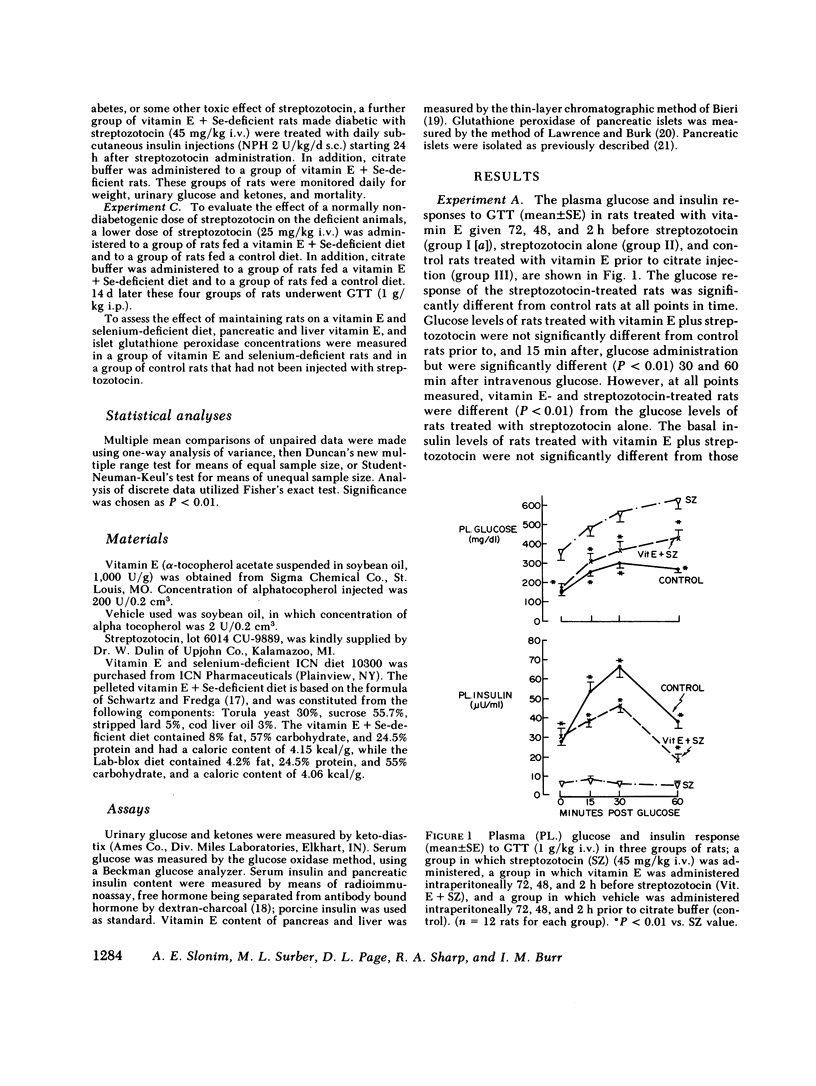
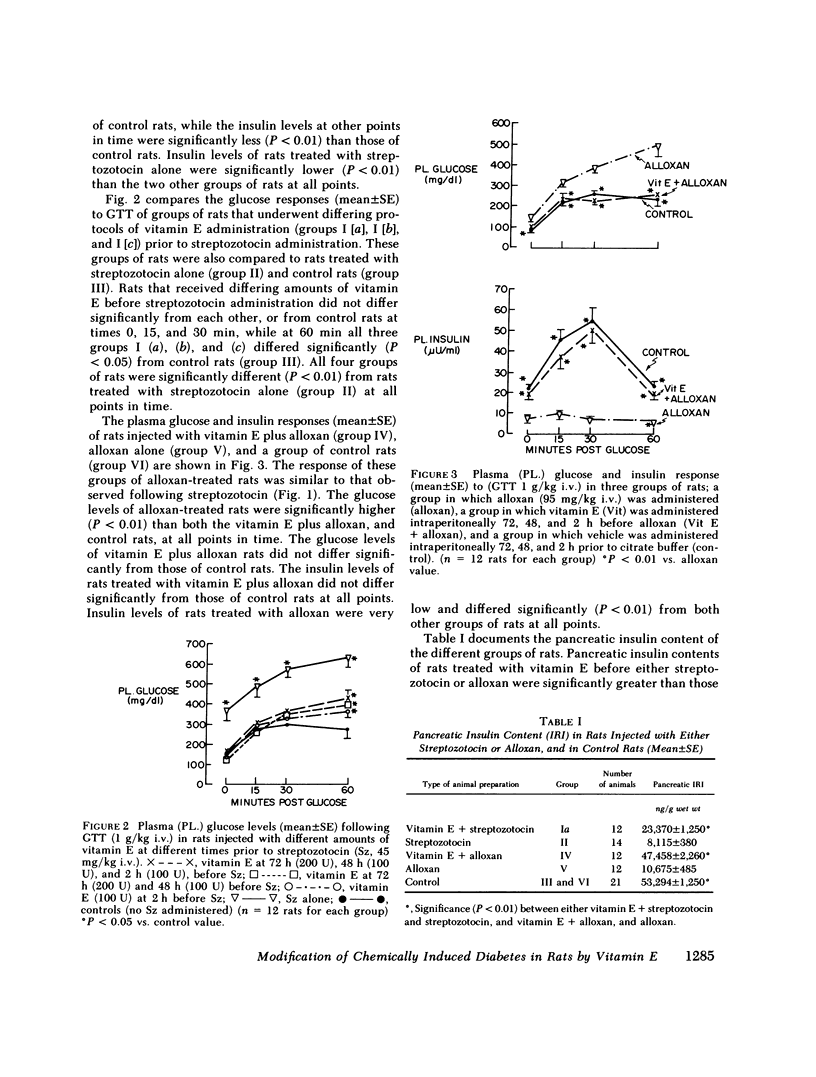
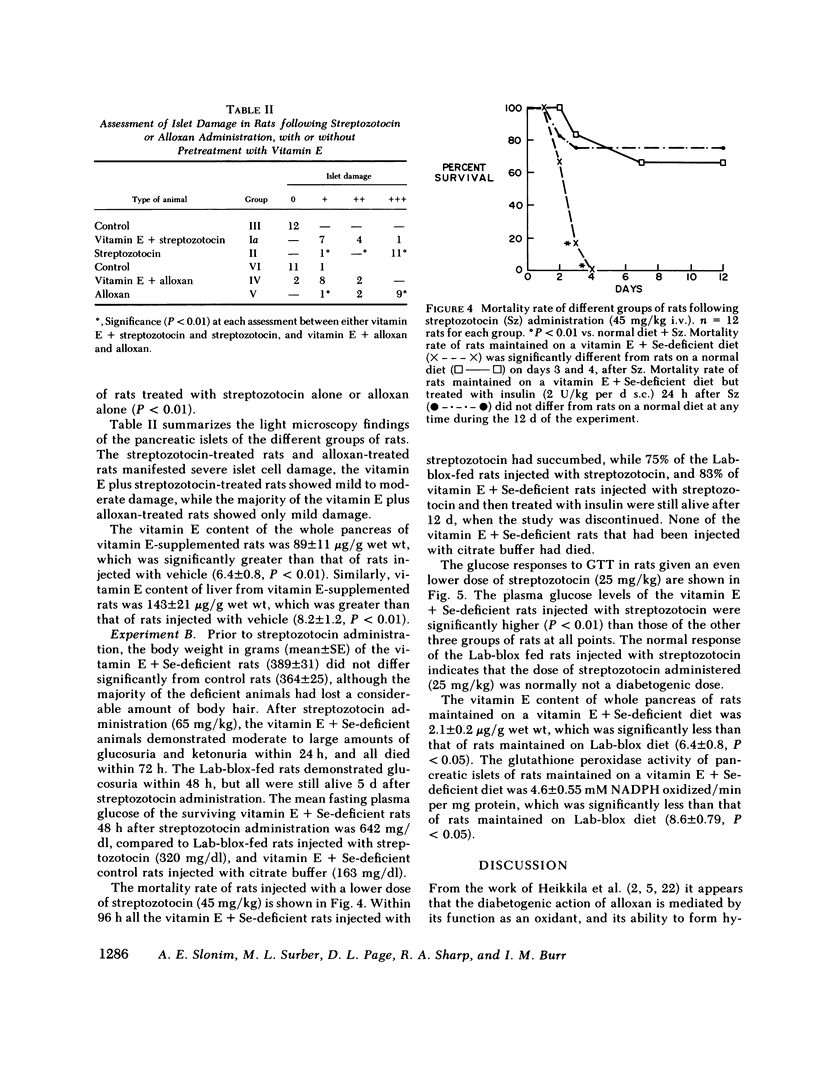
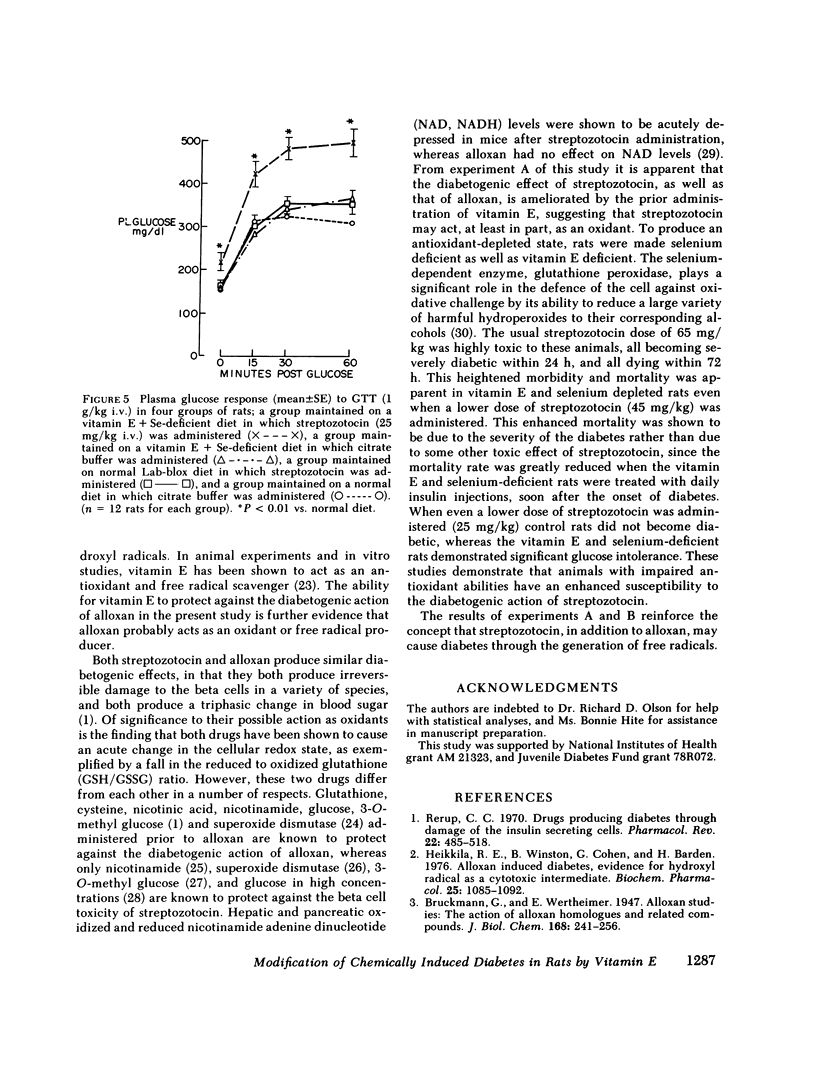
Abstract
Administration of the antioxidant vitamin E to rats, prior to administration of either streptozotocin or alloxan, provided protection against the diabetogenic effect of both these agents. This was demonstrated by their response to a glucose load, their pancreatic insulin content and light microscopy findings. In addition, rats whose antioxidant state was depleted, by being maintained on a vitamin E and selenium-deficient diet, demonstrated increased diabetogenic susceptibility to normally nondiabetogenic doses of streptozotocin. These findings provide indirect support for the suggestion that the chemical agents streptozotocin and alloxan may exert their diabetogenic effect by acting as oxidants or free radical producers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chow C. K., Tappel A. L. An enzymatic protective mechanism against lipid peroxidation damage to lungs of ozone-exposed rats. Lipids. 1972 Aug;7(8):518–524. doi: 10.1007/BF02533017. [DOI] [PubMed] [Google Scholar]
- Fischer L. J., Hamburger S. A. Inhibition of alloxan action in isolated pancreatic islets by superoxide dismutase, catalase, and a metal chelator. Diabetes. 1980 Mar;29(3):213–216. doi: 10.2337/diab.29.3.213. [DOI] [PubMed] [Google Scholar]
- GALLAGHER C. H. Protection by antioxidants against lethal doses of carbon tetrachloride. Nature. 1961 Dec 2;192:881–882. doi: 10.1038/192881a0. [DOI] [PubMed] [Google Scholar]
- Havu N. Sulfhydryl inhibitors and pancreatic islet tissue. Acta Endocrinol Suppl (Copenh) 1969;139:1–231. [PubMed] [Google Scholar]
- Heikkila R. E., Cabbat E. S. Protection against alloxan-induced diabetes in mice by the hydroxyl radical scavenger dimethylurea. Eur J Pharmacol. 1978 Nov 1;52(1):57–60. doi: 10.1016/0014-2999(78)90021-3. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E. The prevention of alloxan-induced diabetes in mice by dimethyl sulfoxide. Eur J Pharmacol. 1977 Jul 15;44(2):191–193. doi: 10.1016/0014-2999(77)90106-6. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Winston B., Cohen G. Alloxan-induced diabetes-evidence for hydroxyl radical as a cytotoxic intermediate. Biochem Pharmacol. 1976 May 1;25(9):1085–1092. doi: 10.1016/0006-2952(76)90502-5. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Marx J. L. Aging research (I): cellular theories of senescence. Science. 1974 Dec 20;186(4169):1105–1107. doi: 10.1126/science.186.4169.1105. [DOI] [PubMed] [Google Scholar]
- McCray P. B., Gibson D. D., Fong K. L., Hornbrook K. R. Effect of glutathione peroxidase activity on lipid peroxidation in biological membranes. Biochim Biophys Acta. 1976 Jun 22;431(3):459–468. [PubMed] [Google Scholar]
- Myers C. E., McGuire W., Young R. Adriamycin: amelioration of toxicity by alpha-tocopherol. Cancer Treat Rep. 1976 Jul;60(7):961–962. [PubMed] [Google Scholar]
- Rerup C. C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970 Dec;22(4):485–518. [PubMed] [Google Scholar]
- Robbins M. J., Sharp R. A., Slonim A. E., Burr I. M. Protection against streptozotocin-induced diabetes by superoxide dismutase. Diabetologia. 1980 Jan;18(1):55–58. doi: 10.1007/BF01228303. [DOI] [PubMed] [Google Scholar]
- Sandler S., Andersson A. The partial protective effect of the hydroxyl radical scavenger dimethyl urea on streptozotocin-induced diabetes in the mouse in vivo and in vitro. Diabetologia. 1982 Oct;23(4):374–378. doi: 10.1007/BF00253747. [DOI] [PubMed] [Google Scholar]
- Schein P. S., Cooney D. A., Vernon M. L. The use of nicotinamide to modify the toxicity of streptozotocin diabetes without loss of antitumor activity. Cancer Res. 1967 Dec;27(12):2324–2332. [PubMed] [Google Scholar]
- Schimmel R. J., Graham D. Inhibition by diphenylhydantoin of the diabetogenic action of streptozotocin. Horm Metab Res. 1974 Nov;6(6):475–477. doi: 10.1055/s-0028-1093806. [DOI] [PubMed] [Google Scholar]
- Schwarz K. Biological potency of organic selenium compounds. I. Aliphatic monoseleno- and diseleno-dicarboxylic acids. J Biol Chem. 1969 Apr 25;244(8):2103–2110. [PubMed] [Google Scholar]
- Sharp R., Culbert S., Cook J., Jennings A., Burr I. M. Cholinergic modification of glucose-induced biphasic insulin release in vitro. J Clin Invest. 1974 Mar;53(3):710–716. doi: 10.1172/JCI107609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonim A. E., Fletcher T., Burke V., Burr I. M. Effect of streptozotocin on red-blood-cell-reduced glutathione: modification by glucose, nicotinamide, and epinephrine. Diabetes. 1976 Mar;25(3):216–222. doi: 10.2337/diab.25.3.216. [DOI] [PubMed] [Google Scholar]
- Smulson M. E., Schein P., Mullins D. W., Jr, Sudhakar S. A putative role for nicotinamide adenine dinucleotide-promoted nuclear protein modification in the antitumor activity of N-methyl-N-nitrosourea. Cancer Res. 1977 Sep;37(9):3006–3012. [PubMed] [Google Scholar]
- Tappel A. L. Vitamin E and free radical peroxidation of lipids. Ann N Y Acad Sci. 1972 Dec 18;203:12–28. doi: 10.1111/j.1749-6632.1972.tb27851.x. [DOI] [PubMed] [Google Scholar]


