Abstract
Background
Neuro-oncologists are familiar with primary brain tumors, intracerebral metastases meningeal carcinomatosis and extracerebral intracranial tumors as meningeoma. For these conditions, and also some other rare tumor entities several treatment options exist. Cancer can also involve structures around the brain as the dura, the base of the skull, the cavities of the skull and tissue around the bony skull, the skin, the tissue of the neck. and either compress, invade or spread in the central or peripheral nervous system.
Methods
A systematic literature research was conducted determining symptoms and signs, tumor sites of nerve invasion, tumor types, diagnostic techniques, mechanisms of nerve invasion, and important differential diagnosis. Additional cases from own experience were added for illustration.
Results
The mechanisms of tumor invasion of cranial nerves is heterogenous and not only involves several types of invasion, but also spread along the cranial nerves in antero- and retrograde fashion and even spread into different nerve territories via anastomosis. In addition the concept of angiosomas may have an influence on the spread of metastases.
Conclusion
In addition to the well described tumor spread in meningeal carcinomatosis and base of the skull metastases, dural spread, lesions of the bony skull, the cavities of the skull and skin of the face and tissue of the neck region need to be considered, and have an impact on therapeutic decisions.
Keywords: anterograde and retrograde spread, base of the skull metastasis, cancer metastasis, cranial nerve infiltration
Neuro-oncologists are familiar with primary brain tumors and extracerebral intracranial tumors such as meningioma, intracerebral metastases, and meningeal carcinomatosis. Many treatment options exist for these more commonly observed conditions as well as for some of the rarer tumor entities within the brain. However, cancer that affects the nervous system may also involve structures external to the brain including the dura, the base of the skull, the cavities and tissue around the bony skull, the skin, and the tissue of the neck where the tumors compress, invade, or spread into the central or peripheral nervous system. This review focuses on different types of tumor entities, sites of tumor growth, detection by imaging, and mechanisms of tumor growth in and along the cranial nerves (CNs) as well as the differential diagnosis of other conditions that must be distinguished from malignancy.
Neurological symptoms and signs of cancer around the brain differ depending upon the site. Metastasis along the base of the skull can cause varied symptoms characterized as a combination of CN lesions and pain, metastasis to the calvaria or dura, and unspecific headache symptoms or remain asymptomatic. Tumors in the neck can affect the extensive anastomotic caudal CNs and cause pain projection into the skull, which can be a misleading sign.
Tumor sites around the central nervous system are not confined to bone but also occur in the skin, the glands (lacrimal, parotid), the palate, the ear/nose/throat (ENT) regions, the orbits, and the tissues of the neck. The incidence of perineural invasion is estimated to occur in 2%–5% of ENT tumors. Most commonly in such cases, the nerves CN V and CN VII are affected by nerve infiltration, but other CNs can be affected. Anatomical studies show that the rich anastomosis of the CNs also enables perineurial tumor spread from one CN territory into another. Apart from the spread of local tumors of the skull, breast, prostate, and lung cancers as well as myeloma seem to be the most frequent tumors that metastasize into the skull and damage the CNs.
For these reasons, diagnosis must not only be based on clinical signs and symptoms but also on imaging such as CT, MRI, and combinations of CT and PET. Ultrasound and electrophysiology may be helpful in some accessible sites. Cerebrospinal fluid (CSF) studies are needed to exclude meningeal spread, and nerve and tissue biopsies are often necessary to confirm the diagnosis.
Nerve damage from these conditions is not limited to compression and invasion but may also involve other mechanisms including perineurial spread, dermatomal spread, the sprouting of nerves in tissue invasion, and other factors that impact tumor growth within the nerves. The involvement of angiosomes, in which a 3-dimensional combination of tissue structures around the vasculature acts jointly to distribute the cancer, is an interesting possibility. Treatment options for these conditions depend upon the site, tumor type, operability, and radio-chemotherapeutic approach.
Symptoms and Signs
Symptoms and signs depend upon the site of the lesion. Calvarial metastases can be diffuse or circumscribed.1 Dural metastases can appear at several sites (Fig. 1) and are often asymptomatic or have unspecific symptoms.2 Metastases occurring at the base of the skull are typically accompanied by a combination of pain and CN symptoms.3,4
Fig. 1.
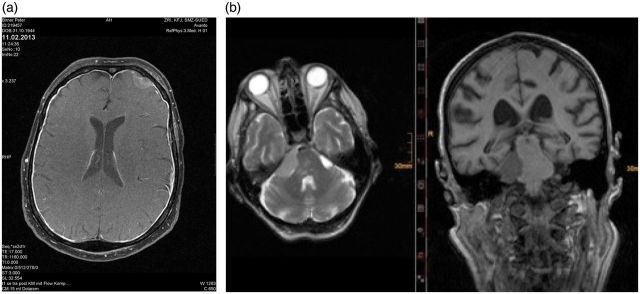
Dural metastasis can present in different locations. (a) A dural metastasis from prostate cancer. (b) A dural metastasis mimicking meningeoma, also from prostate cancer.
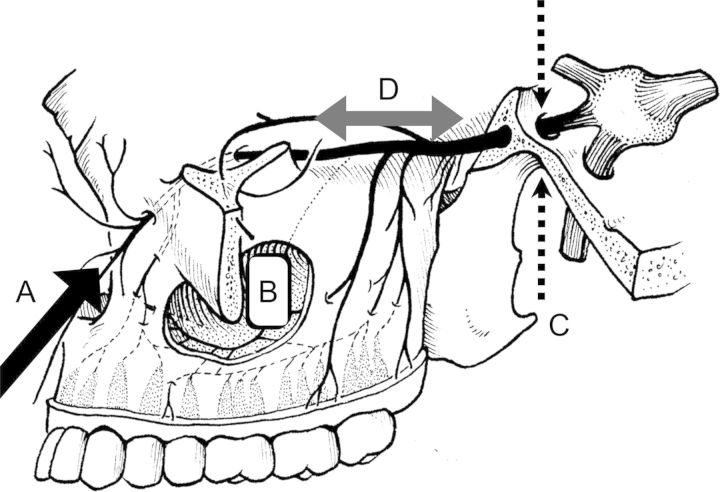
Infiltration of the CNs by perineural spread is often initially asymptomatic and takes time to manifest itself clinically. In the case of perineural invasion, the trigeminal (V2) and facial nerves (VII) seem to be the most frequently affected, although all CNs can be affected. (Fig. 2 and Supplementary Fig. S2). Tumor spread can also occur between the individual nerve territories. One example is the auriculotemporal nerve, which allows tumor spread from CN V to CN VII. The typical presentation of CN infiltration is focal CN V or CN VII dysfunction, resulting in pain, sensory symptoms, or focal motor deficit. Centripetal spread via the trigeminal nerve into the cavernous sinus can occur,5 which results in lesions that can impact extraocular muscle function by affecting branches of the oculomotor nerves and causing facial pain. Infiltration of the caudal CN, including the vagal, accessory, and hypoglossal nerves, is characteristically located either at the site of their exit from the skull or within their course outside of the skull.
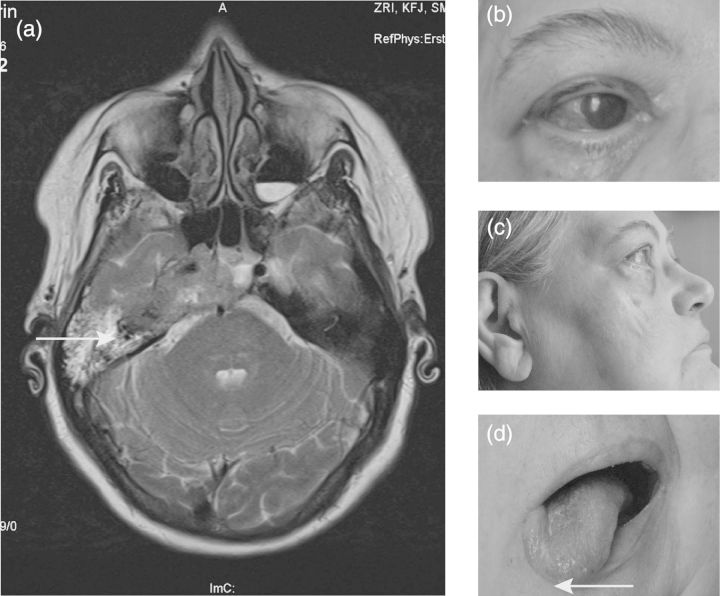
Fig. 2.
Clinical findings of base of the skull metastasis: (a) MRI showing destruction at the base of the skull (arrow). Possible infiltration of cerebral parenchyma. (b) Lesion of CN V 1; reduced blinking and chronic infection resulted. (c) Visible atrophy of masseteric muscle. Chin also moved to the right side when opening the mouth (arrow). (d) CN XII paresis without atrophy at this time.
The pattern of CN lesions accompanying metastases in the base of the skull depends upon the site and nature of the damage. Classically, 5 typical syndromes have been described by Greenberg3 and were recently updated by Mitusuya.1 The inclusion of the controversial anatomical concept of angiosoma6 may prove to be helpful in diagnosis and treatment. Angiosomas divide the tissue compartments of the head and skull according to their vascularization in contrast to the more traditional way of viewing the skull by the distribution of CN nerve trunks and vessels.
ENT tumors and cancer in the neck predominately affect caudal CNs and result in pain that is often projected into the head, hoarseness, swallowing difficulties, and atrophying of the tongue (Fig. 3 and Supplementary Fig. S3).
Fig. 3.
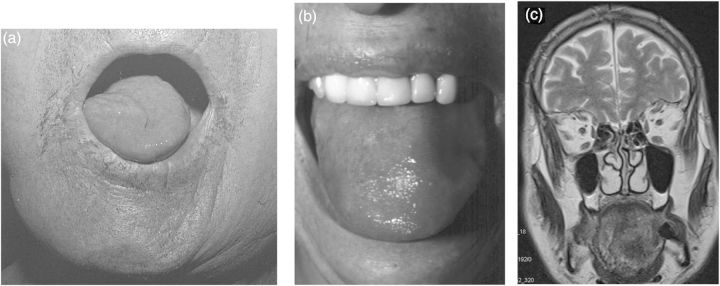
Different types of tongue involvement. (a) Unilateral peripheral CN XII paresis with atrophy in a patient with leptomeningeal carcinomatosis. (b) “Amyloid tongue“ in a patient with paraproteinemia. (c) Local tongue tumor, presenting as a suspected CN XII lesion.
A summary of tumor locations, defined subgroups and CNs, is given on Table 1.
Table 1.
Metastasis around the brain
| Site of metastasis | Subgroups | Involvement of CNs | CN Infiltration |
|---|---|---|---|
| Calvarial metastases | |||
| Dural metastases | (+) | ||
| (Meningeal carcinomatosis) | (Not subject to this review, but meningeal carcinomatosis can be associated with nerve lesions and base of the skull metastases) | ||
| Base of the skull syndromes | Orbital | + | In addition to local compression and infiltration, anterograde and retrograde spread can also occur. |
| Parasellar | |||
| Middle fossa | |||
| Jugular | |||
| Occipital | |||
| Cerbellopontine angle | |||
| Foramen magnum | |||
| Other regions | Orbit | (+) | Invasion into the inner skull by the natural foramina |
| ENT cavities | |||
| Oral cavity | |||
| Miscellaneous | |||
| Skin | (+) | Anterograde and retrograde spread possible | |
| Head and neck tumors | Often local pain syndromes, radiating into the skull. |
Abbreviation: CN, cranial nerve.
Tumor Sites
The most common site of metastases for the cases we are describing is the bony skull, where the calvaria and the base of the skull are most often affected. Dural metastasis occurs less frequently and may mimic subdural hematoma or be associated with effusions.7 In rare cases, dural metastases can appear in the posterior fossa and thus resemble meningioma. A number of tumors spread through cavities such as the nasal cavity, the orbit,8 the oral cavity, and the soft palate9 as well as through other locations including the eyelids,10 the lacrimal glands, the parotid, and several locations in the neck.11 These tumors, in addition to causing symptoms as a consequence of compression, can invade or spread into CNs either anteriorly or in a retrograde fashion. The centrifugal spread of tumors into a dermatomal skin distribution has been described as zosteriform metastasis in melanoma.12 Tumor growth in the skull can also result in the formation of cirrhotic tissue, which could in turn result in enophtalmos in the orbit.13 Local metastasis in the cavernous sinus, the sphenoid bone, and the jugular foramen often causes multiple CN palsies.14 In some rare cases, primary brain tumors may infiltrate the cavernous sinus (Fig. 4 and Supplementary Fig. S4).
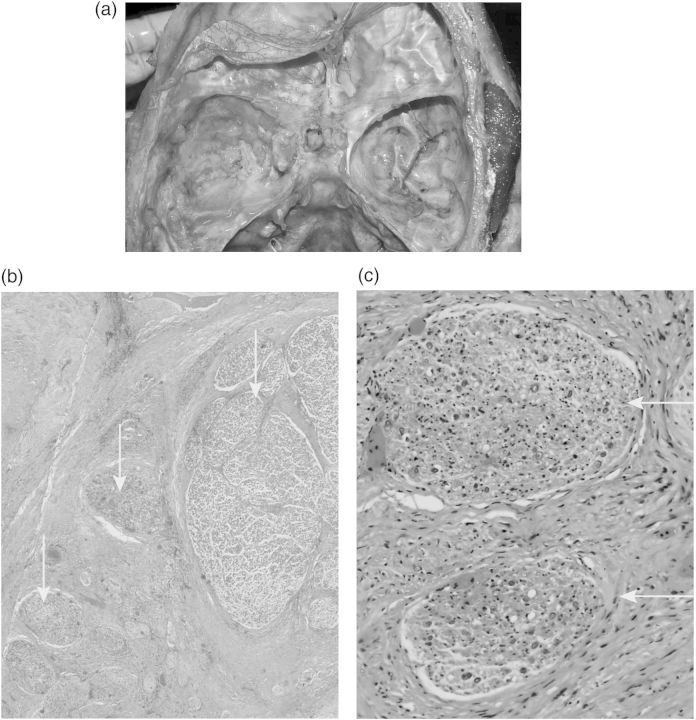
Fig. 4.
Glioma infiltration of nerves in the cavernous sinus. (a) Macroscopic site of the middle cranial fossa and cavernous sinus at autopsy. (b) Nerve twigs from the cavernous sinus. Grey arrow: large fascicle with signs of nerve damage, locally infiltrated nerve twigs. (c) Nerve twig with disarranged tissue (black arrow), suspected infiltration (red arrow).
Tumors
In addition to local tumors, particularly those with an ENT origin,9 several other cancers such as lung, breast, renal, and prostate may metastasize at the base of the skull and result in single or multiple CN palsies. Likewise, hematological malignancies, including lymphoma and multiple myeloma, can metastasize into the skull. Local tumors, including ENT cancers, oral cancers, skin cancer, and melanoma, can spread along the CNs in addition to their local spread.15–19
Usually, the patients who experience the conditions we are describing are aged 50 years and older. A number of these patients may also have brain parenchymal metastases. In the base of the skull, those metastases can be circumscribed or diffusely invasive.
The incidence of perineural tumor spread ranges from 2.5%–5.0% and may occur with a wide variety of head and neck malignancies. In particular, adenoid cystic carcinoma seems to have a high propensity for perineurial spread.20 Cancer of the salivary gland has a nerve invasion rate of up to 60%.21 In melanoma, the infiltration of CN V1 and V2 and multiple CN involvement implies a negative prognosis and is accompanied by a high rate of relapse after treatment.22 In addition to metastatic tumors, malignant nerve-sheath tumors can occur in the skull23 and affect the CNs.
Life expectancy following a diagnosis of metastatic skull tumor resulting from cancer of the prostate is around 2 years, and 1.5 years from breast cancer, 6 months from malignant lymphoma, and 5 months from lung cancer. The incidence is difficult to estimate, as the references originate from different tumor types and tumor locations and are often based on case reports or small series only.
In general, the pathology of nerve invasion of tumors that originate from the pancreas or the prostate is a prognostic factor and may be a key factor in predicting the outcome. Reciprocal signaling interactions between tumor cells and nerves may contribute to tumor spread along nerves.24–26
Diagnostics
The most popular diagnostic approaches for the cases we are discussing include CT, MRI, and the combination of imaging methods such as PET CT.27 New techniques such as MR neurography will likely prove to be helpful.28 Ultrasound can be useful, especially in approachable areas of the skull and neck.29
Indirect diagnostic evidence includes structural lesions, particularly those of the bone, enlargement of foramina, thickening of nerves, and muscle atrophies or muscle tissue changes due to denervation.14 Angiography and MR angiography are useful for assessing the vascular structure, which is necessary to assess the feasibility of surgical or vascular intervention.
An MRI of nerves affected by perineural tumor spread usually shows breakdown of the blood-nerve barrier and leakage of contrast media, which may become apparent even before any thickening of the affected nerves.30 Once the diameter of the nerves has increased, the perineurial fat tissue (particularly that at the foraminal openings) tends to decrease.
The muscle groups of the head can be divided into muscles of mastication,31 tongue and floor of the mouth muscles, ocular muscles, superficial sternocleidomastoid and trapezius muscles, posterior neck muscles, and anterior neck and prevertebral muscles. When motor branches are affected, muscle signal changes that may indicate a CN lesion can be detected in MRIs.32 Over a longer time, fatty infiltration of the muscle occurs. In the chronic phase, muscle atrophy can be detected as hyperintense signals on T1 and in fast spin-echo sequences.
Other methods of investigation include fine needle and open biopsies, surgical explorations, and CSF examinations, especially if meningeal involvement is suspected. When building a differential diagnosis, the search for infections, immune-mediated diseases, and generalized diseases such as neurofibromatosis must be considered.
Mechanisms of Nerve Invasion
Damage to the CNs can result from several mechanical factors such as compression, engulfing, stretching, and pushing. (Table 2, Figs. 5–7) Even without infiltration by malignant cells, these mechanical factors may result in pain, sensory loss, or nerve dysfunction. Direct infiltration of the CNs usually occurs in the vicinity of local mass lesions. The type of spread can be epineural, perineural, or endoneural. In rare cases of lymphoma, an intravascular tumor spread may occur,33 or CN infiltration may result in neurolymphomatosis.34,35 In squamous cell carcinomas of the head and neck, the tumor spread is usually contiguous; skip lesions do not seem to occur,36 as they do in parotid tumors.20 In rare cases of primary brain tumors, continuous nerve infiltration can occur in the cavernous sinus,37 along the CNs, and continuously into the bone and surrounding tissue.
Table 2.
Mechanisms of nerve invasion: several mechanisms of nerve involvement are known and vary from mechanical lesions to different oncological patterns
| Type of nerve growth | Subgroups | Remarks |
|---|---|---|
| Mechanical causes | Compression | |
| Engulfing | ||
| Pushing and stretching of the nerve by mass lesion | ||
| Invasion | Direct invasion (local infiltration) | |
| Perineural | ||
| Endoneurial | ||
| Intravascular spread | ||
| Metastasis | Isolated intranerval (rare) | |
| Perineurial spread | Anterograde | |
| Retrograde | ||
| Particular patterns: | ||
| Spread via nerve scaffolds | ||
| Dermatomal spread | ||
| Anastomotic spread from one nerve region into another | ||
| Tumor invasion — nerve growth | Peripheral nerve sprouting | Observed experimentally |
| Growth factors | NGF, NCAM, other local factors promoting nerve growth | |
| Angiosoma vs common anatomical distribution | Concept of metastatic distribution | The angiosoma concept divides the skull into 13 different angiosomas |
Abbrevations: NGF, Nerve growth factor; NCAM, Neural Cell Adhesion Molecule.
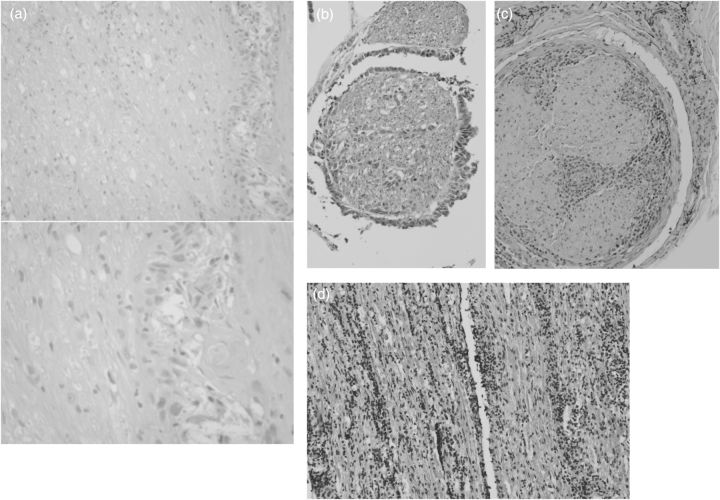
Fig. 5.
Types of nerve infiltration. (a) Biopsy of a facial nerve in a patient with retrograde nerve infiltration in a case of skin tumor (courtesy of Dr. Tissa Wijeratne, Australia). (b) Nerve root infiltration with cuff like tumor cells and intranerval tumor cells (adenocarcinoma of lung). (c) Neurolymphomatosis: lymphoma spread in a peripheral nerve. (d) Diffuse cranial nerve infiltration in lymphoma.
Fig. 6.
Nerve infiltration. (A) Nerve compression and invasion. (B) Intranerval metastasis. (C) Tumor spread within the nerve. (D) Intravascular lymphoma.
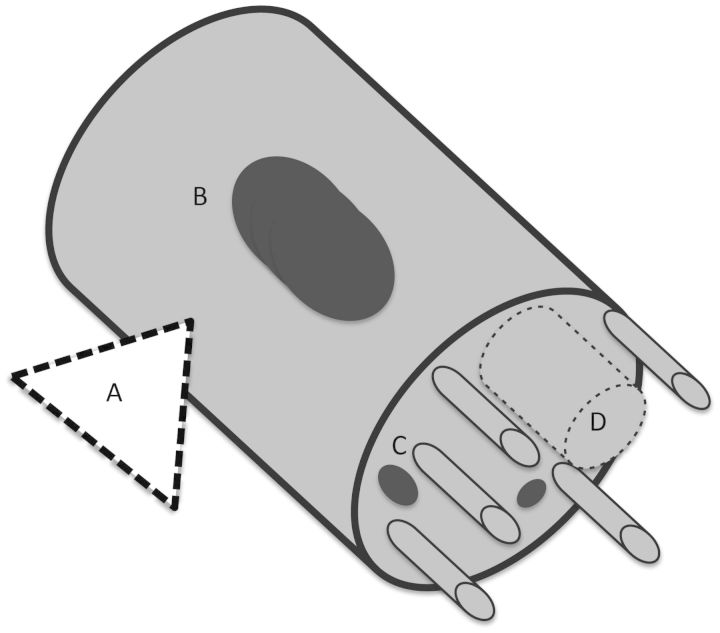
Fig. 7.
Nerve infiltration/ tumor spread. (A) Retrograde infiltration along peripheral nerves (eg, facial nerve or trigeminal nerve) (black arrow). (B) Spread from tumors arising in the cavities of the skull. (C) Nerve infiltration can occur in both directions through the foramina (dotted arrows). (D) Anterograde and retrograde spread in CN (bidirectional arrow). With kind permission of Springer Science+Business Media this figure is reproduced from Feldman EA, Grisold W, Russell JW, Zifko UA. Cranial nerves. In: Atlas of Neuromuscular Disease: A Practical Guideline. Austria: Springer-Verlag/Wien; 2005: 47.
The pattern of peripheral nerve involvement in cancer, including a detailed outline, has been described by Meller et al.38 Intraneural metastases are rare.39 Tumor cells travel via the vasa nervorum through the epineurium, perineurium, and endoneurium, and these vessels anastomose with the vascular system that parallels the nerve fibers.14 In the CNs, perineurial spread occurs in a centripedal or centrifugal fashion, although the literature more typically describes centripetal spread. Spread occurs in the CNs at intracranial and extracranial sites as well as in skin and ENT tumors. The spread can occur inside the skull, outside the skull, or pass through the basal foramina40,41 and thus have a portion both inside and outside of the skull.
Several interesting conclusions emerge from observations of this spread: (a) tumor spread can use the same or neighboring CN as a scaffold; (b) spread from the distribution of one CN into the territory of another can occur via anastomosis (eg, the auriculotemporal nerve), which can be a site of extensive cancer spread, particularly in the richly anastomized areas of the caudal CN , and (c) the spread of cancer along nerves can serve as a valuable prognostic factor in the general pathology of prostate and pancreatic cancer.25
Spread via Anastomosis
In addition to the CNs and nerve trunks described in anatomy textbooks, anastomoses also exist between nerves (eg, the auriculotemporal and greater petrosal nerves and nerves of the tongue)42 that allow the spread of tumors from one area of nerve distribution into another. The anastomoses are often widely distributed, and extensive connectivity between CNs has been demonstrated in the tongue and in the pharynx using the Siloh staining technique.43
Small Nerve Fibers in Cancer Growth
Experiments studying metastases of breast or prostate carcinoma to the bone show the invasion of bony tissue being accompanied by sprouting of sensory nerves, which can be suppressed by anti-nerve growth factor (NGF) treatment in animals. Although those experiments were primarily initiated to investigate cancer pain, they demonstrate the importance of nerve sprouting in cancer growth.44,45
Factors Promoting or Inhibiting Nerve Growth
In addition to mechanical and cellular factors in neoplastic spread to the CNs, several factors, such as NGF, neural cell adhesion molecule, p75, and immune regulators of the nervous system, have been shown to enhance or delay nerve growth and may either facilitate or inhibit cancer spread in nervous tissue. There may well be a strong relationship between the nerve growth found in reinnervation and the growth of cancer tissue in peripheral nerves that could ultimately enhance the treatment of cancer metastases.
Angiosomas
The “angiosome concept,” originally described by Taylor,6 divides the body into 3-dimensional anatomic units of tissue fed by a source artery (the angiosome). The head and neck regions consist of 13 angiosomas, from which 3 are not in contact with the skin but rather exist within the neck and skull region.46 The angiosome concept originally comes from plastic and reconstructive surgery and has significantly improved the success of skin flaps due to increased understanding of vascularization. When applied to the head, skull, and neck, this concept suggests a different anatomical perspective than that used in clinical neurology, which is traditionally based on the CN distribution and nerve trunks stemming from the cervical roots. For example, this concept has previously been used in part to explain vascular ischemic CN lesions.47 When this concept is applied to invasion from cancer within the nerves of the neck and head, this concept may well explain why the upper CNs, which are mostly supplied by branches of the carotid artery, are more prone to metastatic nerve lesions than the lower CN, which is supplied by the ascending pharyngeal artery. A practical implication of this concept might be the application of embolization of tumors of the skull, where the knowledge of the pattern of CN vascularization can prevent damage.
An interesting type of cancer spread into the skin (centrifugal type) has been observed in melanoma, in which the skin mestastases are seen as a dermatomal spread resembling herpes.12 Conversely, skin tumors have also been observed to result in a retrograde spread into the skull.
Other Mechanisms of Cranial Nerve Lesions
CNs can be mechanically involved in tumors of the base of the skull (eg, meningeomas), where they might be injured or sacrificed by surgical interventions or damaged by radiation. Three nerves (the optic, the abducens, and the hypoglossal) seem to be particularly prone to radiation injury that results in radiation neuritis. In clinical practice, the amount of time that passes between application of radiation and development of CN lesions is an important diagnostic factor.48 Vascular interventions, such as embolization or ligation of arteries, can cause CN damage.
Differential Diagnosis
Thickening and enlargement of CNs is not specific to perineurial tumor spread and can also be associated with several other conditions such as infections, inflammatory and granulomatous disorders, schwannomatosis neurofibromatosis, lipomatosis, trauma, and vascular conditions. In addition, rare conditions, such as intracranial perineuromas, lipomatosis of peripheral nerves, pseudohypertropic neuritis, and IgG4-related perineurial disease (Table 3), must be considered when evaluating enlarged CNs.
Table 3.
Differential diagnosis of cranial nerve cancer involvement
| Infections | Inflammatory | Other conditions |
|---|---|---|
| Bacteria, spirochochetes, fungi, viral | Immune mediated: | Craniopharyngeoma |
| IgG4-related perineurial disease,49 pseudotoumoral hypertrophic neuritis50 | Lipomatosis,51 | |
| Churg-Strauss syndrome | Mucocele | |
| Giant cell arteritis | Neurofibromatosis | |
| Wegener`s granulomatosis | Perineuroma52 | |
| Orbital inflammation | Pituitary tumor | |
| Sarcoidosis | Pseudotumor cerebri | |
| Systemic lupus erythematosus | Schwannoma | |
| Tolosa-Hunt syndrome | Trauma | |
| Thyroidal orbitopathy | Vascular |
Practical and Therapeutic Considerations
MRI imaging has significantly improved the diagnosis of cancers arising around the brain and in the skull and neck following the “classical” concept of base of skull metastases. However, the spread of cancer along nerves has proven to be more complex than previously assumed and has increased the importance of innervation and tumor nerve growth along the nerves in the general pathology. In addition to the micronerval factors, the conventional explanation of nerve lesions spreading along nerve trunks needs to be revised to account for the rich anastomoses of CNs and the possible effects of angiosomic structure of vascularization of the skull.
In clinical practice, once nerve infiltration has been identified, it is usually necessary to both confirm the diagnosis and treat the patient's symptoms. Surgery is often feasible in selected situations; embolization of vessels may be performed in others. The mainstay of treatment, however, is radiation therapy in its different forms.53 When adopting surgery and endovascular intervention, the concept of angiosomic vascularization can be applied to prevent additional damage.
Chemotherapy can be used, depending on the tumor type, and either applied systemically or regionally. The choice of drugs depends on the chemosensitivity of the tumor. In particular, lymphoproliferative disorders may be prone to respond to chemotherapy. Concommitant intrathecal chemotherapy may be used in cases involving additional CSF.
Any treatment for metastasis to the skull, and in particular of the CNs, needs to aim for amelioration of symptoms and symptomatic treatment. In addition to straightforward sensory and motor symptoms, more complex symptoms (eg, the patient's speech and communication ability) need to be considered.
Conclusion
In addition to metastases into the brain parenchyma, the meninges, and the base of the skull, several other types of cancer involvement and tumor spread can occur. Tumor involvement of the dura, bony skull, cavities of the skull, and skin and the tissue of the neck can involve CNs at different sites and with different mechanisms. Often, a multidisciplinary approach for diagnosis and treatment will be needed.
Supplementary Material
Funding
None declared.
Acknowledgments
This work has been presented in part at the World Congress of Neurology (WCN 2013), in Vienna, Austria, September 2013.
Conflict of interest statement. None declared.
References
- 1.Mitsuya K, Nakasu Y, Horiguchi S, et al. Endo metastatic skull tumors: MRI features and a new conventional classification. J Neurooncol. 2011;104(1):239–245. doi: 10.1007/s11060-010-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debois J. TxNxM1: The anatomy and clinics of metastatic cancer. New York, Boston: Kluwer Academic Publishers; 2002. [Google Scholar]
- 3.Greenberg HSDM, Vikram B. Metastasis to the base of the skull: clinical findings in 43 patients. Neurology. 1981;31:530–537. doi: 10.1212/wnl.31.5.530. [DOI] [PubMed] [Google Scholar]
- 4.Grisold W, Briani C, Vass A. Malignant cell infiltration in the peripheral nervous system. Handb Clin Neurol. 2013;115:685–712. doi: 10.1016/B978-0-444-52902-2.00040-0. [DOI] [PubMed] [Google Scholar]
- 5.Zelenak M, Doval M, Gorscak JJ. Acute cavernous sinus syndrome from metastasis of lung cancer to sphenoid bone. Case Rep Oncol. 2012;5(1):35–42. doi: 10.1159/000335896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg. 1987;40(2):113–141. doi: 10.1016/0007-1226(87)90185-8. [DOI] [PubMed] [Google Scholar]
- 7.Lawton A, Sudakoff G, Dezelan LC, et al. Presentation, treatment, and outcomes of dural metastases in men with metastatic castrate-resistant prostate cancer: A Case Series. J Palliat Med. 2010;13(9):271–273. doi: 10.1089/jpm.2009.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung WSM, Ahn KJ, Park MR, et al. The radiological spectrum of orbital pathologies that Involve the lacrimal gland and the lacrimal fossa. Korean J Radiol. 2007;8(4):336–342. doi: 10.3348/kjr.2007.8.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HH, Lee LY, Chin SC, et al. Carcinoma ex pleomorphic adenoma of soft palate with cavernous sinus invasion. World J Surg Oncol. 2010;8:24. doi: 10.1186/1477-7819-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gokmen Soysal H, Markoc F. Invasive squamous cell carcinoma of the eyelids and periorbital region. Br J Ophthalmol. 2007;91(3):325–329. doi: 10.1136/bjo.2006.102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramchandren S, Dalmau J. Cervical Plexus: Metastases to the peripheral nervous system. J Neurooncol. 2005;75(1):101–110. doi: 10.1007/s11060-004-8102-9. [DOI] [PubMed] [Google Scholar]
- 12.Evans AV, Russell-Jones R. Zosteriform metastasis from melanoma. BMJ. 2003;326(7397):1025–1026. doi: 10.1136/bmj.326.7397.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson PD, Kendall BE. Enophthalmos and metastatic carcinoma of the breast. J Neurol Neurosurg Psych. 1985;48(12):1305–1306. doi: 10.1136/jnnp.48.12.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong V. Imaging the cranial nerves in cancer. Cancer Imaging. 2004;4 Spec No A:S1–S5. doi: 10.1102/1470-7330.2004.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clouston PD, Sharpe DM, Corbett AJ, et al. Perineural spread of cutaneous head and neck cancer. Its orbital and central neurologic complications. Arch Neurol. 1990;47(1):73–77. doi: 10.1001/archneur.1990.00530010091025. [DOI] [PubMed] [Google Scholar]
- 16.Ong CK, Chong VFH. Imaging of perineural spread in head and neck tumours. Cancer Imaging. 2010;10 Spec No A:S92–S98. doi: 10.1102/1470-7330.2010.9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmalfuss IM, Tart RP, Mukherji S, et al. Perineural tumor spread along the auriculotemporal nerve. Am J Neuroradiol. 2002;23(2):303–311. [PMC free article] [PubMed] [Google Scholar]
- 18.Viken J, Bendtsen Hansen K, et al. Facial pain and multiple cranial palsies in a patient with skin cancer. J Headache Pain. 2011;12(3):381–383. doi: 10.1007/s10194-011-0324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wierzbicka M, Kopec T, Szyfter W, et al. The presence of facial nerve weakness on diagnosis of a parotid gland malignant process. Eur Arch Otorhinolaryngol. 2012;269(4):1177–1182. doi: 10.1007/s00405-011-1882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ettl T, Gosau M, Reichert TE. Salivary gland carcinomas. Oral Maxillofac Surg. 2012;16(3):267–283. doi: 10.1007/s10006-012-0350-9. [DOI] [PubMed] [Google Scholar]
- 21.Binmadi NO, John R, Basile JC. Perineural invasion in oral squamous cell carcinoma: A discussion of significance and review of the literature. Oral Oncol. 2011;47(11):1005–1010. doi: 10.1016/j.oraloncology.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Sun Y, Gao D. Role of the nervous system in cancer metastasis. Oncol Lett. 2013;5(4):1101–1111. doi: 10.3892/ol.2013.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.L'Heureux-Lebeau B, Saliba I. Updates on the diagnosis and treatment of intracranial nerve malignant peripheral nerve sheath tumors. OncoTargets Ther. 2013;6:459–470. doi: 10.2147/OTT.S41397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bilici A, Seker M, Ustaalioglu BB, et al. Prognostic significance of perineural invasion in patients with gastric cancer who underwent curative resection. Ann Surg Oncol. 2010;17(8):2037–2044. doi: 10.1245/s10434-010-1027-y. [DOI] [PubMed] [Google Scholar]
- 25.Gil Z, Cavel O, Kelly K, et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102(2):107–118. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchesi F, Piemonti L, Mantovani A, Allavena P, et al. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010;21(1):77–82. doi: 10.1016/j.cytogfr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Xu L, Zhou Y, Qiu D, et al. Fusion PET-CT detection of neurolymphomatosis originating from primary breast lymphoma: A case report and literature review. Oncol Lett. 2012;4(5):973–975. doi: 10.3892/ol.2012.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chhabra A, Zhao L, Carrino JA, et al. MR Neurography: Advances. Radiology Research and Practice. 2013. Article ID 809568, http://dx.doi.org/10.1155/2013/809568. [DOI] [PMC free article] [PubMed]
- 29.Nemzek WRSH, Gandour-Edwards R, Donald P, et al. Perineural spread of head and neck tumors: How accurate is MR Imaging? Am J Neuroradiol. 1998;19(4):701–706. [PMC free article] [PubMed] [Google Scholar]
- 30.Saremi F, Helmy M, Farzin S, et al. MRI of cranial nerve enhancement. Am J Roentgen. 2005;185(6):1487–1497. doi: 10.2214/AJR.04.1518. [DOI] [PubMed] [Google Scholar]
- 31.Davis SB, Mathews VP, Williams DW., III Masticator muscle enhancement in subacute denervation atrophy. Am J Neuroradiol. 1995;16(6):1292–1294. [PMC free article] [PubMed] [Google Scholar]
- 32.Russo CP, Smoker WR, Weissman JL. MR appearance of trigeminal and hypoglossal motor denervation. Am J Neuroradiol. 1997;18(7):1375–1383. [PMC free article] [PubMed] [Google Scholar]
- 33.Mizutani T. Neurological disturbances caused by intravascular lymphomatosis. Brain Nerve. 2011;63(5):443–449. [PubMed] [Google Scholar]
- 34.Santos E, Scolding NJ. Neurolymphomatosis mimicking neurosarcoidosis: a case report. J Med Case Rep. 2010;4:5. doi: 10.1186/1752-1947-4-5. doi:10.1186/1752-1947-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada S, Tanimoto A, Nabeshima A, et al. Diffuse large B-cell lymphoma presenting with neurolymphomatosis and intravascular lymphoma: a unique autopsy case with diverse neurological symptoms. Diagn Pathol. 2012;7:94. doi: 10.1186/1746-1596-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panizza BWT, Solares CA, Boyle GM, et al. Histopathological features of clinical perineural invasion of cutaneous squamous cell carcinoma of the head and neck and the potential implications for treatment [published online ahead of print September 30, 2013] Head Neck. 2013 doi: 10.1002/hed.23509. doi:101002/hed23509. [DOI] [PubMed] [Google Scholar]
- 37.Oberndorfer S, Woehrer A, Hainfellner JA, et al. Secondary gliosarcoma with massive invasion of meninges, skull base, and soft tissue, and systemic metastasis. Clin Neuropathol. 2013;32(6):522–524. doi: 10.5414/NP300643. [DOI] [PubMed] [Google Scholar]
- 38.Meller I, Alkalay D, Mozes M, et al. Isolated metastases to peripheral nerves. Report of five cases involving the brachial plexus. Cancer. 1995;76(10):1829–1832. doi: 10.1002/1097-0142(19951115)76:10<1829::aid-cncr2820761023>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Grisold W, Piza-Katzer H, Jahn R, et al. Intraneural nerve metastasis with multiple mononeuropathies. J Peripher Nerv Syst. 2000;5(3):163–167. doi: 10.1046/j.1529-8027.2000.00016.x. [DOI] [PubMed] [Google Scholar]
- 40.Ballantyne AJ, McCarten AB, Ibanez ML. The extension of cancer of the head and neck through peripheral nerves. Am J Surg. 1963;106:651–667. doi: 10.1016/0002-9610(63)90074-6. [DOI] [PubMed] [Google Scholar]
- 41.Laine FJ, Braun IF, Jensen ME, et al. Perineural tumor extension through the foramen ovale: evaluation with MR imaging. Radiology. 1990;174(1):65–71. doi: 10.1148/radiology.174.1.2152985. [DOI] [PubMed] [Google Scholar]
- 42.Ginsberg LE, De Monte F, Gillenwater AM. Greater superficial petrosal nerve: anatomy and MR findings in perineural tumor spread. Am J Neuroradiol. 1996;17(2):389–393. [PMC free article] [PubMed] [Google Scholar]
- 43.Mu L, Sanders I. Sihler's whole mount nerve staining technique: a review. Biotech Histochem. 2010;85(1):19–42. doi: 10.1080/10520290903048384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bloom AP, Jimenez-Andrade JM, Taylor RN, et al. Breast cancer-induced bone remodeling, skeletal pain and sprouting of sensory nerve fibers. J Pain. 2011;12(6):698–711. doi: 10.1016/j.jpain.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jimenez-Andrade JM, Ghilardi JR, Castaneda-Corrala G, et al. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation and cancer pain. Pain. 2011;152(11):2564–2574. doi: 10.1016/j.pain.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Houseman ND, Taylor GI, Pan WR. The angiosomes of the head and neck: anatomic study and clinical applications. Plast Reconstr Surg. 2000;105(7):2287–2313. doi: 10.1097/00006534-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Lapresle J, Lasjaunias P. Cranial nerve ischaemic arterial syndromes. Brain. 1986;109(Pt1):207–216. doi: 10.1093/brain/109.1.207. A review. [DOI] [PubMed] [Google Scholar]
- 48.Chong VF, Khoo JB, Chan LL, et al. Neurological changes following radiation therapy for head and neck tumours. Eur J Radiol. 2002;44(2):120–129. doi: 10.1016/s0720-048x(02)00066-9. [DOI] [PubMed] [Google Scholar]
- 49.Inoue D, Zen Y, Sato Y, et al. IgG4-Related Perineural Disease. Int J Rheumatol. 2012 doi: 10.1155/2012/401890. 2012:401890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zanoletti E, Mazzoni A, Barbo R. Pseudotumoural hypertrophic neuritis of the facial nerve neurite ipertrofica pseudotumorale del nervo facciale. ACTA Otorhinolaryngol Ital. 2008;28(2):55–60. [PMC free article] [PubMed] [Google Scholar]
- 51.Maratos EC, Goicochea MT, Condomi-Alcorta S, et al. Lipomatosis of the Trigeminal Nerve Causing Trigeminal Neuralgia: Case Report and Literature Review. Skull Base. 2010;20(4):293–299. doi: 10.1055/s-0030-1249245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsang WY CJ, Chow LT, Tse CC. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16(8):756–763. doi: 10.1097/00000478-199208000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Bakst RL, Lee N, He S, et al. Radiation impairs perineural invasion by modulating the nerve microenvironment. PLoS ONE. 2012;7(6):e39925. doi: 10.1371/journal.pone.0039925. [DOI] [PMC free article] [PubMed] [Google Scholar]