Abstract
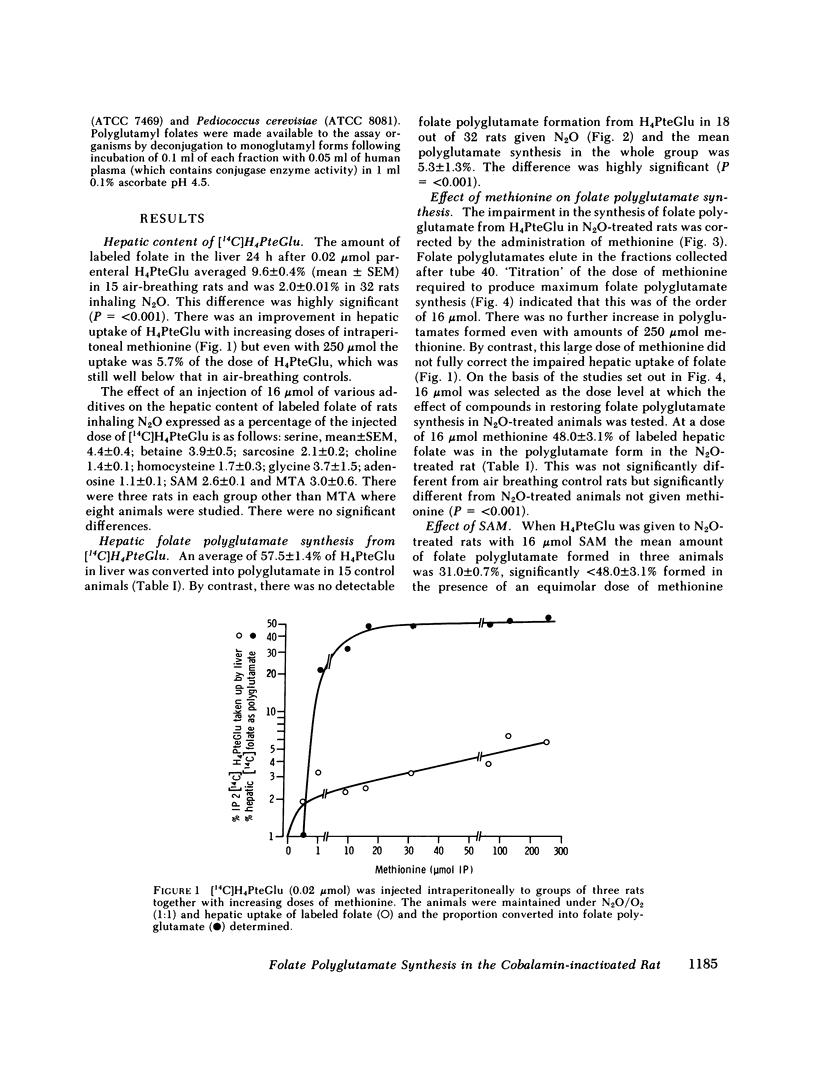
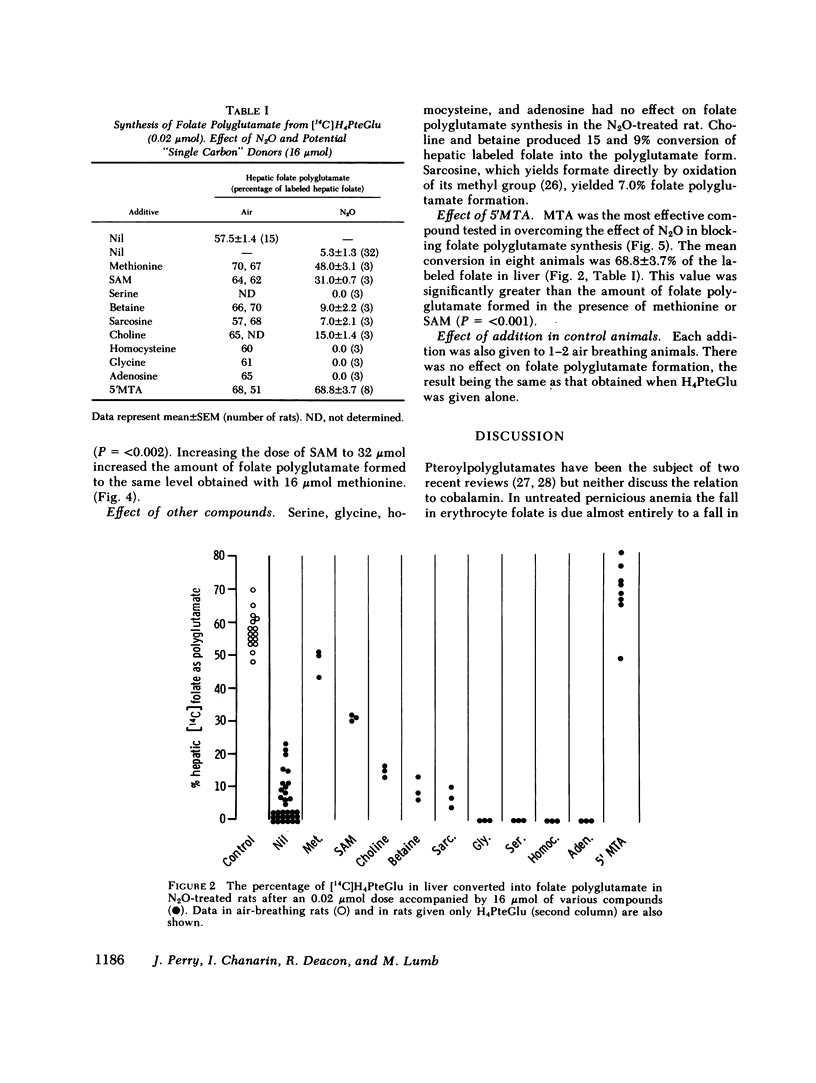
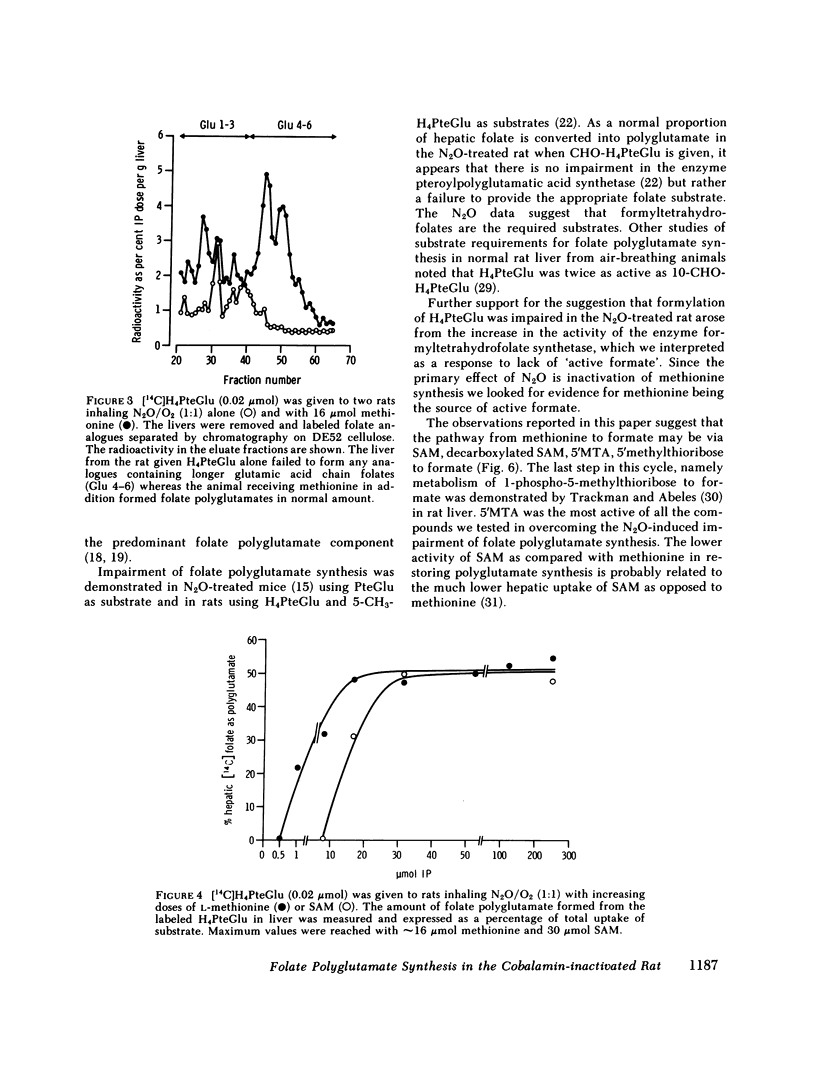
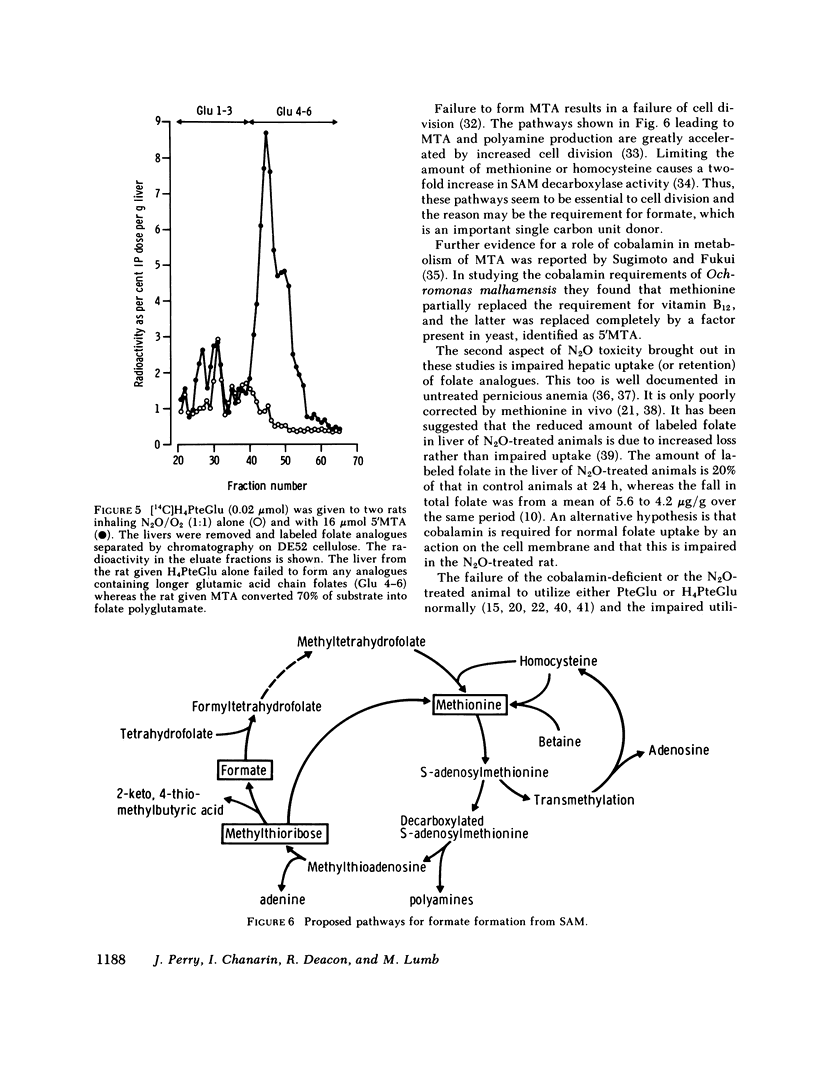
Nitrous oxide, by inactivating cobalamin in vivo, produces a suitable animal model for cobalamin 'deficiency.' The synthesis of folate polyglutamate with tetrahydrofolate as substrate is severely impaired in the N2O-treated rat, but is normal with formyltetrahydrofolate as substrate. Methionine restores the capacity of the N2O-treated rat to utilize tetrahydrofolate the minimum effective dose being 16 mumol. S-Adenosylmethionine was somewhat less effective than methionine but 5'methylthioadenosine, a product of S-adenosylmethionine metabolism, was significantly more effective than methionine in correcting the defect in folate polyglutamate synthesis. 5'Methylthioadenosine is metabolised to yield formate. It is suggested that these compounds have their effect in correcting folate polyglutamate synthesis by supplying formate for the formylation of tetrahydrofolate. Formyltetrahydrofolate, at least in the cobalamin-inactivated animal, is the required substrate for folate polyglutamate synthesis. Cobalamin is concerned with the maintenance of normal levels of methionine and this in turn is a major source of formate through S-adenosylmethionine and 5'methylthioadenosine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amess J. A., Burman J. F., Rees G. M., Nancekievill D. G., Mollin D. L. Megaloblastic haemopoiesis in patients receiving nitrous oxide. Lancet. 1978 Aug 12;2(8085):339–342. doi: 10.1016/s0140-6736(78)92941-0. [DOI] [PubMed] [Google Scholar]
- Backlund P. S., Jr, Smith R. A. Methionine synthesis from 5'-methylthioadenosine in rat liver. J Biol Chem. 1981 Feb 25;256(4):1533–1535. [PubMed] [Google Scholar]
- Branford White C. J., Mullins D. J. Fucose incorporation into synaptic mitochondrial and plasma-membrane glycoproteins. Biochem Soc Trans. 1980 Oct;8(5):619–620. doi: 10.1042/bst0080619. [DOI] [PubMed] [Google Scholar]
- Buehring K. U., Tamura T., Stokstad E. L. Folate coenzymes of Lactobacillus casei and Streptococcus faecalis. J Biol Chem. 1974 Feb 25;249(4):1081–1089. [PubMed] [Google Scholar]
- Chanarin I., Perry J., Lumb M. The biochemical lesion in vitamin-B12 deficiency in man. Lancet. 1974 Jun 22;1(7869):1251–1252. doi: 10.1016/s0140-6736(74)90005-1. [DOI] [PubMed] [Google Scholar]
- Das K. C., Hoffbrand A. V. Studies of folate uptake by phytohaemagglutinin-stimulated lymphocytes. Br J Haematol. 1970 Aug;19(2):203–221. doi: 10.1111/j.1365-2141.1970.tb01617.x. [DOI] [PubMed] [Google Scholar]
- Deacon R., Chanarin I., Perry J., Lumb M. A comparison of tetrahydrofolate and 5-formyltetrahydrofolate in correcting the impairment of thymidine synthesis in pernicious anaemia. Scand J Haematol. 1982 Apr;28(4):289–292. doi: 10.1111/j.1600-0609.1982.tb00529.x. [DOI] [PubMed] [Google Scholar]
- Deacon R., Chanarin I., Perry J., Lumb M. Impaired deoxyuridine utilization in the B12-inactivated rat and its correction by folate analogues. Biochem Biophys Res Commun. 1980 Mar 28;93(2):516–520. doi: 10.1016/0006-291x(80)91107-9. [DOI] [PubMed] [Google Scholar]
- Deacon R., Chanarin I., Perry J., Lumb M. Marrow cells from patients with untreated pernicious anaemia cannot use tetrahydrofolate normally. Br J Haematol. 1980 Dec;46(4):523–528. doi: 10.1111/j.1365-2141.1980.tb06008.x. [DOI] [PubMed] [Google Scholar]
- Deacon R., Lumb M., Perry J., Chanarin I., Minty B., Halsey M. J., Nunn J. F. Selective inactivation of vitamin B12 in rats by nitrous oxide. Lancet. 1978 Nov 11;2(8098):1023–1024. doi: 10.1016/s0140-6736(78)92341-3. [DOI] [PubMed] [Google Scholar]
- Deacon R., Perry J., Lumb M., Chanarin I. The effect of nitrous oxide-induced inactivation of vitamin B12 on thymidylate synthetase activity of rat bone marrow cells. Biochem Biophys Res Commun. 1981 Sep 16;102(1):215–218. doi: 10.1016/0006-291x(81)91509-6. [DOI] [PubMed] [Google Scholar]
- Frisell W. R., Randolph V. M. Effect of adenine nucleotides on the oxidation of methyl groups in liver mitochondria. Biochim Biophys Acta. 1973 Feb 22;292(2):360–365. doi: 10.1016/0005-2728(73)90042-x. [DOI] [PubMed] [Google Scholar]
- Gawthorne J. M., Smith R. M. The synthesis of pteroylpolyglutamates by sheep liver enzymes in vitro. Biochem J. 1973 Oct;136(2):295–301. doi: 10.1042/bj1360295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawthorne J. M., Stokstad E. L. The effect of vitamin B 12 and methionine on folic acid uptake by rat liver. Proc Soc Exp Biol Med. 1971 Jan;136(1):42–46. doi: 10.3181/00379727-136-35188. [DOI] [PubMed] [Google Scholar]
- HERBERT V., ZALUSKY R. Interrelations of vitamin B12 and folic acid metabolism: folic acid clearance studies. J Clin Invest. 1962 Jun;41:1263–1276. doi: 10.1172/JCI104589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEEJEEBHOY K. N., PATHARE S. M., NORONHA J. M. OBSERVATIONS ON CONJUGATED AND UNCONJUGATED BLOOD FOLATE LEVELS IN MEGALOBLASTIC ANEMIA AND THE EFFECTS OF VITAMIN B 12. Blood. 1965 Sep;26:354–359. [PubMed] [Google Scholar]
- Kisliuk R. L. Pteroylpolyglutamates. Mol Cell Biochem. 1981 Sep 25;39:331–345. doi: 10.1007/BF00232583. [DOI] [PubMed] [Google Scholar]
- Kondo H., Osborne M. L., Kolhouse J. F., Binder M. J., Podell E. R., Utley C. S., Abrams R. S., Allen R. H. Nitrous oxide has multiple deleterious effects on cobalamin metabolism and causes decreases in activities of both mammalian cobalamin-dependent enzymes in rats. J Clin Invest. 1981 May;67(5):1270–1283. doi: 10.1172/JCI110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASSEN H. C., HENRIKSEN E., NEUKIRCH F., KRISTENSEN H. S. Treatment of tetanus; severe bone-marrow depression after prolonged nitrous-oxide anaesthesia. Lancet. 1956 Apr 28;270(6922):527–530. doi: 10.1016/s0140-6736(56)90593-1. [DOI] [PubMed] [Google Scholar]
- LAST P. M., MARTIN F. I., WILSON P. Bone-marrow depression in tetanus; report of a fatal case. Lancet. 1956 Sep 1;271(6940):442–443. doi: 10.1016/s0140-6736(56)91920-1. [DOI] [PubMed] [Google Scholar]
- Layzer R. B. Myeloneuropathy after prolonged exposure to nitrous oxide. Lancet. 1978 Dec 9;2(8102):1227–1230. doi: 10.1016/s0140-6736(78)92101-3. [DOI] [PubMed] [Google Scholar]
- Leslie G. I., Baugh C. M. The uptake of pteroyl(14-C)glutamic acid into rat liver and its incorporation into the natural pteroyl poly-gamma-glutamates of that organ. Biochemistry. 1974 Nov 19;13(24):4957–4961. doi: 10.1021/bi00721a013. [DOI] [PubMed] [Google Scholar]
- Lumb M., Deacon R., Perry J., Chanarin I., Minty B., Halsey M. J., Nunn J. F. The effect of nitrous oxide inactivation of vitamin B12 on rat hepatic folate. Implications for the methylfolate-trap hypothesis. Biochem J. 1980 Mar 15;186(3):933–936. doi: 10.1042/bj1860933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumb M., Perry J., Deacon R., Chanarin I. Changes in plasma folate levels in rats inhaling nitrous oxide. Scand J Haematol. 1981 Jan;26(1):61–64. doi: 10.1111/j.1600-0609.1981.tb01625.x. [DOI] [PubMed] [Google Scholar]
- Lumb M., Perry J., Deacon R., Chanarin I. Changes in tissue folates accompanying nitrous oxide-induced inactivation of vitamin B12 in the rat. Am J Clin Nutr. 1981 Nov;34(11):2412–2417. doi: 10.1093/ajcn/34.11.2412. [DOI] [PubMed] [Google Scholar]
- Lumb M., Perry J., Deacon R., Chanarin I. Recovery of tissue folates after inactivation of cobalamin by nitrous oxide. The significance of dietary folate. Am J Clin Nutr. 1981 Nov;34(11):2418–2422. doi: 10.1093/ajcn/34.11.2418. [DOI] [PubMed] [Google Scholar]
- Lumb M., Perry J., Deacon R., Chanarin I. Urinary folate loss following inactivation of vitamin B12 by nitrous oxide in rats. Br J Haematol. 1982 Jun;51(2):235–242. [PubMed] [Google Scholar]
- McGing P. G., Scott J. M. Evidence that the decreased liver folate status following vitamin B-12 inactivation in the mouse is due to increased loss rather than impaired uptake. Biochim Biophys Acta. 1981 Apr 3;673(4):594–597. doi: 10.1016/0304-4165(81)90488-8. [DOI] [PubMed] [Google Scholar]
- McGing P. G., Scott J. M. The role of methionine and vitamin B12 in folate incorporation by rat liver. Br J Nutr. 1980 Jan;43(1):235–237. doi: 10.1079/bjn19800082. [DOI] [PubMed] [Google Scholar]
- McGuire J. J., Bertino J. R. Enzymatic synthesis and function of folylpolyglutamates. Mol Cell Biochem. 1981 Aug 11;38(Spec No)(Pt 1):19–48. doi: 10.1007/BF00235686. [DOI] [PubMed] [Google Scholar]
- McGuire J. J., Hsieh P., Coward J. K., Bertino J. R. Enzymatic synthesis of folylpolyglutamates. Characterization of the reaction and its products. J Biol Chem. 1980 Jun 25;255(12):5776–5788. [PubMed] [Google Scholar]
- Nixon P. F., Bertino J. R. Enzymic preparations of radiolabeded (+)-L-5-methyltetrahydrofolate and (+)-L-5-formyltetrahydrofolate. Anal Biochem. 1971 Sep;43(1):162–172. doi: 10.1016/0003-2697(71)90121-7. [DOI] [PubMed] [Google Scholar]
- Perry J., Chanarin I., Deacon R., Lumb M. The substrate for folate polyglutamate biosynthesis in the vitamin B12-inactivated rat. Biochem Biophys Res Commun. 1979 Nov 28;91(2):678–684. doi: 10.1016/0006-291x(79)91575-4. [DOI] [PubMed] [Google Scholar]
- Perry J., Deacon R., Lumb M., Chanarin I. The effect of nitrous oxide-induced inactivation of vitamin B12 on the activity of formyl-methenyl-methylenetetrahydrofolate synthetase, methylene-tetrahydrofolate reductase and formiminotetrahydrofolate transferase. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1329–1333. doi: 10.1016/s0006-291x(80)80012-x. [DOI] [PubMed] [Google Scholar]
- Scott J. M., Dinn J. J., Wilson P., Weir D. G. Pathogenesis of subacute combined degeneration: a result of methyl group deficiency. Lancet. 1981 Aug 15;2(8242):334–337. doi: 10.1016/s0140-6736(81)90649-8. [DOI] [PubMed] [Google Scholar]
- Shane B., Watson J. E., Stokstad E. L. Uptake and metabolism of [3H]folate by normal and by vitamin B-12- and methionine-deficient rats. Biochim Biophys Acta. 1977 Mar 29;497(1):241–252. doi: 10.1016/0304-4165(77)90157-x. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Osborne-White W. S. Folic acid metabolism in vitamin B12-deficient sheep. Depletion of liver folates. Biochem J. 1973 Oct;136(2):279–293. doi: 10.1042/bj1360279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y., Fukui S. Studies on biological functions of 5'-methylthioadenosine vitamin B12-replacing effect for Ochromonas malhamensis. J Nutr Sci Vitaminol (Tokyo) 1976;22(2):89–100. doi: 10.3177/jnsv.22.89. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Taheri M. R., Wickremasinghe R. G., Jackson B. F., Hoffbrand A. V. The effect of folate analogues and vitamin B12 on provision of thymine nucleotides for DNA synthesis in megaloblastic anemia. Blood. 1982 Mar;59(3):634–640. [PubMed] [Google Scholar]
- Tisdale M. J. Effect of methionine deprivation on S-adenosylmethionine decarboxylase of tumour cells. Biochim Biophys Acta. 1981 Jul 17;675(3-4):366–372. doi: 10.1016/0304-4165(81)90027-1. [DOI] [PubMed] [Google Scholar]
- Tisman G., Herbert V. B 12 dependence of cell uptake of serum folate: an explanation for high serum folate and cell folate depletion in B 12 deficiency. Blood. 1973 Mar;41(3):465–469. [PubMed] [Google Scholar]
- Toohey J. I. Methylthioadenosine nucleoside phosphorylase deficiency in methylthio-dependent cancer cells. Biochem Biophys Res Commun. 1978 Jul 14;83(1):27–35. doi: 10.1016/0006-291x(78)90393-5. [DOI] [PubMed] [Google Scholar]
- Trackman P. C., Abeles R. H. The metabolism of 1-phospho-5-methylthioribose. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1238–1244. doi: 10.1016/0006-291x(81)90255-2. [DOI] [PubMed] [Google Scholar]
- Zappia V., Galletti P., Porcelli M., Ruggiero G., Andreana A. Uptake of adenosylmethionine and related sulfur compounds by isolated rat liver. FEBS Lett. 1978 Jun 15;90(2):331–335. doi: 10.1016/0014-5793(78)80398-6. [DOI] [PubMed] [Google Scholar]


