Abstract
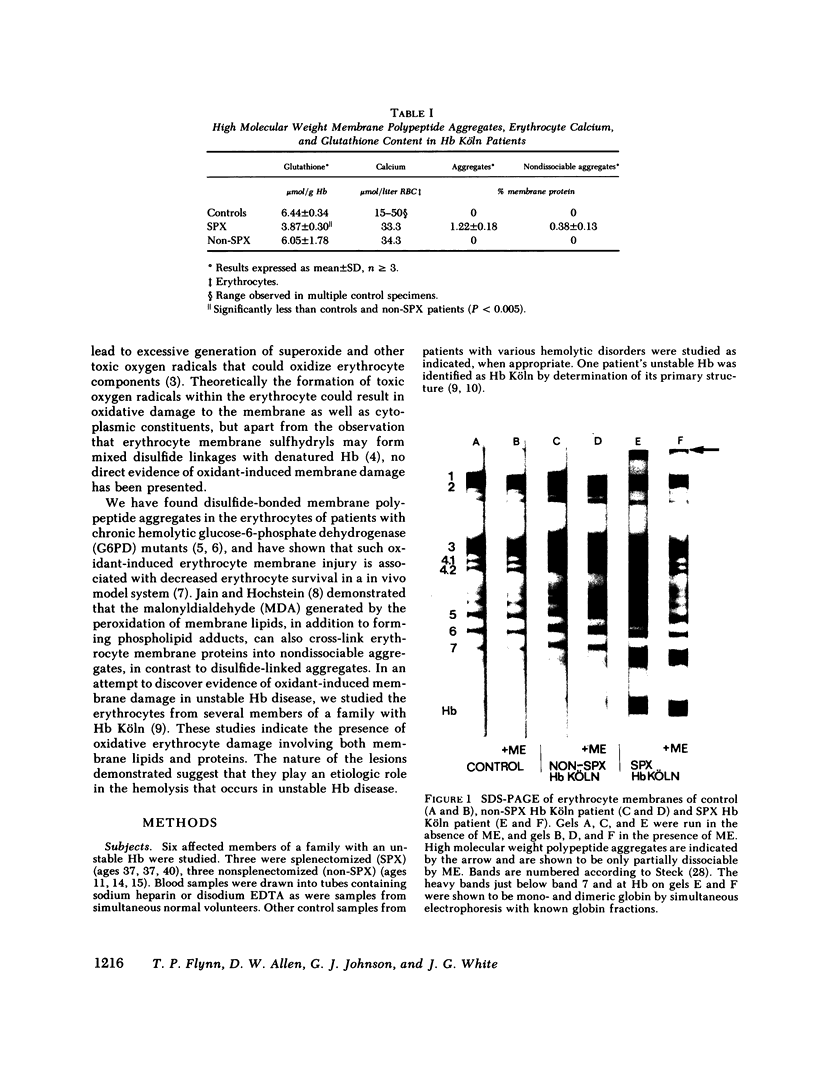
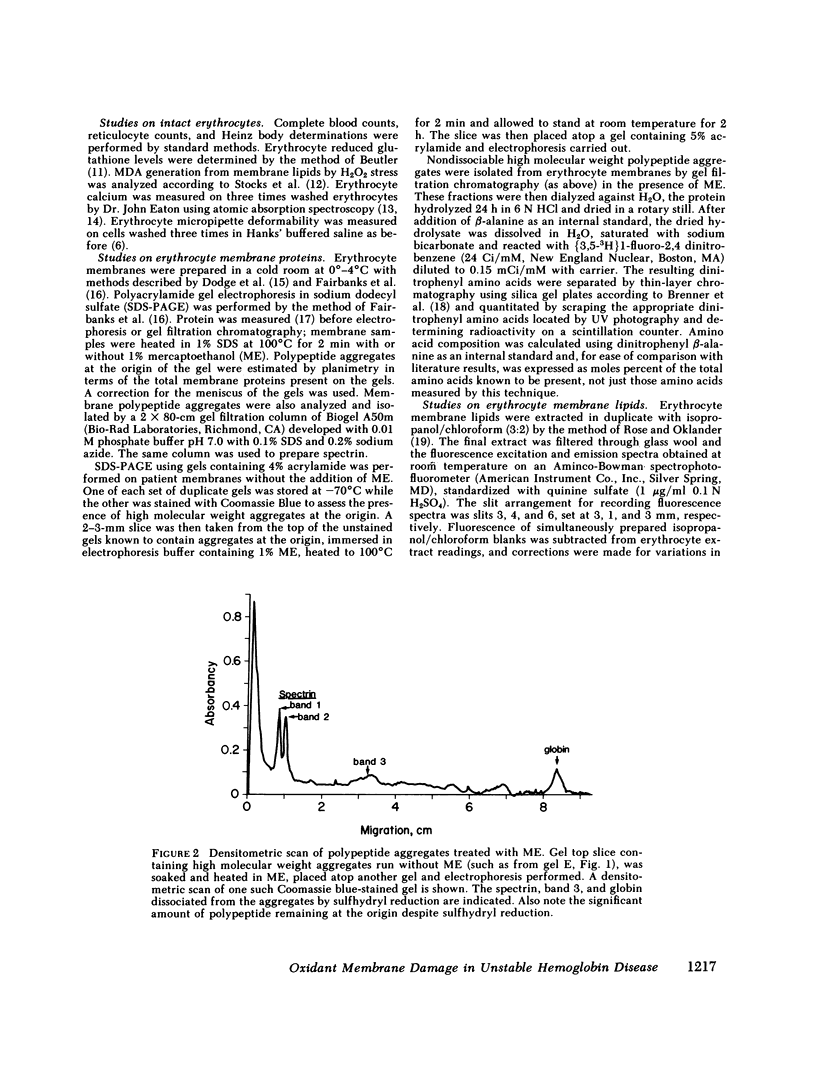
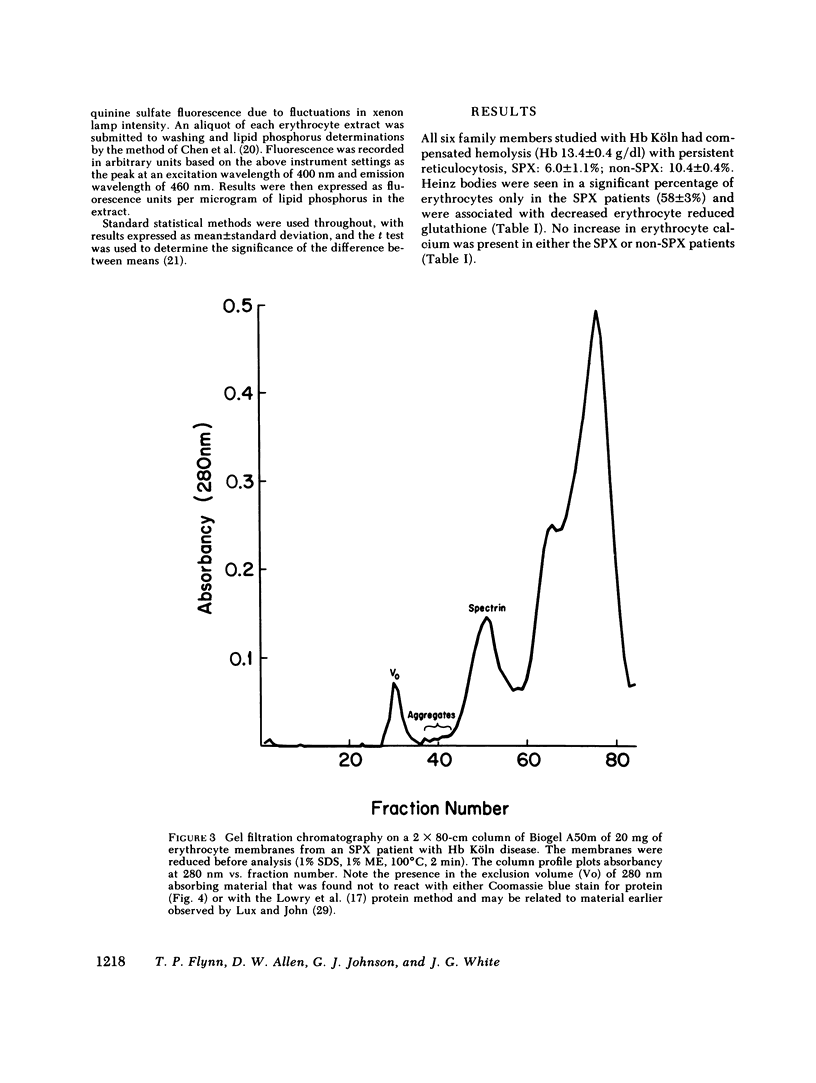
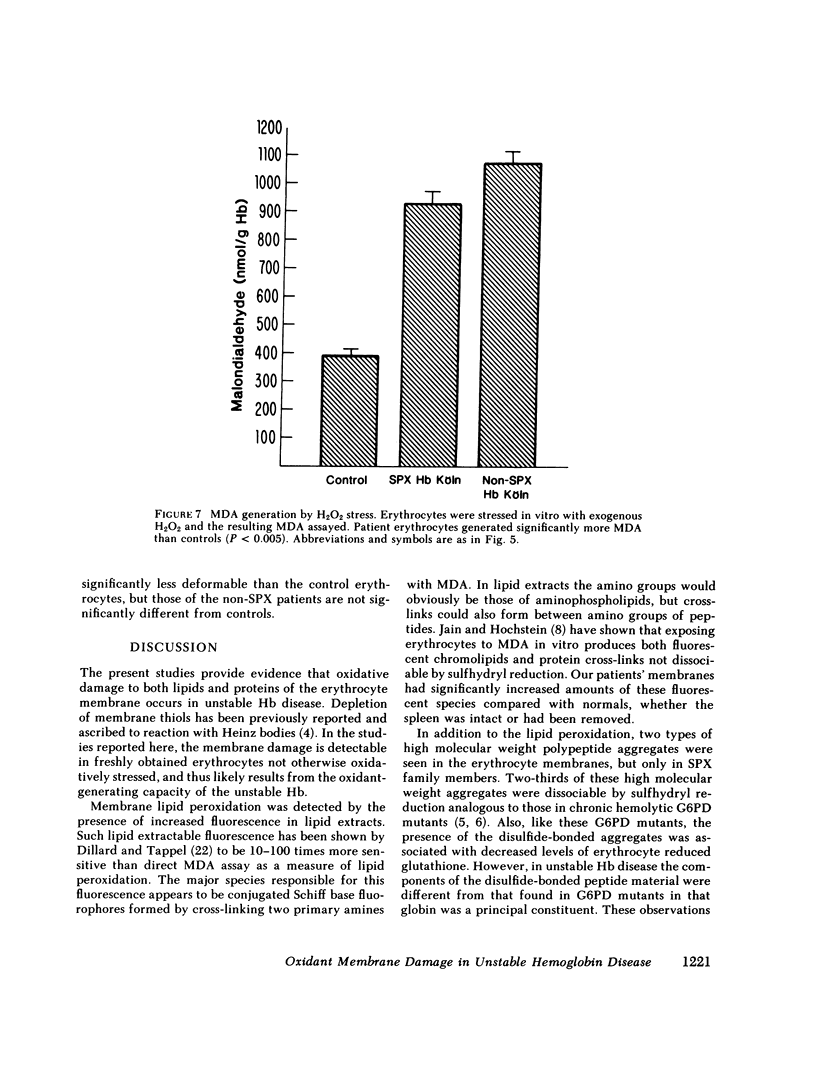
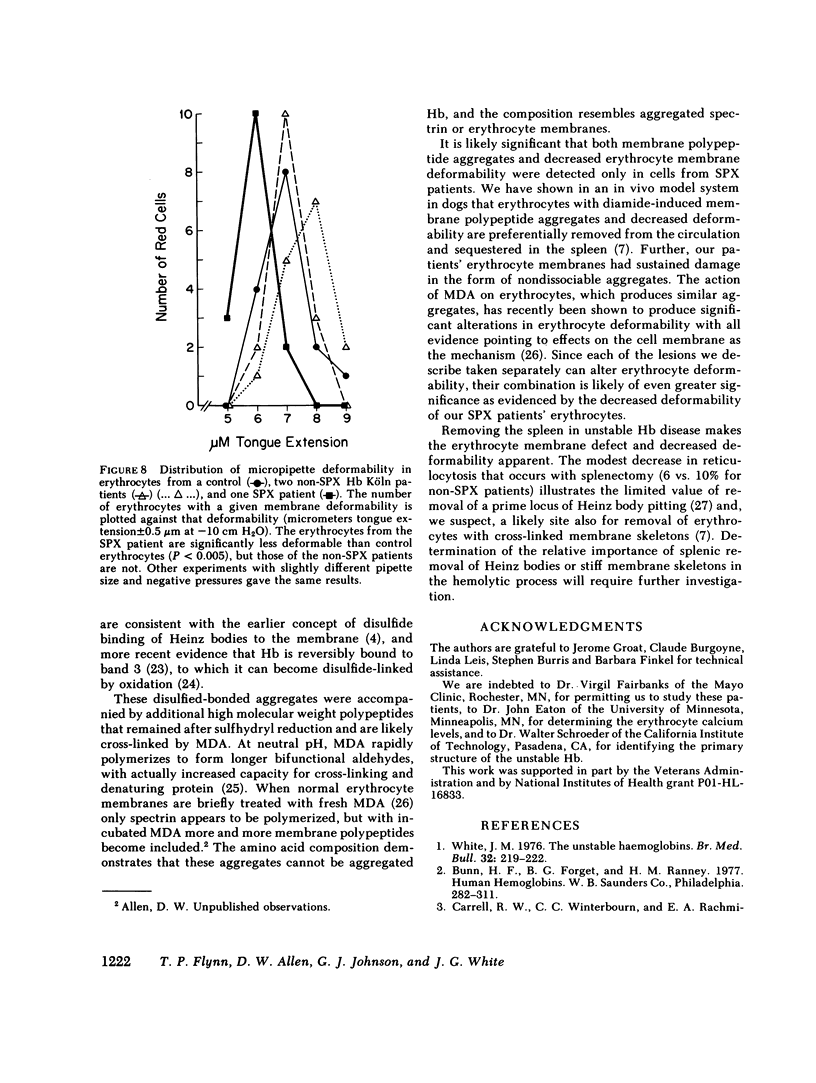
Since unstable hemoglobins have been considered a source of reactive oxygen radicals, and oxidative membrane damage a prehemolytic event, we examined the erythrocyte membranes of six patients (three splenectomized) with hemoglobin Köln disease. In the hydrogen peroxide stress test, the patients' erythrocytes generated more than twice the malonyldialdehyde (a lipid peroxidative product) than control erythrocytes. Fluorescence spectra of lipid extracts of the patients' erythrocytes showed an excitation maximum at 400 nm and an emission maximum of 460 nm, characteristic of malonyldialdehyde lipid adducts. Two types of membrane polypeptide aggregates were found in the erythrocytes of the splenectomized patients. The first, which were dissociable by treatment with mercaptoethanol, contained disulfide-linked spectrin, band 3 and globin. The second, not dissociable by mercaptoethanol, had an amino acid composition similar to that of erythrocyte membranes and spectrin (unlike globin) and like that of aggregates produced by the action of malonyldialdehyde on normal erythrocyte membranes. Atomic absorption spectroscopy of hemoglobin Köln erythrocytes showed no increase in calcium content implying that these cross-links were not due to calcium-stimulated transglutaminase. Using a micropipette technique, we demonstrated that erythrocytes containing membrane aggregates from splenectomized patients were less deformable while aggregate-free erythrocytes from non-splenectomized patients had normal deformability. We conclude that the erythrocyte membranes in hemoglobin Köln disease show evidence of lipid peroxidation with production of malonyldialdehyde, and that the nondissociable membrane aggregates formed in this disease are likely cross-linked by malonyldialdehyde. Because the erythrocytes containing membrane aggregates from splenectomized patients with unstable hemoglobin disease show decreased membrane deformability, we hypothesize that this abnormality results in premature erythrocyte destruction in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. W., Johnson G. J., Cadman S., Kaplan M. E. Membrane polypeptide aggregates in glucose 6-phosphate dehydrogenase-deficient and in vitro aged red blood cells. J Lab Clin Med. 1978 Feb;91(2):321–327. [PubMed] [Google Scholar]
- Carrell R. W., Lehmann H., Hutchison H. E. Haemoglobin Köln (beta-98 valine--methionine): an unstable protein causing inclusion-body anaemia. Nature. 1966 May 28;210(5039):915–916. doi: 10.1038/210915a0. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dillard C. J., Tappel A. L. Fluorescent products of lipid peroxidation of mitochondria and microsomes. Lipids. 1971 Oct;6(10):715–721. doi: 10.1007/BF02531296. [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Skelton T. D., Swofford H. S., Kolpin C. E., Jacob H. S. Elevated erythrocyte calcium in sickle cell disease. Nature. 1973 Nov 9;246(5428):105–106. doi: 10.1038/246105a0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Jacob H. S., Brain M. C., Dacie J. V. Altered sulfhydryl reactivity of hemoglobins and red blood cell membranes in congenital Heinz body hemolytic anemia. J Clin Invest. 1968 Dec;47(12):2664–2677. doi: 10.1172/JCI105950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. K., Hochstein P. Polymerization of membrane components in aging red blood cells. Biochem Biophys Res Commun. 1980 Jan 15;92(1):247–254. doi: 10.1016/0006-291x(80)91545-4. [DOI] [PubMed] [Google Scholar]
- Johnson G. J., Allen D. W., Cadman S., Fairbanks V. F., White J. G., Lampkin B. C., Kaplan M. E. Red-cell-membrane polypeptide aggregates in glucose-6-phosphate dehydrogenase mutants with chronic hemolytic disease. A clue to the mechanism of hemolysis. N Engl J Med. 1979 Sep 6;301(10):522–527. doi: 10.1056/NEJM197909063011004. [DOI] [PubMed] [Google Scholar]
- Johnson G. J., Allen D. W., Flynn T. P., Finkel B., White J. G. Decreased survival in vivo of diamide-incubated dog erythrocytes. A model of oxidant-induced hemolysis. J Clin Invest. 1980 Nov;66(5):955–961. doi: 10.1172/JCI109964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lux S. E., John K. M. Isolation and partial characterization of a high molecular weight red cell membrane protein complex normally removed by the spleen. Blood. 1977 Oct;50(4):625–641. [PubMed] [Google Scholar]
- Pfafferott C., Meiselman H. J., Hochstein P. The effect of malonyldialdehyde on erythrocyte deformability. Blood. 1982 Jan;59(1):12–15. [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Rifkind R. A. Heinz body anemia: an ultrastructural study. II. Red cell sequestration and destruction. Blood. 1965 Oct;26(4):433–448. [PubMed] [Google Scholar]
- Sayare M., Fikiet M. Cross-linking of hemoglobin to the cytoplasmic surface of human erythrocyte membranes. Identification of band 3 as a site for hemoglobin binding in Cu2+-o-phenanthroline catalyzed cross-linking. J Biol Chem. 1981 Dec 25;256(24):13152–13158. [PubMed] [Google Scholar]
- Schroeder W. A., Shelton J. B., Shelton J. R., Powars D., Friedman S., Baker J., Finklestein J. Z., Miller B., Johnson C. S., Sharpsteen J. R. Identification of eleven human hemoglobin variants by high-performance liquid chromatography: additional data on functional properties and clinical expression. Biochem Genet. 1982 Feb;20(1-2):133–152. doi: 10.1007/BF00484942. [DOI] [PubMed] [Google Scholar]
- Shaklai N., Yguerabide J., Ranney H. M. Classification and localization of hemoglobin binding sites on the red blood cell membrane. Biochemistry. 1977 Dec 13;16(25):5593–5597. doi: 10.1021/bi00644a032. [DOI] [PubMed] [Google Scholar]
- Shin B. C., Huggins J. W., Carraway K. L. Effects of pH, concentration and aging on the malonaldehyde reaction with proteins. Lipids. 1972 Apr;7(4):229–233. doi: 10.1007/BF02533218. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocks J., Offerman E. L., Modell C. B., Dormandy T. L. The susceptibility to autoxidation of human red cell lipids in health and disease. Br J Haematol. 1972 Dec;23(6):713–724. doi: 10.1111/j.1365-2141.1972.tb03486.x. [DOI] [PubMed] [Google Scholar]
- White J. M. The unstable haemoglobins. Br Med Bull. 1976 Sep;32(3):219–222. doi: 10.1093/oxfordjournals.bmb.a071366. [DOI] [PubMed] [Google Scholar]