Abstract
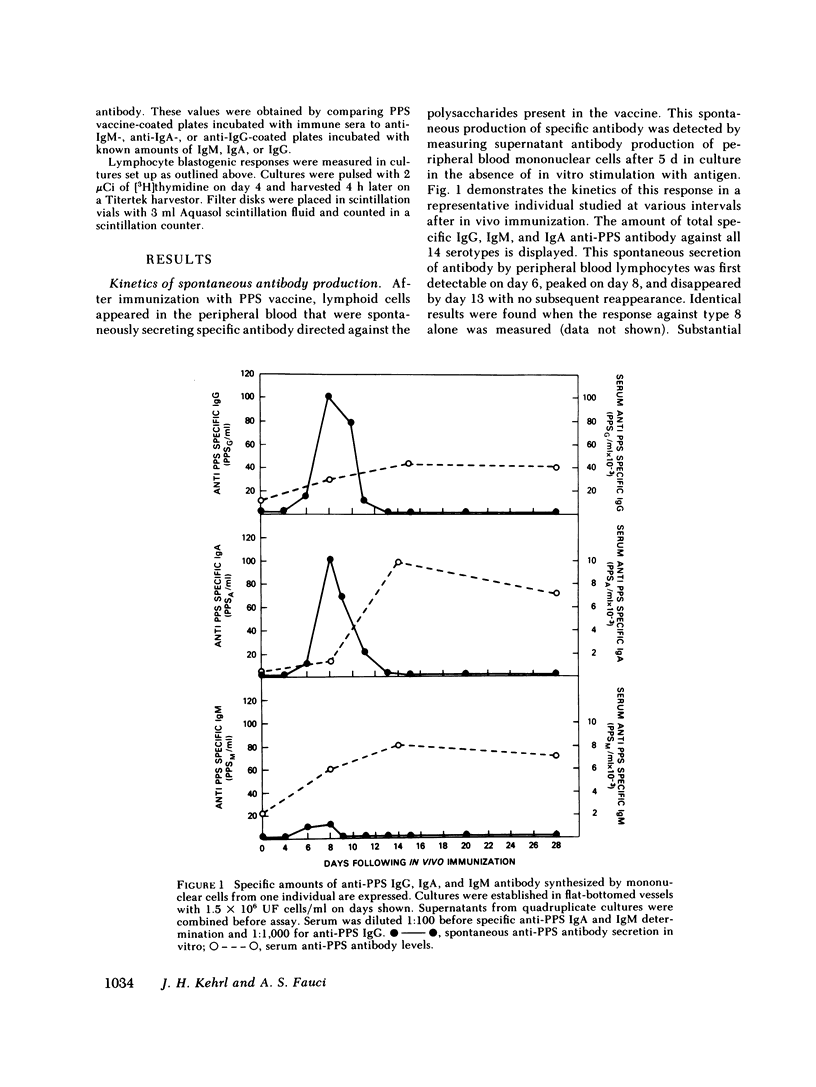
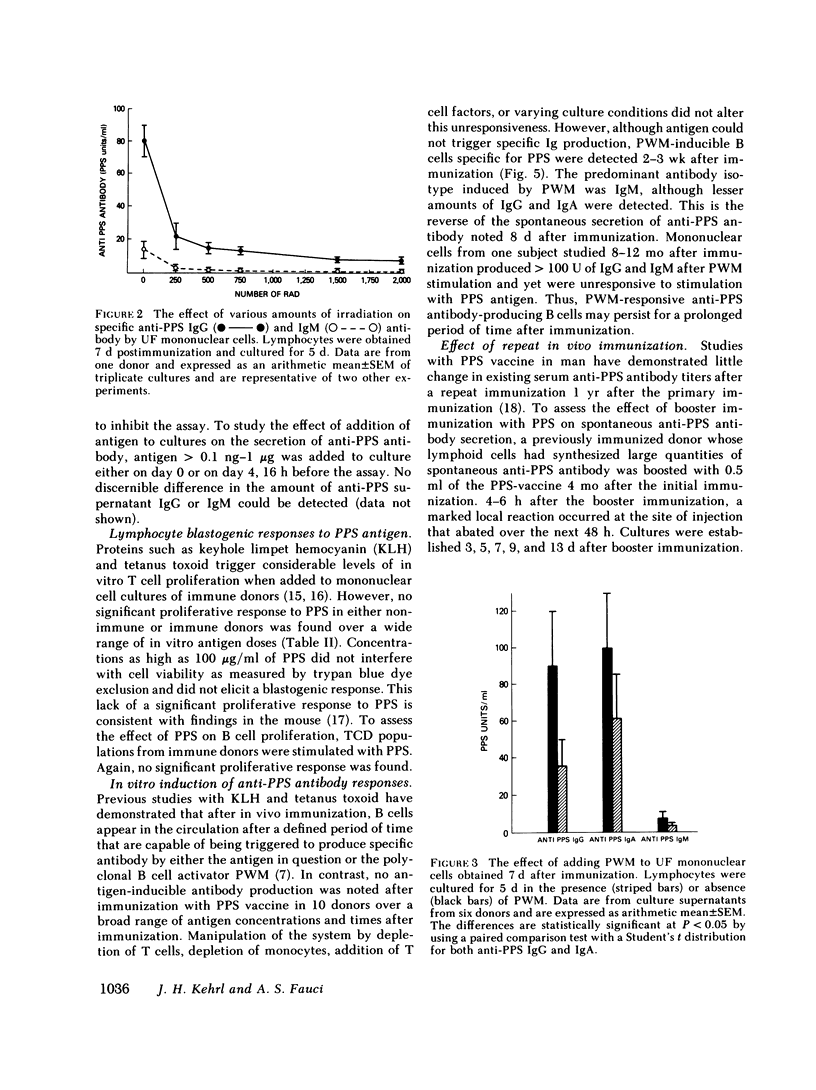
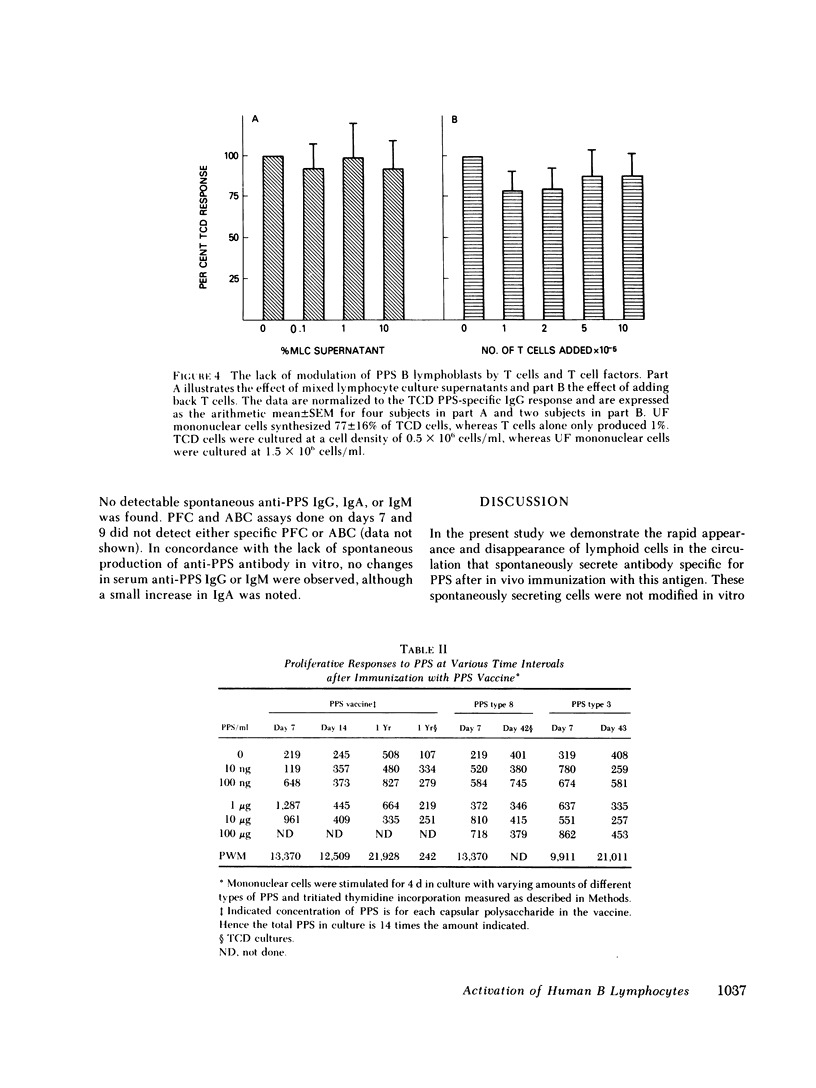
The in vivo and in vitro immune response after in vivo immunization with pneumococcal polysaccharides (PPS) has been analyzed in man. Substantial differences were noted in this system when compared with human responses to soluble protein antigens. Within 6 d after immunization, specific PPS antigen-binding cells (ABC), specific plaque-forming cells (PFC), and cells capable of spontaneously synthesizing in vitro large amounts of specific anti-PPS immunoglobulin (Ig) G. IgA, and lesser amounts of specific IgM appeared in the peripheral blood. The ABC, PFC, and the total amount of specific spontaneous antibody production followed nearly identical kinetics after immunization. Low doses of irradiation markedly inhibited spontaneous anti-PPS antibody production by lymphocytes obtained 7 or 8 d after immunization, suggesting a requirement for in vitro proliferation for full expression of antibody-secreting capability of these cells that are activated in vivo and are capable of spontaneous antibody production in vitro. Spontaneous secretion by B lymphocytes in vitro was independent of T cells, unmodified by the addition of T cell factors, and readily suppressible by pokeweed mitogen (PWM).
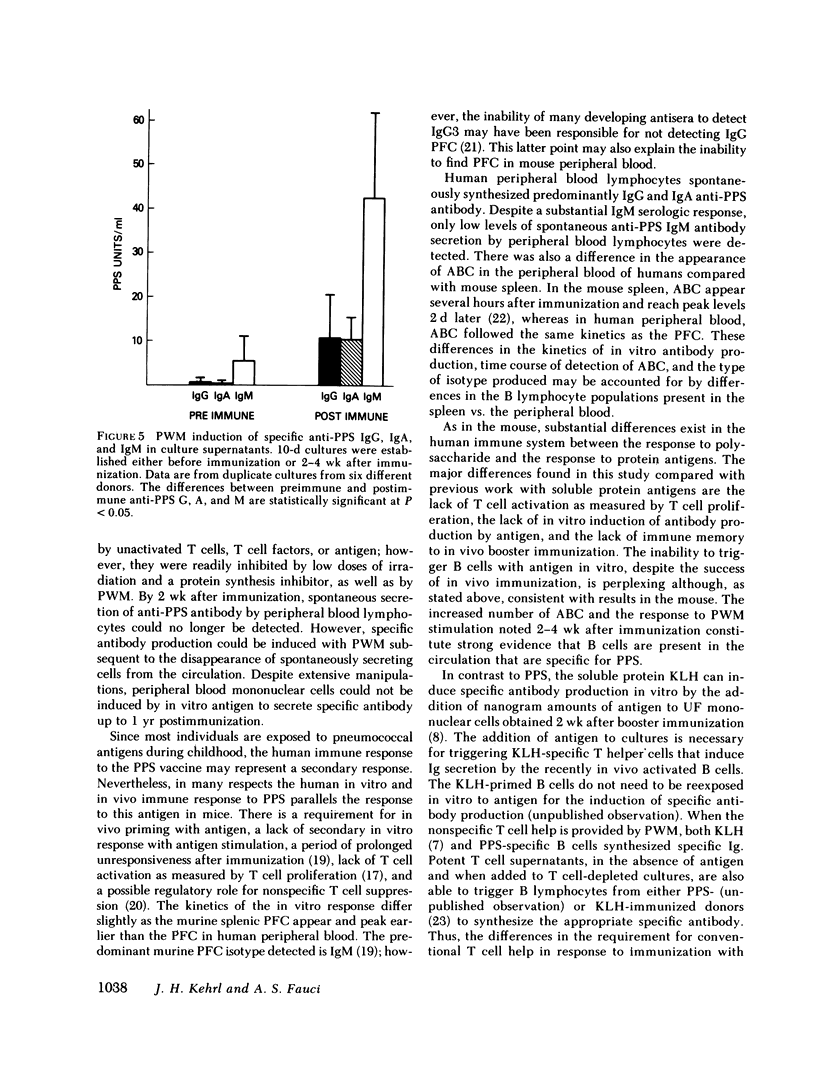
By 2 wk after immunization, spontaneous anti-PPS antibody production in vitro was no longer detected. Subsequent stimulation of lymphocytes in culture with a wide range of concentrations of specific antigen did not trigger either proliferation or specific antibody synthesis. Despite the unresponsiveness of these cells to antigenic stimulation at this time, they were capable of specific antiPPS antibody production after stimulation with PWM. In vivo booster immunization 4 mo after an initial immunization did not reproduce the increased numbers of ABC, PFC, or in vitro specific antibody production that had been found 4 mo earlier. The dichotomy in capacity for activation of PPS-specific B cells by PWM vs. specific antigen, and the in vivo and in vitro unresponsiveness to in vivo booster immunization with PPS, contrast sharply with previous studies in man with soluble protein antigens such as keyhole limpet hemocyanin and tetanus toxoid. Furthermore, the lack of T cell activation by PPS also contrasts with previous results with tetanus toxoid and other protein antigens. This system should prove useful in delineating certain aspects of human B cell physiology not readily approachable with standard soluble protein antigens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsbaugh D. F., Hansen C. T., Prescott B., Stashak P. W., Barthold D. R., Baker P. J. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J Exp Med. 1972 Oct 1;136(4):931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Landy M. Brevity of the inductive phase in the immune response of mice to capsular polysaccharide antigens. J Immunol. 1967 Oct;99(4):687–694. [PubMed] [Google Scholar]
- Baker P. J., Reed N. D., Stashak P. W., Amsbaugh D. F., Prescott B. Regulation of the antibody response to type 3 pneumococcal polysaccharide. I. Nature of regulatory cells. J Exp Med. 1973 Jun 1;137(6):1431–1441. doi: 10.1084/jem.137.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Stashak P. W., Amsbaugh D. F., Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose-response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971 Apr;20(4):469–480. [PMC free article] [PubMed] [Google Scholar]
- Benner R., Hijmans W., Haaijman J. J. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin Exp Immunol. 1981 Oct;46(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Braley-Mullen H. Antigen requirements for priming of IgG producing B memory cells specific for Type III pneumococcal polysaccharide. Immunology. 1980 Aug;40(4):521–527. [PMC free article] [PubMed] [Google Scholar]
- Carlson A. J., Davidson W. L., McLean A. A., Vella P. P., Weibel R. E., Woodhour A. F., Hilleman M. R. Pneumococcal vaccine: dose, revaccination, and coadministration with influenza vaccine. Proc Soc Exp Biol Med. 1979 Sep;161(4):558–563. doi: 10.3181/00379727-161-40596. [DOI] [PubMed] [Google Scholar]
- Curtis J. E., Hersh E. M., Harris J. E., McBride C., Freireich E. J. The human primary immune response to keyhole limpet haemocyanin: interrelationships of delayed hypersensitivity, antibody response and in vitro blast transformation. Clin Exp Immunol. 1970 Apr;6(4):473–491. [PMC free article] [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Role of T lymphocytes in the humoral immune response. I. Proliferation of B lymphocytes in thymus-deprived mice. J Immunol. 1974 Nov;113(5):1438–1445. [PubMed] [Google Scholar]
- Dresser D. W., Popham A. M. The influence of T cells on the initiation and expression of immunological memory. Immunology. 1979 Oct;38(2):265–274. [PMC free article] [PubMed] [Google Scholar]
- Falkoff R. J., Zhu L. P., Fauci A. S. Separate signals for human B cell proliferation and differentiation in response to Staphylococcus aureus: evidence for a two-signal model of B cell activation. J Immunol. 1982 Jul;129(1):97–102. [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. Alternate-day prednisone therapy and human lymphocyte subpopulations. J Clin Invest. 1975 Jan;55(1):22–32. doi: 10.1172/JCI107914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Whalen G., Burch C. Activation of human B lymphocytes XVI. Cellular requirements, interactions, and immunoregulation of pokeweed mitogen-induced total-immunoglobulin producing plaque-forming cells in peripheral blood. Cell Immunol. 1980 Aug 15;54(1):230–240. doi: 10.1016/0008-8749(80)90204-x. [DOI] [PubMed] [Google Scholar]
- Geha R. S., Merler E. Response of human thymus-derived (T) and non-thymus-derived (B) lymphocytes to mitogenic stimulation in vitro. Eur J Immunol. 1974 Mar;4(3):193–199. doi: 10.1002/eji.1830040308. [DOI] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Hosea S. W., Burch C. G., Brown E. J., Berg R. A., Frank M. M. Impaired immune response of splenectomised patients to polyvalent pneumococcal vaccine. Lancet. 1981 Apr 11;1(8224):804–807. doi: 10.1016/s0140-6736(81)92681-7. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Volkman D. J., Whalen G., Fauci A. S. In vitro antigen-induced, antigen-specific antibody production in man. Specific and polyclonal components, kinetics, and cellular requirements. J Exp Med. 1981 Oct 1;154(4):1043–1057. doi: 10.1084/jem.154.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Zitron I. M., Mond J. J., Ahmed A., Scher I., Paul W. E. Surface immunoglobulin D as a functional receptor for a subclass of B lymphocytes. Immunol Rev. 1977;37:89–104. doi: 10.1111/j.1600-065x.1977.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Musson R. A., Henson P. M. Humoral and formed elements of blood modulate the response of peripheral blood monocytes. I. Plasma and serum inhibit and platelets enhance monocyte adherence. J Immunol. 1979 May;122(5):2026–2031. [PubMed] [Google Scholar]
- Paul W. E., Siskind G. W., Benacerraf B., Ovary Z. Secondary antibody responses in haptenic systems: cell population selection by antigen. J Immunol. 1967 Oct;99(4):760–770. [PubMed] [Google Scholar]
- Peltola H., Käyhty H., Sivonen A., Mäkelä H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977 Nov;60(5):730–737. [PubMed] [Google Scholar]
- Perlmutter R. M., Hansburg D., Briles D. E., Nicolotti R. A., Davie J. M. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978 Aug;121(2):566–572. [PubMed] [Google Scholar]
- Peters M., Fauci A. S. Selective activation of antigen-specific human B cells in recently immunized individuals by nonspecific factors in the absence of antigen. J Immunol. 1983 Feb;130(2):678–680. [PubMed] [Google Scholar]
- Sharon R., McMaster P. R., Kask A. M., Owens J. D., Paul W. E. DNP-Lys-ficoll: a T-independent antigen which elicits both IgM and IgG anti-DNP antibody-secreting cells. J Immunol. 1975 May;114(5):1585–1589. [PubMed] [Google Scholar]
- Slack J., Der-Balian G. P., Nahm M., Davie J. M. Subclass restriction of murine antibodies. II. The IgG plaque-forming cell response to thymus-independent type 1 and type 2 antigens in normal mice and mice expressing an X-linked immunodeficiency. J Exp Med. 1980 Apr 1;151(4):853–862. doi: 10.1084/jem.151.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. H., Macy E., Morrow C., Saxon A. Characterization of a circulating subpopulation of spontaneous antitetanus toxoid antibody producing B cells following in vivo booster immunization. J Immunol. 1979 Jun;122(6):2498–2504. [PubMed] [Google Scholar]
- Stevens R. H., Saxon A. Antigen-induced suppression of human in vitro pokeweed mitogen-stimulated antibody production. Cell Immunol. 1980 Sep 15;55(1):85–93. doi: 10.1016/0008-8749(80)90139-2. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Saxon A. Immunoregulation in humans: control of antitetanus toxoid antibody production after booster immunization. J Clin Invest. 1978 Dec;62(6):1154–1160. doi: 10.1172/JCI109234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. H., Saxon A. Reduced in vitro production of anti-tetanus toxoid antibody after repeated in vivo immunization with tetanus toxoid. J Immunol. 1979 Feb;122(2):592–598. [PubMed] [Google Scholar]
- Thomson P. D., Harris N. S. Detection of plaque-forming cells in the peripheral blood of actively immunized humans. J Immunol. 1977 Apr;118(4):1480–1482. [PubMed] [Google Scholar]
- Volkman D. J., Allyn S. P., Fauci A. S. Antigen-induced in vitro antibody production in humans: tetanus toxoid-specific antibody synthesis. J Immunol. 1982 Jul;129(1):107–112. [PubMed] [Google Scholar]
- Volkman D. J., Lane H. C., Fauci A. S. Antigen-induced in vitro antibody production in humans: a model for B cell activation and immunoregulation. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2528–2531. doi: 10.1073/pnas.78.4.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan R., Murphy B. R., Strober W., Clements M. L., Nelson D. L. In vitro production of anti-influenza virus antibody after intranasal inoculation with cold-adapted influenza virus. J Immunol. 1981 Nov;127(5):1958–1963. [PubMed] [Google Scholar]
- Yount W. J., Dorner M. M., Kunkel H. G., Kabat E. A. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968 Mar 1;127(3):633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]


