Abstract
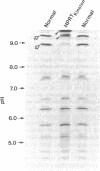
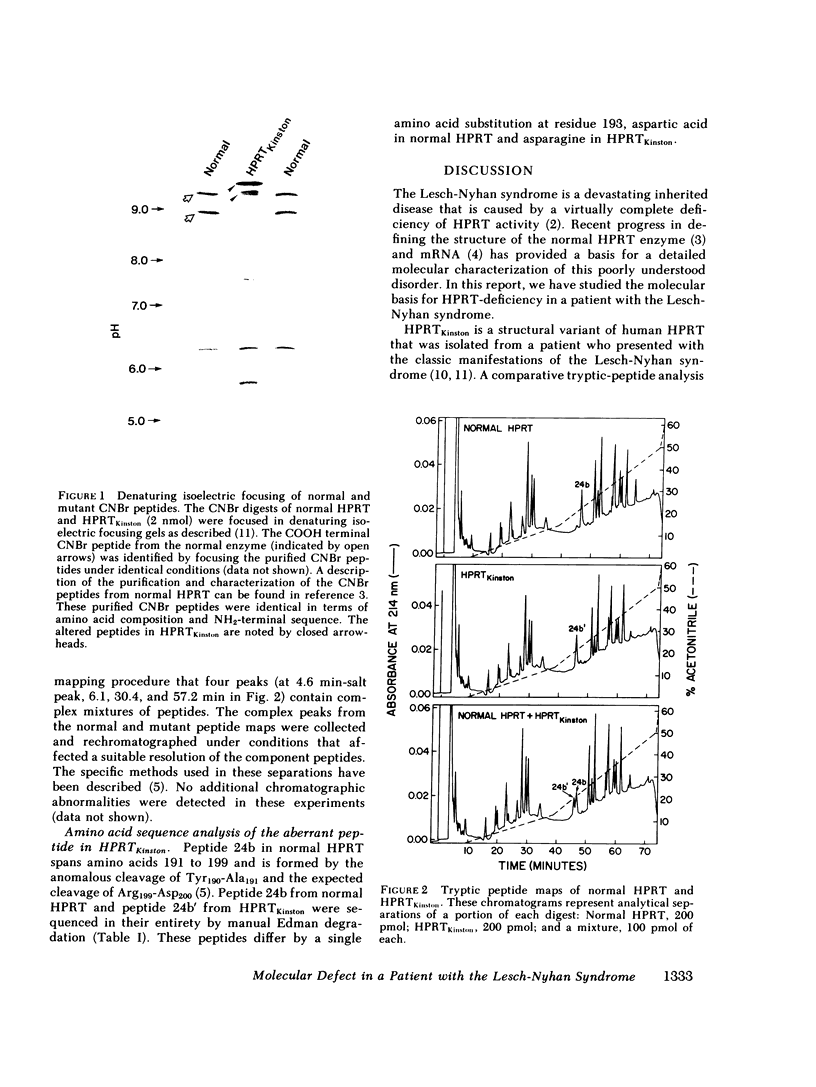
We have investigated the molecular basis of hypoxanthine-guanine phosphoribosyltransferase (HPRT) deficiency in a patient who presented with the Lesch-Nyhan syndrome. A catalytically incompetent form of HPRT has been isolated from this patient's erythrocytes and lymphoblasts. This enzyme variant, which we have termed HPRTKinston, is indistinguishable from the normal enzyme in terms of its intracellular concentration and maximal velocity, but differs with respect to its isoelectric point (more basic) and Michaelis constants for both substrates (markedly elevated). The tryptic peptides of HPRTKinston were mapped by reverse-phase high pressure liquid chromatography in an attempt to define the precise abnormality in its primary structure. Sequence analysis of the single aberrant tryptic peptide in HPRTKinston revealed an aspartic acid to asparagine amino acid substitution at position 193. Electrophoretic analysis of the CNBr peptides of HPRTKinston confirmed the location of the proposed mutation. This amino acid substitution can be explained by a single nucleotide change in the codon for aspartic acid 193 (GAC leads to AAC). This is the first specific mutation described at the molecular level in a patient with the Lesch-Nyhan syndrome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennand J., Chinault A. C., Konecki D. S., Melton D. W., Caskey C. T. Cloned cDNA sequences of the hypoxanthine/guanine phosphoribosyltransferase gene from a mouse neuroblastoma cell line found to have amplified genomic sequences. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1950–1954. doi: 10.1073/pnas.79.6.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghangas G. S., Milman G. Hypoxanthine phosphoribosyltransferase: two-dimensional gels from normal and Lesch-Nyhan hemolyzates. Science. 1977 Jun 3;196(4294):1119–1120. doi: 10.1126/science.870972. [DOI] [PubMed] [Google Scholar]
- Ghangas G. S., Milman G. Radioimmune determination of hypoxanthine phosphoribosyltransferase crossreacting material in erythrocytes of Lesch-Nyhan patients. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4147–4150. doi: 10.1073/pnas.72.10.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden J. A., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Evidence for tetrameric structure. J Biol Chem. 1978 Jun 25;253(12):4459–4463. [PubMed] [Google Scholar]
- Jolly D. J., Okayama H., Berg P., Esty A. C., Filpula D., Bohlen P., Johnson G. G., Shively J. E., Hunkapillar T., Friedmann T. Isolation and characterization of a full-length expressible cDNA for human hypoxanthine phosphoribosyl transferase. Proc Natl Acad Sci U S A. 1983 Jan;80(2):477–481. doi: 10.1073/pnas.80.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley W. N., Rosenbloom F. M., Henderson J. F., Seegmiller J. E. A specific enzyme defect in gout associated with overproduction of uric acid. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1735–1739. doi: 10.1073/pnas.57.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A., Kelley W. N. Lesch-Nyhan syndrome: altered kinetic properties of mutant enzyme. Science. 1971 Feb 19;171(3972):689–691. doi: 10.1126/science.171.3972.689. [DOI] [PubMed] [Google Scholar]
- Rijksen G., Staal G. E., van der Vlist M. J., Beemer F. a., Troost J., Gutensohn W., van Laarhoven J. P., de Bruyn C. H. Partial hypoxanthine-guanine phosphoribosyl transferase deficiency with full expression of the Lesch-Nyhan syndrome. Hum Genet. 1981;57(1):39–47. doi: 10.1007/BF00271165. [DOI] [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Upchurch K. S., Leyva A., Arnold W. J., Holmes E. W., Kelley W. N. Hypoxanthine phosphoribosyltransferase deficiency: association of reduced catalytic activity with reduced levels of immunologically detectable enzyme protein. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4142–4146. doi: 10.1073/pnas.72.10.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Baugher B. W., Landa L., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Purification and characterization of mutant forms of the enzyme. J Biol Chem. 1981 Oct 25;256(20):10306–10312. [PubMed] [Google Scholar]
- Wilson J. M., Baugher B. W., Mattes P. M., Daddona P. E., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Demonstration of structural variants in lymphoblastoid cells derived from patients with a deficiency of the enzyme. J Clin Invest. 1982 Mar;69(3):706–715. doi: 10.1172/JCI110499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Landa L. E., Kobayashi R., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Tryptic peptides and post-translational modification of the erythrocyte enzyme. J Biol Chem. 1982 Dec 25;257(24):14830–14834. [PubMed] [Google Scholar]
- Wilson J. M., Tarr G. E., Kelley W. N. Human hypoxanthine (guanine) phosphoribosyltransferase: an amino acid substitution in a mutant form of the enzyme isolated from a patient with gout. Proc Natl Acad Sci U S A. 1983 Feb;80(3):870–873. doi: 10.1073/pnas.80.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. M., Tarr G. E., Mahoney W. C., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Complete amino acid sequence of the erythrocyte enzyme. J Biol Chem. 1982 Sep 25;257(18):10978–10985. [PubMed] [Google Scholar]