Abstract
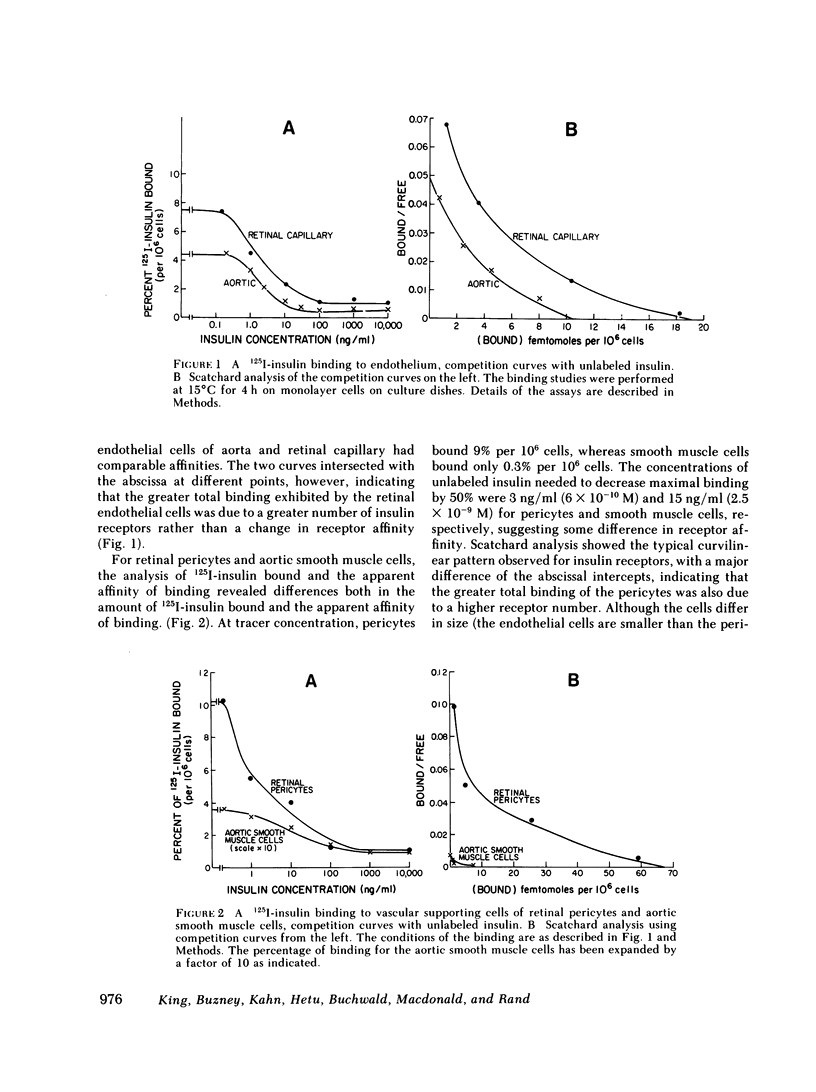
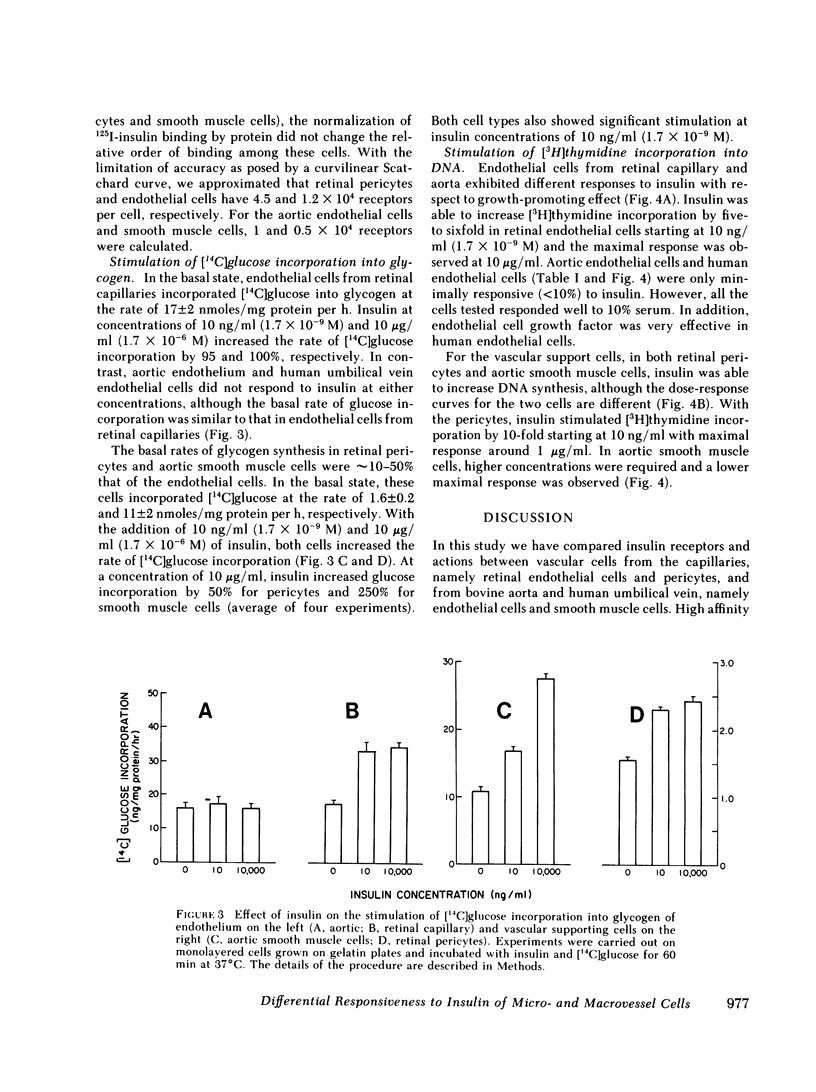
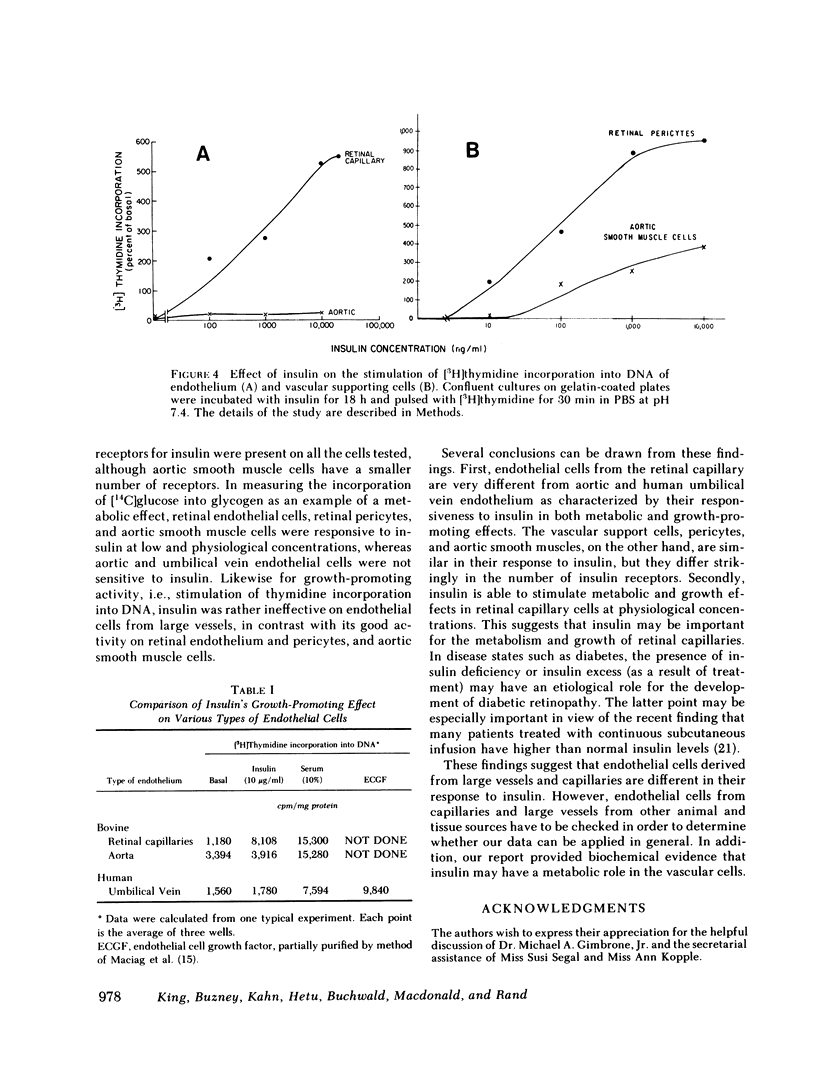
The pathologies of diabetic micro- and macroangiopathy are different, suggesting that diabetes affects these two types of vascular tissue in a dissimilar manner. We have compared insulin receptors and the effects of insulin on cultured endothelium from calf retinal capillaries and aorta, and the vascular supporting cells, retinal pericytes, and aortic smooth muscle cells. 125I-insulin binds to high affinity insulin receptors on all four cell types. Receptor concentrations were similar except for aortic smooth muscle cells, which have 10-fold fewer receptors than the other cell types. Insulin at a concentration of 10 ng/ml stimulated [14C]glucose incorporation into glycogen in retinal endothelial cells and pericytes and aortic smooth muscle cells, but had no effect on aortic endothelium. Insulin over a concentration range of 10 ng/ml-10 microgram/ml, stimulated [3H]thymidine incorporation into the DNA of retinal pericytes, and endothelial cells and aortic smooth muscle cells but had no effect on aortic endothelial cells. These data suggested that a differential response to insulin may exist between endothelium of micro- and macrovasculature, and suggest that retinal capillary endothelium and retinal pericytes are both very insulin-sensitive tissues.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buzney S. M., Massicotte S. J. Retinal vessels: proliferation of endothelium in vitro. Invest Ophthalmol Vis Sci. 1979 Nov;18(11):1191–1195. [PubMed] [Google Scholar]
- COGAN D. G., TOUSSAINT D., KUWABARA T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol. 1961 Sep;66:366–378. doi: 10.1001/archopht.1961.00960010368014. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr The Banting Memorial Lecture 1971. Physiology of insulin in man. Diabetes. 1971 Dec;20(12):785–799. doi: 10.2337/diab.20.12.785. [DOI] [PubMed] [Google Scholar]
- Cuendet G. S., Loten E. G., Jeanrenaud B., Renold A. E. Decreased basal, noninsulin-stimulated glucose uptake and metabolism by skeletal soleus muscle isolated from obese-hyperglycemic (ob/ob) mice. J Clin Invest. 1976 Nov;58(5):1078–1088. doi: 10.1172/JCI108559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P. Molecular basis of insulin action. Annu Rev Biochem. 1977;46:359–384. doi: 10.1146/annurev.bi.46.070177.002043. [DOI] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. C., Zetter B. R. Long-term culture of capillary endothelial cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank H. J., Pardridge W. M. A direct in vitro demonstration of insulin binding to isolated brain microvessels. Diabetes. 1981 Sep;30(9):757–761. doi: 10.2337/diab.30.9.757. [DOI] [PubMed] [Google Scholar]
- Freychet P., Kahn R., Roth J., Neville D. M., Jr Insulin interactions with liver plasma membranes. Independence of binding of the hormone and its degradation. J Biol Chem. 1972 Jun 25;247(12):3953–3961. [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974 Mar;60(3):673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S. Human vascular smooth muscle in culture. Growth and ultrastructure. Lab Invest. 1975 Jul;33(1):16–27. [PubMed] [Google Scholar]
- Hanna A. K., Zinman B., Nakhooda A. F., Minuk H. L., Stokes E. F., Albisser A. M., Leibel B. S., Marliss E. B. Insulin, glucagon, and amino acids during glycemic control by the artificial pancreas in diabetic man. Metabolism. 1980 Apr;29(4):321–332. doi: 10.1016/0026-0495(80)90005-0. [DOI] [PubMed] [Google Scholar]
- King G. L., Kahn C. R., Rechler M. M., Nissley S. P. Direct demonstration of separate receptors for growth and metabolic activities of insulin and multiplication-stimulating activity (an insulinlike growth factor) using antibodies to the insulin receptor. J Clin Invest. 1980 Jul;66(1):130–140. doi: 10.1172/JCI109826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maciag T., Hoover G. A., Stemerman M. B., Weinstein R. Serial propagation of human endothelial cells in vitro. J Cell Biol. 1981 Nov;91(2 Pt 1):420–426. doi: 10.1083/jcb.91.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock M. L., Bar R. S., Goldsmith J. Interactions of insulin with bovine endothelium. Metabolism. 1982 Jan;31(1):52–56. [PubMed] [Google Scholar]
- Rand L. I. Recent advances in diabetic retinopathy. Am J Med. 1981 Mar;70(3):595–602. doi: 10.1016/0002-9343(81)90581-7. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Podskalny J. M., Goldfine I. D., Wells C. A. DNA synthesis in human fibroblasts: stimulation by insulin and by nonsuppressible insulin-like activity (NSILA-S). J Clin Endocrinol Metab. 1974 Sep;39(3):512–521. doi: 10.1210/jcem-39-3-512. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M. Selection and characterization of bovine aortic endothelial cells. In Vitro. 1978 Dec;14(12):966–980. doi: 10.1007/BF02616210. [DOI] [PubMed] [Google Scholar]
- Zetter B. R. The endothelial cells of large and small blood vessels. Diabetes. 1981;30(Suppl 2):24–28. doi: 10.2337/diab.30.2.s24. [DOI] [PubMed] [Google Scholar]


