectin. J Neurosci. 1985;5:2205–11. doi: 10.1523/JNEUROSCI.05-08-02205.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci U S A. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–86. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Roybon L, Lamas NJ, Garcia-Diaz A, Yang EJ, Sattler R, Jackson-Lewis V, Kim YA, Kachel CA, Rothstein JD, Przedborski S, Wichterle H, Henderson CE. Human stem cell-derived spinal cord astrocytes with defined mature or reactive phenotypes. Cell Rep. 2013;4:1035–48. doi: 10.1016/j.celrep.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge JS, Alderson RF, Pasnikowski E, McClain J, Ip NY, Lindsay RM. Expression of Ciliary Neurotrophic Factor and the Neurotrophins-Nerve Growth Factor, Brain-Derived Neurotrophic Factor and Neurotrophin 3-in Cultured Rat Hippocampal Astrocytes. Eur J Neurosci. 1992;4:459–471. doi: 10.1111/j.1460-9568.1992.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Sarafian TA, Montes C, Imura T, Qi J, Coppola G, Geschwind DH, Sofroniew MV. Disruption of astrocyte STAT3 signaling decreases mitochondrial function and increases oxidative stress in vitro. PLoS One. 2010;5:e9532. doi: 10.1371/journal.pone.0009532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A, Westergaard N, Sonnewald U, Petersen SB, Yu AC, Hertz L. Regulatory role of astrocytes for neuronal biosynthesis and homeostasis of glutamate and GABA. Prog Brain Res. 1992;94:199–211. doi: 10.1016/s0079-6123(08)61751-3. [DOI] [PubMed] [Google Scholar]
- Shih CH, Lacagnina M, Leuer-Bisciotti K, Proschel C. Astroglial-derived periostin promotes axonal regeneration after spinal cord injury. J Neurosci. 2014;34:2438–43. doi: 10.1523/JNEUROSCI.2947-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GM, Miller RH, Silver J. Changing role of forebrain astrocytes during development, regenerative failure, and induced regeneration upon transplantation. J Comp Neurol. 1986;251:23–43. doi: 10.1002/cne.902510103. [DOI] [PubMed] [Google Scholar]
- Smith GM, Rutishauser U, Silver J, Miller RH. Maturation of astrocytes in vitro alters the extent and molecular basis of neurite outgrowth. Dev Biol. 1990;138:377–90. doi: 10.1016/0012-1606(90)90204-v. [DOI] [PubMed] [Google Scholar]
- Smith GM, Miller RH. Immature type-1 astrocytes suppress glial scar formation, are motile and interact with blood vessels. Brain Res. 1991;543:111–22. doi: 10.1016/0006-8993(91)91054-5. [DOI] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–30. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–47. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struzynska L, Bubko I, Walski M, Rafalowska U. Astroglial reaction during the early phase of acute lead toxicity in the adult rat brain. Toxicology. 2001;165:121–31. doi: 10.1016/s0300-483x(01)00415-2. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr Mol Med. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- Takano T, Kang J, Jaiswal JK, Simon SM, Lin JH, Yu Y, Li Y, Yang J, Dienel G, Zielke HR, Nedergaard M. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc Natl Acad Sci U S A. 2005;102:16466–71. doi: 10.1073/pnas.0506382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99:3024–9. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli KJ, Neugebauer KM, Bixby JL, Lilien J, Reichardt LF. N-cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron. 1988;1:33–43. doi: 10.1016/0896-6273(88)90207-3. [DOI] [PubMed] [Google Scholar]
- Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, Kitamura K, Kumagai G, Nishino M, Tomisato S, Higashi H, Nagai T, Katoh H, Kohda K, Matsuzaki Y, Yuzaki M, Ikeda E, Toyama Y, Nakamura M, Yamanaka S, Okano H. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:12704–9. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol. 2008;86:342–67. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GM, Catlin G, Cossins JA, Mangan M, Ward GA, Miller KM, Clements JM. Quantitation of matrix metalloproteinases in cultured rat astrocytes using the polymerase chain reaction with a multi-competitor cDNA standard. Glia. 1996;18:332–40. [PubMed] [Google Scholar]
- Wender R, Brown AM, Fern R, Swanson RA, Farrell K, Ransom BR. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J Neurosci. 2000;20:6804–10. doi: 10.1523/JNEUROSCI.20-18-06804.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard N, Sonnewald U, Schousboe A. Metabolic trafficking between neurons and astrocytes: the glutamate/glutamine cycle revisited. Dev Neurosci. 1995;17:203–11. doi: 10.1159/000111288. [DOI] [PubMed] [Google Scholar]
- Zador Z, Stiver S, Wang V, Manley GT. Role of aquaporin-4 in cerebral edema and stroke. Handb Exp Pharmacol. 2009:159–70. doi: 10.1007/978-3-540-79885-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]