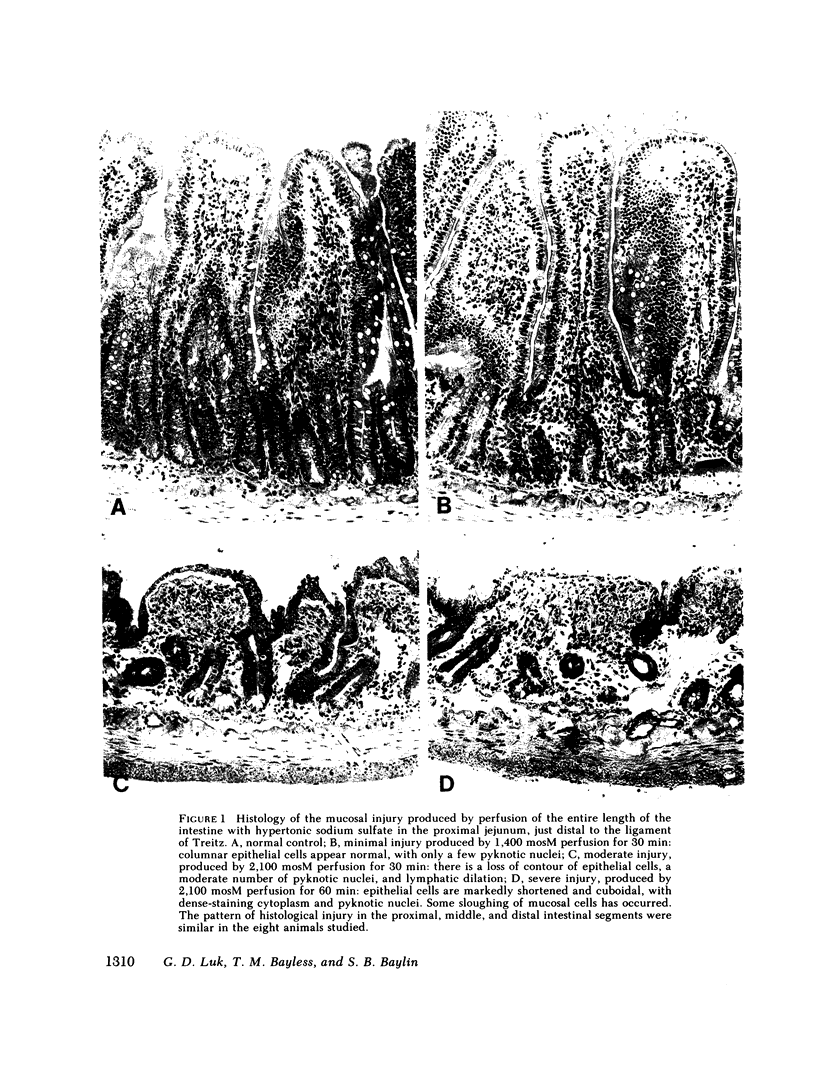
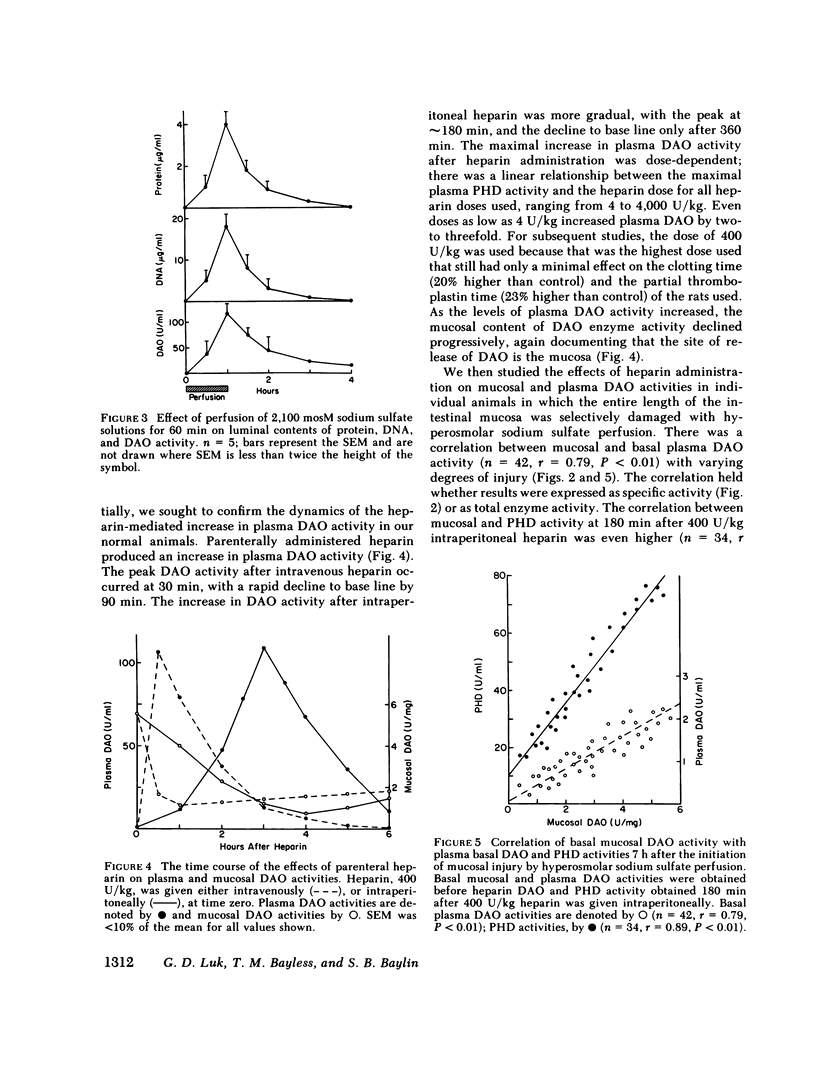
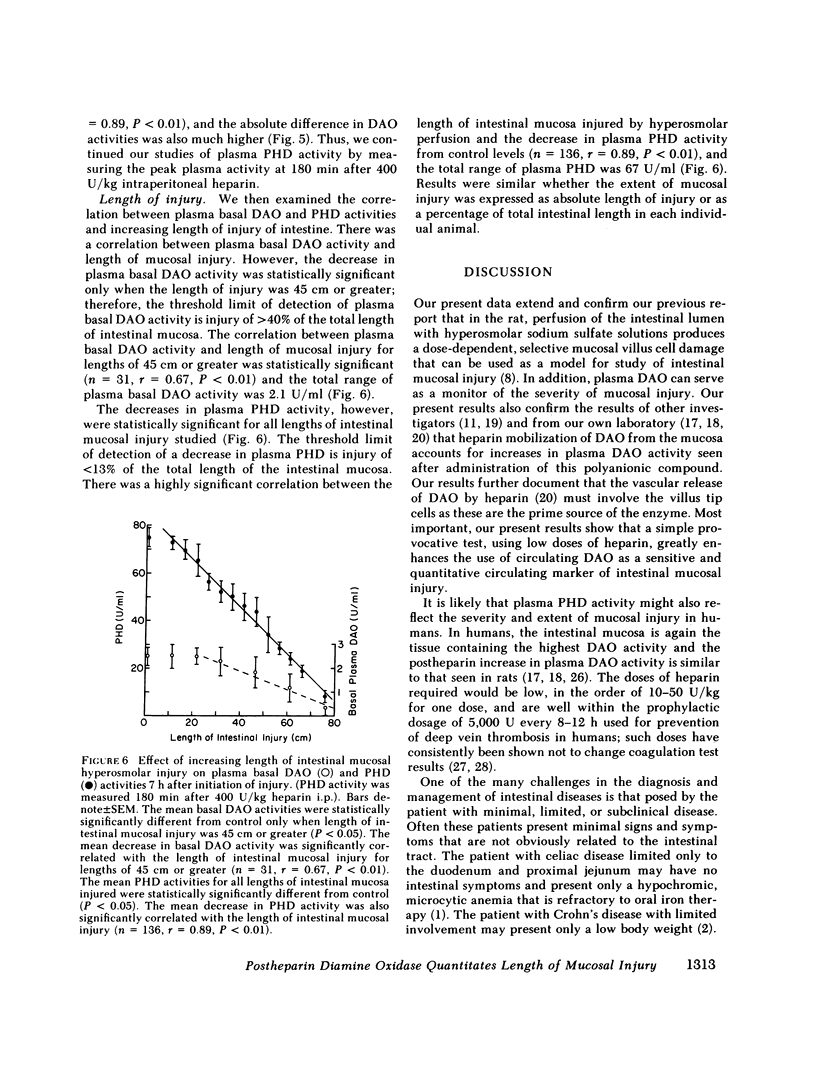
Abstract
Diamine oxidase (DAO; EC 1.4.3.6) is an enzyme found in high activity in the mature cells of the upper villus of rat small intestinal mucosa and in very much lower activity in all other tissues in the nonpregnant rat. This study was designed to determine whether a provocative test for increasing the level of plasma DAO activity by heparin administration could be used to monitor the extent and severity of acute, severe, small intestinal mucosal injury. In adult rats, small intestinal loops of varying lengths were perfused with 2,100 mosM sodium sulfate solution for 60 min to produce selective damage to villus epithelium. Plasma postheparin DAO (PHD) activity (180 min after 400 U/kg i.p. heparin) was measured 7 h after initiation of perfusion. With increasing length of intestinal mucosal injury, there was a progressive decrease in both basal and plasma PHD activity. The decrease in plasma PHD activity closely reflected the length of intestinal mucosa injured (n = 128, r = 0.86, P less than 0.001), and it was much more sensitive (threshold limit of detection = 13% of total length, range = 67 U/ml for 100% length of injury) than unstimulated basal levels of plasma DAO (threshold = 40%, range = 2.1 U/ml). Our previous data have suggested that DAO is unique among intestinal mucosal enzymes in that circulating levels can serve as a marker of mucosal injury; this study illustrates that the addition of a low-dose heparin administration enhances the use of DAO even further as a sensitive, quantitative, circulating marker for monitoring the extent of small intestinal mucosal injury in the rat.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylin S. B., Beaven M. A., Buja L. M., Keiser H. R. Histaminase activity: a biochemical marker for medullary carcinoma of the thyroid. Am J Med. 1972 Dec;53(6):723–733. doi: 10.1016/0002-9343(72)90189-1. [DOI] [PubMed] [Google Scholar]
- Baylin S. B., Beaven M. A., Krauss R. M., Keiser H. R. Response of plasma histaminase activity to small doses of heparin in normal subjects and patients with hyperlipoproteinemia. J Clin Invest. 1973 Aug;52(8):1985–1993. doi: 10.1172/JCI107383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylin S. B., Stevens S. A., Shakir K. M. Association of diamine oxidase and ornithine decarboxylase with maturing cells in rapidly proliferating epithelium. Biochim Biophys Acta. 1978 Jul 3;541(3):415–419. doi: 10.1016/0304-4165(78)90200-3. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Jacobsen S. A new assay for histaminase activity: measurement of tritiated water from beta (side chain label)-H 3-histamine. J Pharmacol Exp Ther. 1971 Jan;176(1):52–64. [PubMed] [Google Scholar]
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Best W. R., Becktel J. M., Singleton J. W. Rederived values of the eight coefficients of the Crohn's Disease Activity Index (CDAI). Gastroenterology. 1979 Oct;77(4 Pt 2):843–846. [PubMed] [Google Scholar]
- Buffoni F. Histaminase and related amine oxidases. Pharmacol Rev. 1966 Dec;18(4):1163–1199. [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Dahlbäck O., Hansson R., Tibbling G., Tryding N. The effect of heparin on diamine oxidase and lipoprotein lipase in human lymph and blood plasma. Scand J Clin Lab Invest. 1968;21(1):17–25. doi: 10.3109/00365516809076972. [DOI] [PubMed] [Google Scholar]
- Ettinger D. S., Baylin S. B., Minaberry D., Abeloff M. D., Mellits E. D. Response of plasma histaminase activity to heparin in normal subjects and in patients with small cell carcinoma of the lung. J Natl Cancer Inst. 1978 Jun;60(6):1239–1242. doi: 10.1093/jnci/60.6.1239. [DOI] [PubMed] [Google Scholar]
- Hansson R., Holmberg C. G., Tibbling G., Tryding N., Westling H., Wetterqvist H. Heparin-induced diamine oxidase increase in human blood plasma. Acta Med Scand. 1966 Nov;180(5):533–536. doi: 10.1111/j.0954-6820.1966.tb02866.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Kupelian J., Maudsley D. V. Release of diamine oxidase by heparin in the rat. Biochem Pharmacol. 1969 Jul;18(7):1585–1591. doi: 10.1016/0006-2952(69)90145-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Luk G. D., Bayless T. M., Baylin S. B. Diamine oxidase (histaminase). A circulating marker for rat intestinal mucosal maturation and integrity. J Clin Invest. 1980 Jul;66(1):66–70. doi: 10.1172/JCI109836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk G. D., Vaughan W. P., Burke P. J., Baylin S. B. Diamine oxidase as a plasma marker of rat intestinal mucosal injury and regeneration after administration of 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1981 Jun;41(6):2334–2337. [PubMed] [Google Scholar]
- Present D. H., Korelitz B. I., Wisch N., Glass J. L., Sachar D. B., Pasternack B. S. Treatment of Crohn's disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980 May 1;302(18):981–987. doi: 10.1056/NEJM198005013021801. [DOI] [PubMed] [Google Scholar]
- Roggin G. M., Banwell J. G., Yardley J. H., Hendrix T. R. Unimpaired response of rabbit jejunum to cholera toxin after selective damage to villus epithelium. Gastroenterology. 1972 Dec;63(6):981–989. [PubMed] [Google Scholar]
- Rosenberg R. D. Biologic actions of heparin. Semin Hematol. 1977 Oct;14(4):427–440. [PubMed] [Google Scholar]
- Shaff R. E., Beaven M. A. Turnover and synthesis of diamine oxidase (DAO) in rat tissues. Studies with heparin and cycloheximide. Biochem Pharmacol. 1976 May 1;25(9):1057–1062. doi: 10.1016/0006-2952(76)90496-2. [DOI] [PubMed] [Google Scholar]
- Shakir K. M., Margolis S., Baylin S. B. Localization of histamine (diamine oxidase) in rat small intestinal mucosa: site of release by heparin. Biochem Pharmacol. 1977 Dec 15;26(24):2343–2347. doi: 10.1016/0006-2952(77)90438-5. [DOI] [PubMed] [Google Scholar]
- Taylor S. L., Lieber E. R. Subcellular distribution and properties of intestinal histamine-metabolizing enzymes from rats, guinea pigs and rhesus monkeys. Comp Biochem Physiol C. 1979;63C(1):21–26. doi: 10.1016/0306-4492(79)90124-2. [DOI] [PubMed] [Google Scholar]
- Whittington P. F., Barnes H. V., Bayless T. M. Medical management of Crohn's disease in adolescence. Gastroenterology. 1977 Jun;72(6):1338–1344. [PubMed] [Google Scholar]