Abstract
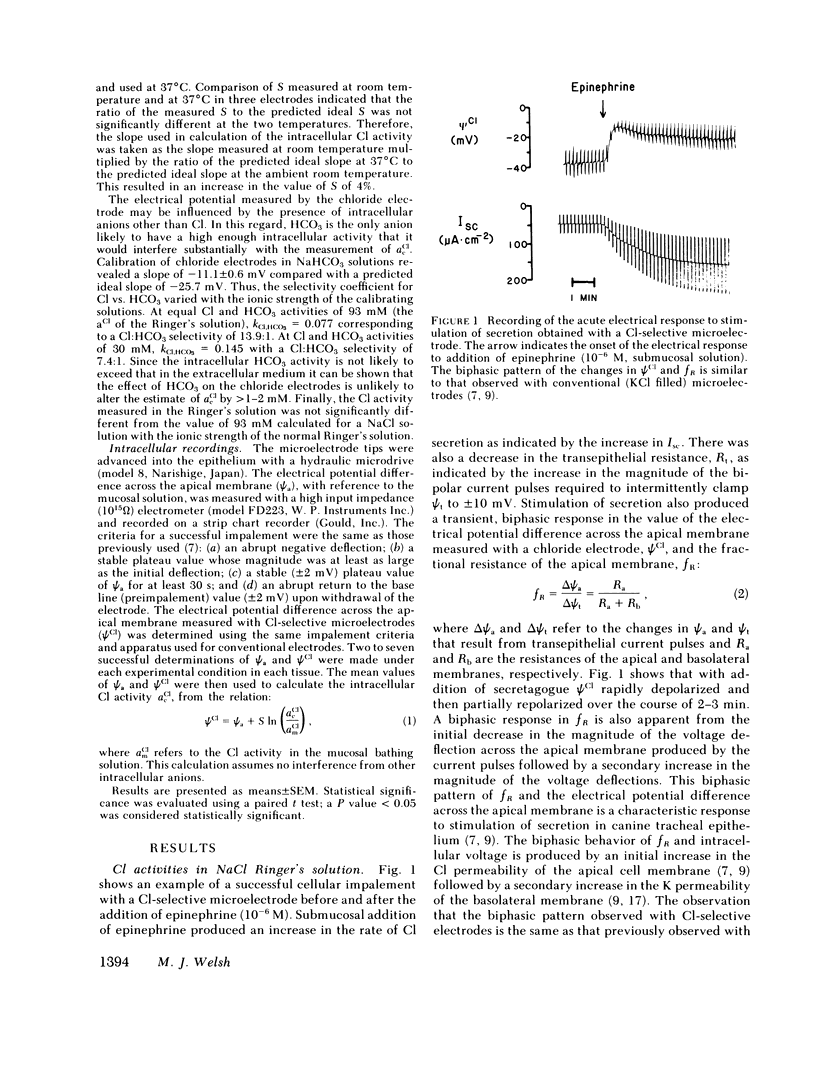
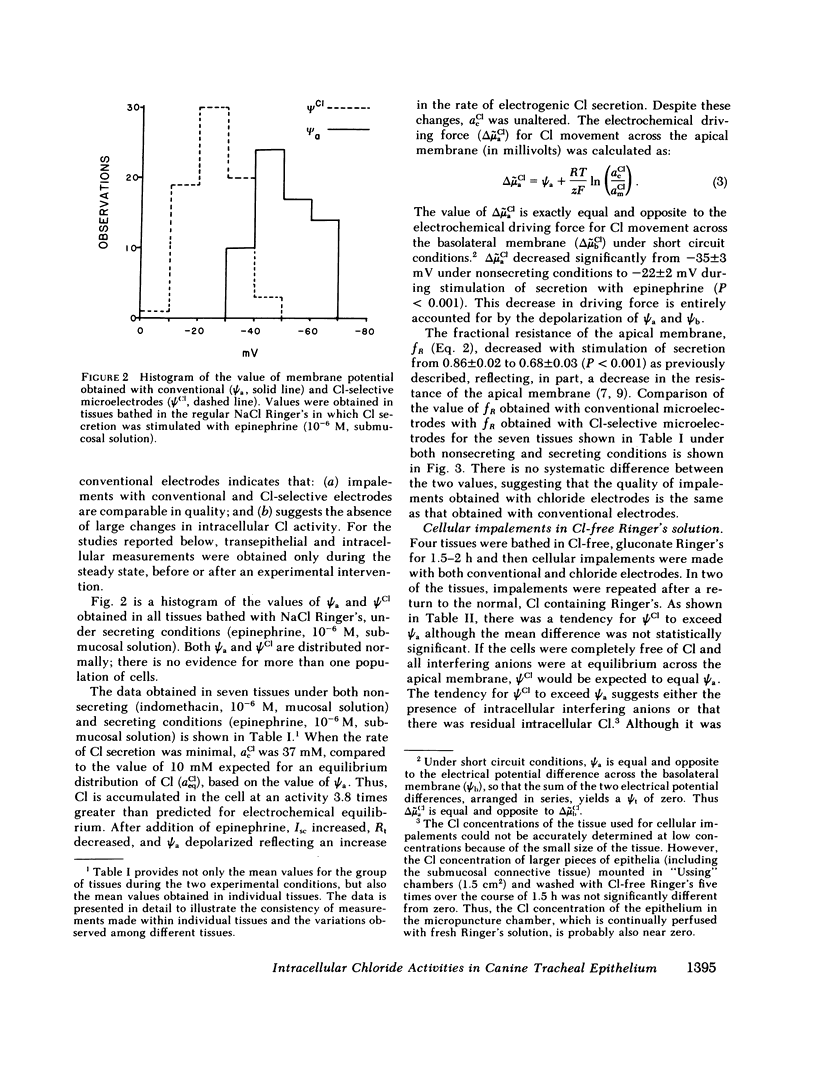
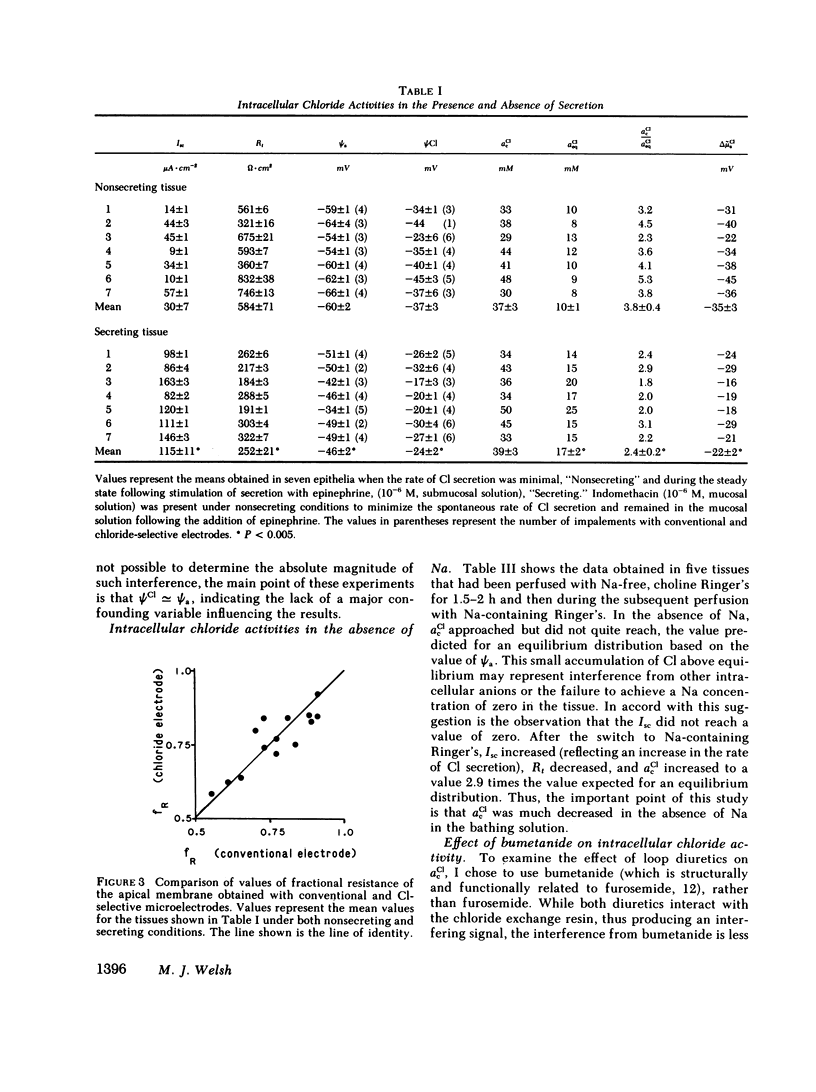
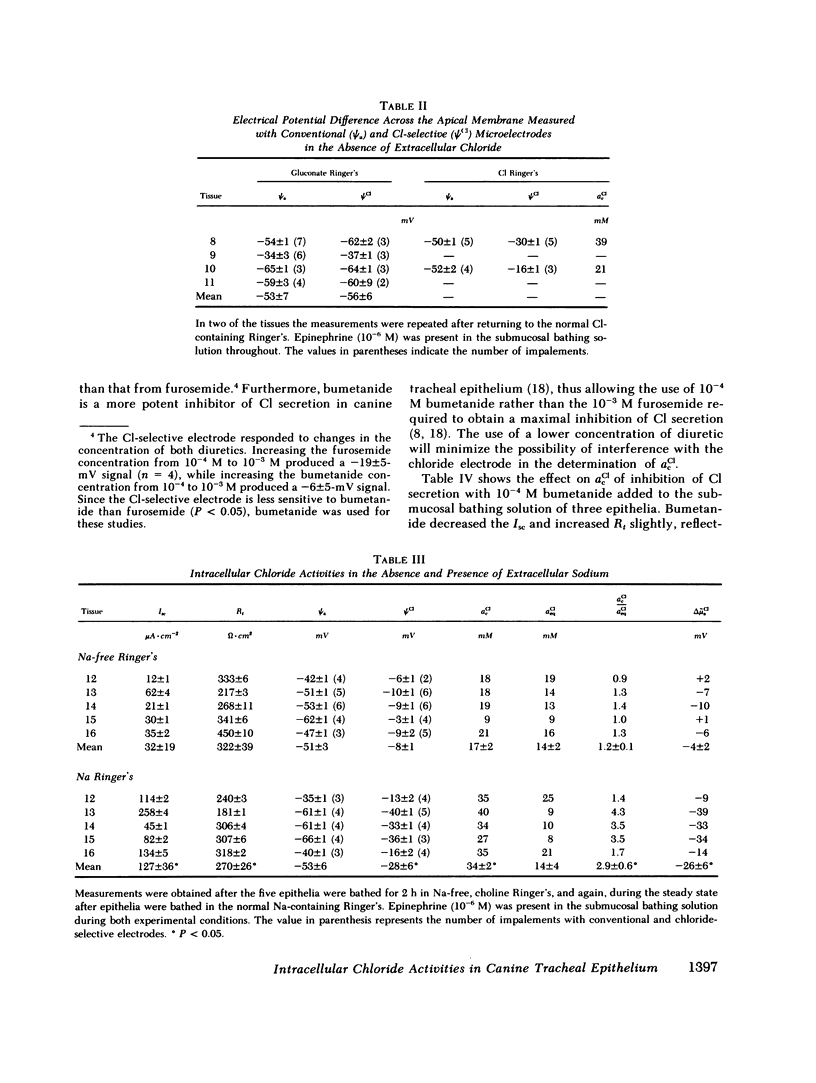
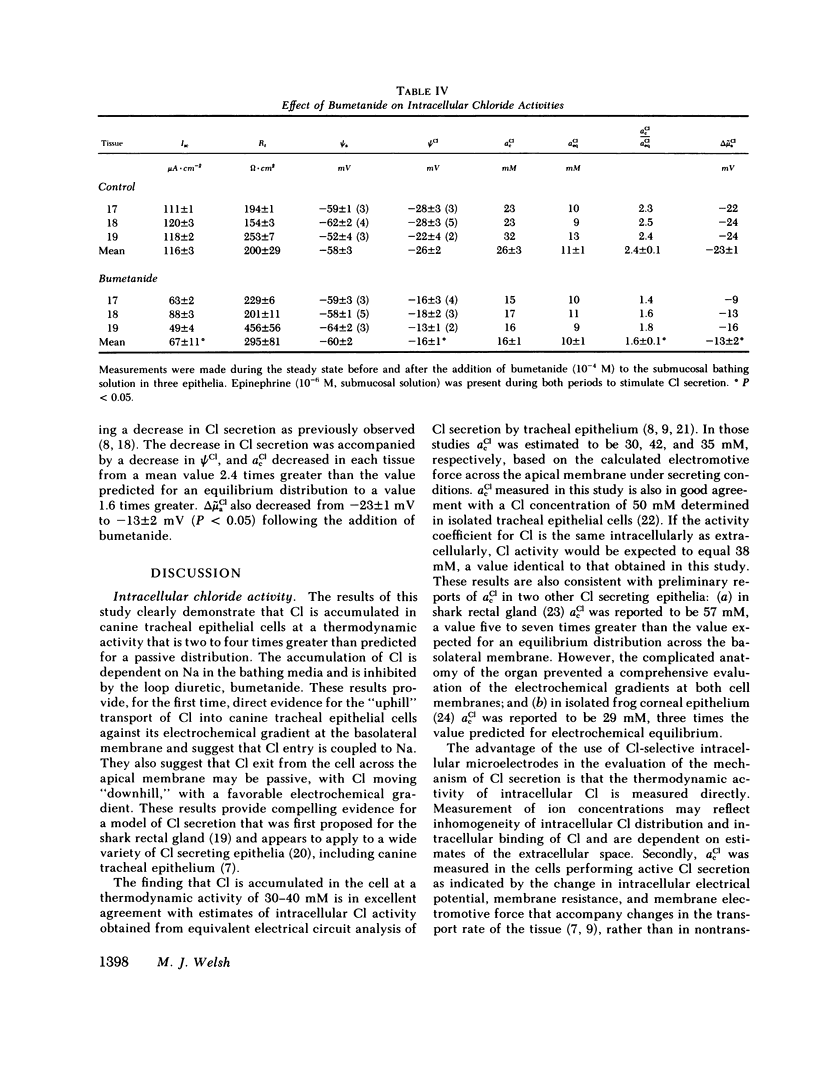
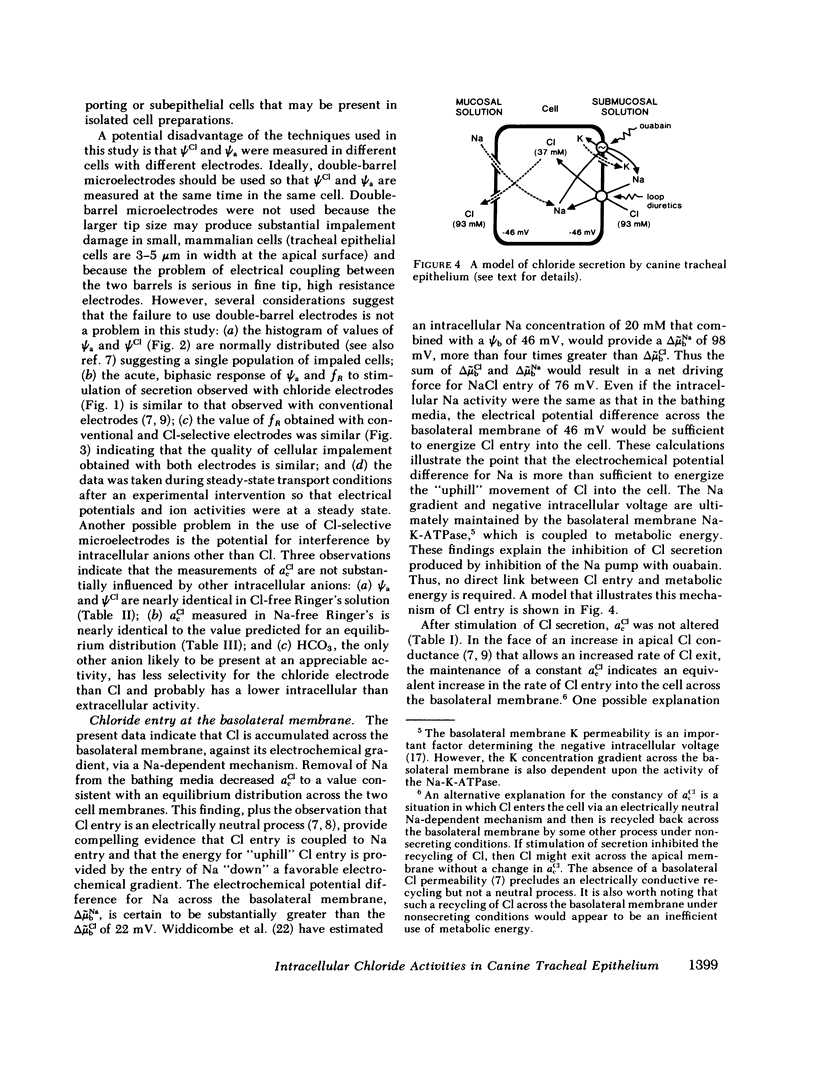
Canine tracheal epithelium secretes Cl via an electrogenic transport process that appears to apply to a wide variety of secretory epithelia. To examine the mechanisms involved, intracellular chloride activity, acCl, was measured with Cl-selective intracellular microelectrodes. The results indicate that when the rate of secretion was minimal acCl was 37 mM; with stimulation of secretion the intracellular voltage depolarized, but acCl was not significantly altered, at 39 mM. These findings indicate that: (a) Cl is accumulated across the basolateral membrane under nonsecreting and secreting conditions at an activity 3.8 and 2.4 times, respectively, that predicted for an equilibrium distribution; (b) Cl exit across the apical membrane may be passive with an electrochemical driving force of 22 mV; and (c) stimulation of secretion enhanced the rate of Cl entry across the basolateral membrane, since Cl transport increased without a change in acCl. In the absence of Na in the extracellular fluid, acCl approached the value expected for an equilibrium distribution. This finding suggests that "uphill" entry of Cl into the cell against its electrochemical gradient is dependent upon, and energized by, the entry of Na down its gradient. Submucosal bumetanide, a loop diuretic, also decreased the rate of Cl secretion and decreased acCl, indicating an inhibition of Cl entry. These findings indicate that Cl entry into the cell is directed against its electrochemical gradient and is mediated by a Na-coupled, bumetanide-inhibitable, transport process at the basolateral membrane and that Cl may exit passively down a favorable electrochemical gradient across the apical membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Bazzaz F. J., Al-Awqati Q. Interaction between sodium and chloride transport in canine tracheal mucosa. J Appl Physiol Respir Environ Exerc Physiol. 1979 Jan;46(1):111–119. doi: 10.1152/jappl.1979.46.1.111. [DOI] [PubMed] [Google Scholar]
- Al-Bazzaz F., Yadava V. P., Westenfelder C. Modification of Na and Cl transport in canine tracheal mucosa by prostaglandins. Am J Physiol. 1981 Feb;240(2):F101–F105. doi: 10.1152/ajprenal.1981.240.2.F101. [DOI] [PubMed] [Google Scholar]
- Baumgarten C. M. An improved liquid ion exchanger for chloride ion-selective microelectrodes. Am J Physiol. 1981 Nov;241(5):C258–C263. doi: 10.1152/ajpcell.1981.241.5.C258. [DOI] [PubMed] [Google Scholar]
- Duffey M. E., Thompson S. M., Frizzell R. A., Schultz S. G. Intracellular chloride activities and active chloride absorption in the intestinal epithelium of the winter flounder. J Membr Biol. 1979 Nov 30;50(3-4):331–341. doi: 10.1007/BF01868896. [DOI] [PubMed] [Google Scholar]
- Duffey M. E., Turnheim K., Frizzell R. A., Schultz S. G. Intracellular chloride activities in rabbit gallbladder: direct evidence for the role of the sodium-gradient in energizing "uphill" chloride transport. J Membr Biol. 1978 Sep 19;42(3):229–245. doi: 10.1007/BF01870360. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Field M., Schultz S. G. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979 Jan;236(1):F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Smith P. L., Vosburgh E., Field M. Coupled sodium-chloride influx across brush border of flounder intestine. J Membr Biol. 1979 Apr 12;46(1):27–39. doi: 10.1007/BF01959973. [DOI] [PubMed] [Google Scholar]
- Fromm M., Schultz S. G. Some properties of KCl-filled microelectrodes: correlation of potassium "leakage" with tip resistance. J Membr Biol. 1981;62(3):239–244. doi: 10.1007/BF01998169. [DOI] [PubMed] [Google Scholar]
- Olver R. E., Davis B., Marin M. G., Nadel J. A. Active transport of Na+ and Cl- across the canine tracheal epithelium in vitro. Am Rev Respir Dis. 1975 Dec;112(6):811–815. doi: 10.1164/arrd.1975.112.6.811. [DOI] [PubMed] [Google Scholar]
- Palfrey H. C., Feit P. W., Greengard P. cAMP-stimulated cation cotransport in avian erythrocytes: inhibition by "loop" diuretics. Am J Physiol. 1980 Mar;238(3):C139–C148. doi: 10.1152/ajpcell.1980.238.3.C139. [DOI] [PubMed] [Google Scholar]
- Reuss L., Grady T. P. Effects of external sodium and cell membrane potential on intracellular chloride activity in gallbladder epithelium. J Membr Biol. 1979 Dec 12;51(1):15–31. doi: 10.1007/BF01869341. [DOI] [PubMed] [Google Scholar]
- Silva P., Stoff J., Field M., Fine L., Forrest J. N., Epstein F. H. Mechanism of active chloride secretion by shark rectal gland: role of Na-K-ATPase in chloride transport. Am J Physiol. 1977 Oct;233(4):F298–F306. doi: 10.1152/ajprenal.1977.233.4.F298. [DOI] [PubMed] [Google Scholar]
- Smith P. L., Welsh M. J., Stoff J. S., Frizzell R. A. Chloride secretion by canine tracheal epithelium: I. Role of intracellular c AMP levels. J Membr Biol. 1982;70(3):217–226. doi: 10.1007/BF01870564. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Inhibition of chloride secretion by furosemide in canine tracheal epithelium. J Membr Biol. 1983;71(3):219–226. doi: 10.1007/BF01875463. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Smith P. L., Frizzell R. A. Chloride secretion by canine tracheal epithelium: II. The cellular electrical potential profile. J Membr Biol. 1982;70(3):227–238. doi: 10.1007/BF01870565. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Smith P. L., Frizzell R. A. Chloride secretion by canine tracheal epithelium: III. Membrane resistances and electromotive forces. J Membr Biol. 1983;71(3):209–218. doi: 10.1007/BF01875462. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Widdicombe J. H. Pathways of ion movement in the canine tracheal epithelium. Am J Physiol. 1980 Sep;239(3):F215–F221. doi: 10.1152/ajprenal.1980.239.3.F215. [DOI] [PubMed] [Google Scholar]
- Westenfelder C., Earnest W. R., Al-Bazzaz F. Characterization of Na-K-ATPase in dog tracheal epithelium: enzymatic and ion transport measurements. J Appl Physiol Respir Environ Exerc Physiol. 1980 Jun;48(6):1008–1019. doi: 10.1152/jappl.1980.48.6.1008. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H., Basbaum C. B., Highland E. Ion contents and other properties of isolated cells from dog tracheal epithelium. Am J Physiol. 1981 Nov;241(5):C184–C192. doi: 10.1152/ajpcell.1981.241.5.C184. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H., Basbaum C. B., Yee J. Y. Localization of Na pumps in the tracheal epithelium of the dog. J Cell Biol. 1979 Aug;82(2):380–390. doi: 10.1083/jcb.82.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdicombe J. H., Ueki I. F., Bruderman I., Nadel J. A. The effects of sodium substitution and ouabain on ion transport by dog tracheal epithelium. Am Rev Respir Dis. 1979 Aug;120(2):385–392. doi: 10.1164/arrd.1979.120.2.385. [DOI] [PubMed] [Google Scholar]


