Abstract
Myeloperoxidase (MPO), a heme enzyme present in the azurophilic granules of human polymorphonuclear neutrophils (PMN), is important in the oxygen-dependent microbicidal activity of PMN. MPO deficiency, defined as the lack of PMN peroxidative activity, is a common genetic defect of human PMN. The purpose of our study was to characterize the structural basis for this loss of enzymatic activity, using protein biochemical and immunochemical techniques to examine PMN from three subjects with partial MPO deficiency and from five subjects with complete MPO deficiency.
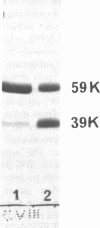
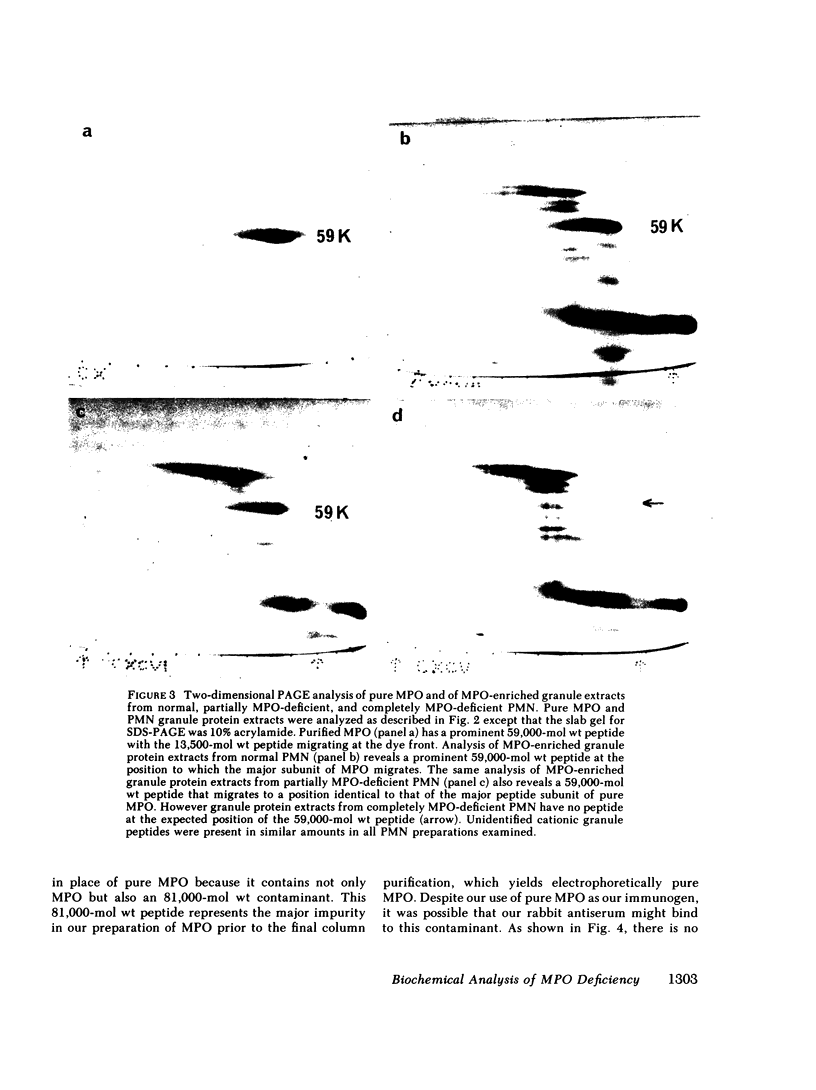
We purified MPO from normal PMN and defined its electrophoretic mobility after two-dimensional electrophoretic separation, using nondenaturing acidic polyacrylamide gel electrophoresis (PAGE) followed by sodium dodecyl sulfate (SDS) denaturation and SDS-PAGE separation of MPO subunit peptides. In agreement with previous studies, we found that normal MPO had subunits of 59,000 and 13,500 mol wt when subjected to SDS-PAGE under reducing conditions.
Granule protein extracts of normal PMN, partially MPO-deficient PMN, and completely MPO-deficient PMN were analyzed with two-dimensional PAGE. Partially MPO-deficient PMN granules contained electrophoretically normal MPO in less than normal amounts, whereas completely MPO-deficient PMN granules contain no protein with the electrophoretic mobility of normal MPO.
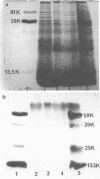
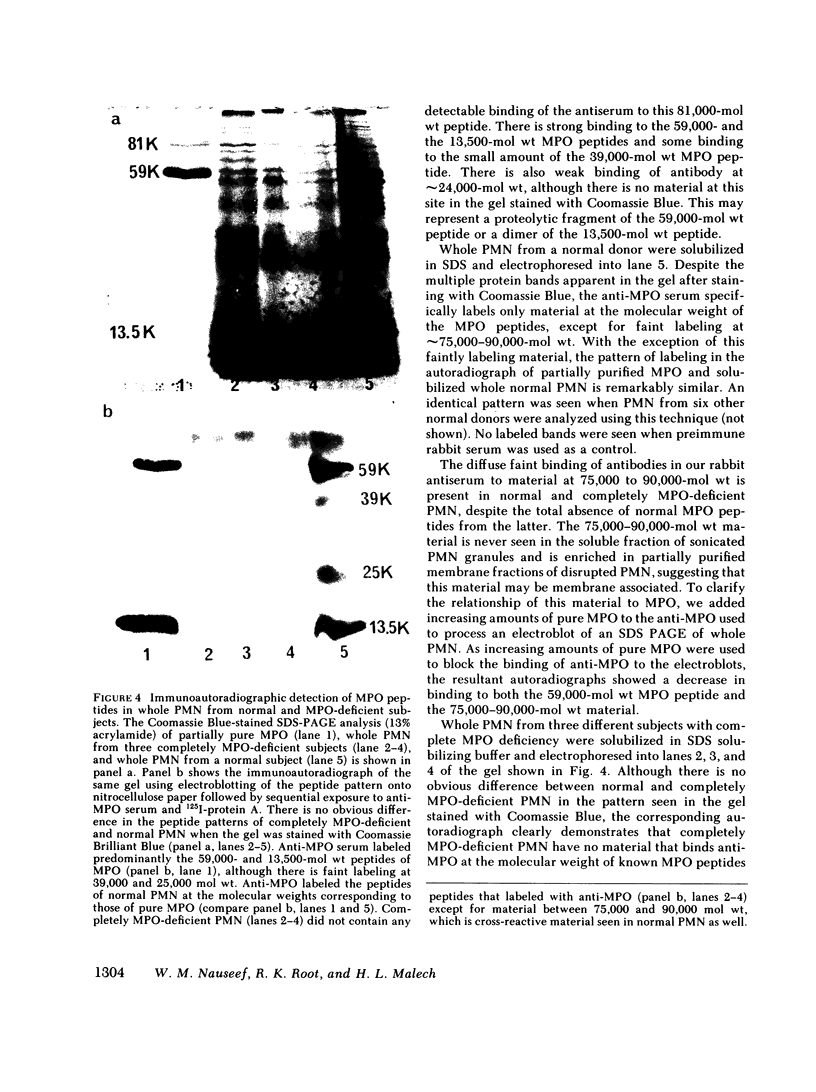
Using rabbit antiserum against purified MPO, we used immunoautoradiographic analysis to examine whole PMN for peptides immunochemically related to MPO. PMN from normal, partially MPO-deficient, and completely MPO-deficient subjects were solubilized in SDS and component peptides separated by SDS-PAGE. The peptides were electroblotted onto nitrocellulose paper that was exposed sequentially to rabbit anti-MPO and 125I-protein A before autoradiography. Radiolabeled bands were identical when partially purified MPO or normal PMN were compared except that whole PMN contained a small amount of an immunologically cross-reactive membrane associated material of 75,000-90,000 mol wt. Using a modification of this immunoautoradiographic analysis, we quantitated the relative amounts of MPO peptides in PMN. PMN from MPO-deficient subjects contain 41.0-52.3% the amount of MPO peptides present in normal PMN. Similar analysis showed that completely MPO-deficient PMN lacked any peptides corresponding to MPO peptides.
We conclude that partial MPO deficiency is characterized by the presence of electrophoretically and immunologically normal MPO in amounts approximately one-half that seen in PMN from normal subjects. Completely MPO-deficient PMN lack any normal MPO peptides. No MPO-deficient subject studied had an immunologically cross-reacting variant of MPO. Since this deficiency is associated with the absence of more than one peptide, it is possible that the underlying genetic defect may involve: (a) failure to synthesize a single precursor peptide; (b) abnormal regulation of the synthesis of two separate peptides; or (c) an aberration in postsynthetic processing or packaging into azurophilic granules.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrein P. C., Stossel T. P. Prevention of degradation of human polymorphonuclear leukocyte proteins by diisopropylfluorophosphate. Blood. 1980 Sep;56(3):442–447. [PubMed] [Google Scholar]
- Andrews P. C., Krinsky N. I. The reductive cleavage of myeloperoxidase in half, producing enzymically active hemi-myeloperoxidase. J Biol Chem. 1981 May 10;256(9):4211–4218. [PubMed] [Google Scholar]
- Bakkenist A. R., Wever R., Vulsma T., Plat H., van Gelder B. F. Isolation procedure and some properties of myeloperoxidase from human leucocytes. Biochim Biophys Acta. 1978 May 11;524(1):45–54. doi: 10.1016/0005-2744(78)90101-8. [DOI] [PubMed] [Google Scholar]
- Catovsky D., Galton D. A., Robinson J. Myeloperoxidase-deficient neutrophils in acute myeloid leukaemia. Scand J Haematol. 1972;9(2):142–148. doi: 10.1111/j.1600-0609.1972.tb00923.x. [DOI] [PubMed] [Google Scholar]
- Cech P., Papathanassiou A., Boreux G., Roth P., Miescher P. A. Hereditary myeloperoxidase deficiency. Blood. 1979 Mar;53(3):403–411. [PubMed] [Google Scholar]
- Cech P., Stalder H., Widmann J. J., Rohner A., Miescher P. A. Leukocyte myeloperoxidase deficiency and diabetes mellitus associated with Candida albicans liver abscess. Am J Med. 1979 Jan;66(1):149–153. doi: 10.1016/0002-9343(79)90507-2. [DOI] [PubMed] [Google Scholar]
- Clift R. A., Sanders J. E., Thomas E. D., Williams B., Buckner C. D. Granulocyte transfusions for the prevention of infection in patients receiving bone-marrow transplants. N Engl J Med. 1978 May 11;298(19):1052–1057. doi: 10.1056/NEJM197805112981904. [DOI] [PubMed] [Google Scholar]
- Davis A. T., Brunning R. D., Quie P. G. Polymorphonuclear leukocyte myeloperoxidase deficiency in a patient with myelomonocytic leukemia. N Engl J Med. 1971 Sep 30;285(14):789–790. doi: 10.1056/NEJM197109302851410. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Pabalan S., Schultz J. The subunit structure of crystalline canine myeloperoxidase. Biochim Biophys Acta. 1977 Aug 23;493(2):247–259. doi: 10.1016/0005-2795(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Higashi O., Katsuyama N., Satodate R. A case with hematological abnormality characterized by the absence of peroxidase activity in blood polymorphonuclear leukocytes. Tohoku J Exp Med. 1965 Oct 25;87(1):77–89. doi: 10.1620/tjem.87.77. [DOI] [PubMed] [Google Scholar]
- Hunh D., Belohradsky B. H., Haas R. Familiärer Myeloperoxidasedefekt und akute myeloische Leukämie. Acta Haematol. 1978;59(3):129–143. doi: 10.1159/000207756. [DOI] [PubMed] [Google Scholar]
- Kitahara M., Eyre H. J., Simonian Y., Atkin C. L., Hasstedt S. J. Hereditary myeloperoxidase deficiency. Blood. 1981 May;57(5):888–893. [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970 Sep 11;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Mikkelsen R. B., Corfman D. H., André-Schwartz J. Neutrophil plasma membranes. I. High-yield purification of human neutrophil plasma membrane vesicles by nitrogen cavitation and differential centrifugation. J Cell Biol. 1980 Jul;86(1):21–28. doi: 10.1083/jcb.86.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Goldberg L. S., Apple M. A., Rosenthal N. P. Refractory megaloblastic anemia with myeloperoxidase-deficient neutrophils. Ann Intern Med. 1972 Mar;76(3):447–453. doi: 10.7326/0003-4819-76-3-447. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Isolation and properties of human neutrophil myeloperoxidase. Biochemistry. 1981 Jan 20;20(2):325–330. doi: 10.1021/bi00505a015. [DOI] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmann K., Bojanovsky A. Rezidivierende Candidosis bei Myeloperoxydasemangel. Monatsschr Kinderheilkd. 1975 May;123(5):408–409. [PubMed] [Google Scholar]
- Olsson I., Olofsson T., Odeberg H. Myeloperoxidase-mediated iodination in granulocytes. Scand J Haematol. 1972;9(5):483–491. doi: 10.1111/j.1600-0609.1972.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Parry M. F., Root R. K., Metcalf J. A., Delaney K. K., Kaplow L. S., Richar W. J. Myeloperoxidase deficiency: prevalence and clinical significance. Ann Intern Med. 1981 Sep;95(3):293–301. doi: 10.7326/0003-4819-95-3-293. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon S. E., Cline M. J., Schultz J., Lehrer R. I. Myeloperoxidase deficiency. Immunologic study of a genetic leukocyte defect. N Engl J Med. 1970 Jan 29;282(5):250–253. doi: 10.1056/NEJM197001292820505. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undritz E. Die Alius-Grignaschi-Anomalie: Der erblich-konstitutionelle Peroxydasedefekt der Neutrophilen und Monozyten. Blut. 1966 Dec;14(3):129–136. doi: 10.1007/BF01631534. [DOI] [PubMed] [Google Scholar]
- Verdot J. J., Bayle J., Juhan I., Aillaud M. F., Guitard A. M., Muratore R. Hémogramme automatisé et déficit en myélopéroxydase. Analyse de trente-trois observations. Sem Hop. 1981 Mar 8;57(9-10):450–457. [PubMed] [Google Scholar]
- Yamada M., Mori M., Sugimura T. Purification and characterization of small molecular weight myeloperoxidase from human promyelocytic leukemia HL-60 cells. Biochemistry. 1981 Feb 17;20(4):766–771. doi: 10.1021/bi00507a018. [DOI] [PubMed] [Google Scholar]