Abstract
OBJECTIVE
Impaired glucose effectiveness (GE) plays a role in the deterioration of glucose metabolism. Our aim was to validate a surrogate of GE derived from an oral glucose tolerance test (OGTT) and to assess the impact of degrees of obesity and of glucose tolerance on it.
RESEARCH DESIGN AND METHODS
The OGTT-derived surrogate of GE (oGE) was validated in obese adolescents who underwent an OGTT and an intravenous glucose tolerance test (IVGTT). We then evaluated anthropometric determinants of the oGE and its impact on the dynamics of glucose tolerance in a cohort of children with varying degrees of obesity.
RESULTS
The correlation of oGE and IVGTT-derived GE in 98 obese adolescents was r = 0.35 (P < 0.001) as a whole and r = 0.51 (P < 0.001) in subjects with normal glucose tolerance. In a cohort of 1,418 children, the adjusted GE was associated with increasing obesity (P < 0.001 for each category of obesity). Quartiles of oGE and the oral disposition index were associated with 2-h glucose levels (P < 0.001 for both). Among 421 nondiabetic obese subjects (276 subjects with normal glucose tolerance/145 subjects with impaired glucose tolerance who repeated their OGTT after a mean time of 28 ± 16 months), oGE changes were tightly associated with weight (r = 0.83, P < 0.001) and waist circumference changes (r = 0.67, P < 0.001). Baseline oGE and changes in oGE over time emerged as significant predictors of the change in 2-h glucose levels (standardized B = −0.76 and B = −0.98 respectively, P < 0.001 for both).
CONCLUSIONS
The oGE is associated with the degree of and changes in weight and waist circumference and is an independent predictor of glucose tolerance dynamics.
Introduction
Glucose has the ability to facilitate its own uptake in peripheral tissues and to suppress hepatic glucose production at basal concentrations of insulin (1). This property of glucose is known as “glucose effectiveness” (GE), and it increases with increasing insulin concentrations (2). GE makes a major contribution to glucose disposal under fasting conditions (basal insulin concentrations) estimated at ∼70%, while during exposure to postabsorptive insulin concentrations imposed during a hyperinsulinemic-euglycemic clamp the relative contribution of GE drops to ∼30%. After oral ingestion of glucose and an increasingly dynamic insulin concentration, the relative effect of GE on glucose disposal is expected to be greater than that achieved during a clamp and is estimated at 50% (2).
Patients with type 2 diabetes mellitus (T2DM) and impaired fasting glucose (IFG), as well as siblings of patients with T2DM have been shown to have reduced GE (3–6). The molecular mechanism by which glucose is able to suppress its liver production and to enhance its peripheral uptake is not yet clear, although recent publications (7) have postulated a role of circulating free fatty acids (FFAs), which are typically increased in conditions of insulin resistance. Indeed, elevated levels of FFAs promote gluconeogenesis in the liver, driving increased glucose production and reduced glucose oxidation (8). In addition, the ability of glucose to stimulate peripheral glucose uptake is impaired in the face of increased levels of FFAs and may be increased by GLP-1 (9,10). Importantly, the normalization of FFA concentration restores GE in patients with T2DM (11). In addition, the brain has been shown to affect GE in rodent models, as a compensatory mechanism to insulin resistance and T2DM (12).
Measurement of GE involves sophisticated clinical protocols such as the graded hyperglycemic clamp with somatostatin, insulin and glucagon infusion clamp, or the frequently sampled intravenous glucose tolerance test (FSIVGTT). This prevents the assessment of this important factor in large cohorts. Nagasaka et al. (13) have recently described a surrogate of GE derived from the oral glucose tolerance test (OGTT) that shows a good correlation with GE as measured by an IVGTT. GE has been shown to be increased in obese children (14) and differs by ethnicity in the pediatric age group (15). The aim of this analysis was to validate this surrogate in obese adolescents and to assess the impact of varying degrees of obesity and glucose tolerance on the surrogate of GE in a large multiethnic cohort of children. We hypothesized that worsening glucose tolerance will be associated with reduced OGTT-derived index of GE (oGE).
Research Design and Methods
Cohort Used for Validation of the oGE
The validation cohort was a group of obese (BMI ≥95th percentile for age and sex), pubertal (Tanner stage >1) adolescents who were 12–17 years of age. Nondiabetic participants were recruited from primary care clinics affiliated with The Children’s Hospital of Philadelphia (CHOP) in Philadelphia, PA. A small number of participants presenting with obesity were recruited from the CHOP outpatient Endocrinology Clinic as well. In addition, obese, pubertal adolescents in the same age range with T2DM were recruited from the CHOP Diabetes Center for Children. T2DM was diagnosed in these participants based on American Diabetes Association (ADA) criteria (16). Of note, HbA1c was not used to categorize individuals, as the addition of this criterion in the ADA guidelines occurred after the onset of this study. Exclusion criteria were the existence of the following: 1) major chronic illness (other than T2DM), 2) pregnancy, 3) a genetic syndrome known to affect glucose tolerance, 4) known familial hypercholesterolemia, 5) treatment with medications known to affect insulin sensitivity (systemic steroids or metformin in nondiabetic participants in the last 1 month) or lipid profiles (statins, high-dose vitamin A), 6) previous diagnosis of impaired glucose tolerance (IGT) and/or IFG, and 7) treatment with high doses of inhaled steroids (>1,000 μg/day). Written informed consent and age-appropriate assent were obtained on the day of the study visit from all subjects before participation, and the study was approved by the CHOP Institutional Review Board.
Study visits took place at the Clinical Translational Research Center of CHOP and the Hospital of the University of Pennsylvania. Weight was measured with the subject wearing a light gown without shoes by use of a digital scale (Scale-Tronix, White Plains, NY), which was calibrated daily. Height was measured using a wall-mounted stadiometer (Holtain Inc., Crymych, U.K.). BMI was calculated as the weight in kilograms divided by height in square meters, and BMI percentiles were assessed using age- and sex-specific BMI reference data (17). Measurements were repeated three times, and average values were used.
Participants were instructed to have 3 days of a high carbohydrate diet prior to the study visit in preparation for a 2-h OGTT. After a 10- to 12-h overnight fast, a blood sample was obtained for the measurement of glucose and insulin levels. Subjects were then asked to ingest a glucose solution (1.75 g/kg up to a maximum of 75 g) over 2 min. Blood was again drawn for the measurement of glucose and insulin levels at 30, 60, 90, and 120 min. Among the obese adolescents, participants were categorized as having normal glucose tolerance (NGT) or IFG/IGT per ADA criteria (16).
Participants came back the subsequent day, after a fast of 10–12 h, to undergo a FSIVGTT. A baseline sample was obtained for measurement of glucose and insulin levels. A bolus of dextrose (D25) (0.25 g/kg i.v.) was infused over 30 s at t = 0, and an IV bolus of regular human insulin (Eli Lilly, Indianapolis, IN) (18) was infused over 30 s at t = 20 min. Nondiabetic subjects received 0.015 units/kg, and T2DM patients received 0.05 units/kg to accommodate for insufficient insulin secretion in patients with diabetes (19–21). Blood samples were obtained for glucose and insulin at t = −5, 2, 4, 8, 19, 22, 30, 40, 50, 70, 100, and 180 min. MINMOD software was used to calculate GE from these samples (22).
Cohort Used for Analysis of the oGE
Subjects were recruited from the Yale Pathophysiology of Type 2 Diabetes in Youth Study, a long-term, multiethnic cohort, aimed to study the early alternations in glucose metabolism in obese youth. In this study, obese youth are followed longitudinally and OGTTs are repeated. Glucose tolerance (NGT, IGT, IFG, or T2DM) was classified according to ADA definitions (23). The study was approved by the Human Investigations Committee of the Yale School of Medicine. Subjects were eligible if they were healthy, were aged 4–20 years, and had a BMI that exceeded the 97th percentile for their age and sex. Additional participants included in this analysis were lean or overweight siblings of the obese children (23). In addition to patient and parental consent, complete medical histories and thorough physical examinations were obtained from each participant. All subjects were negative for autoimmune markers for type 1 diabetes (insulin antibody, GAD65, and islet cell antibody 512). Obesity categories were defined as normal weight (BMI z score <1.65, overweight 1.65 < BMI z score < 2), moderately obese (2 < BMI z score < 2.5), and severely obese (BMI z score >2.5), as previously described (23).
Calculations
Insulin sensitivity was calculated using the OGTT-derived Matsuda index (24), and the acute insulin response was calculated using the insulinogenic index (25). The oral disposition index (oDI) was calculated as previously described and validated (26,27).
As described by Nagasaka et al. (13), calculation of the oGE is derived from the following three components: postload plasma glucose level without insulin and GE, postload plasma glucose level without insulin/with GE (using the oDI for the calculation), and the expected 2-h plasma glucose level based on equations for the linear regression of the oDI and 2-h glucose level. All calculations for the oGE are provided in the Supplementary Data.
Statistical Analysis
Data are presented as the mean ± SD. Parameters not normally distributed were natural log transformed for the analysis. For the sake of the analysis, subjects with IFG, IGT, or both were considered as a single group. Group comparisons were performed using ANCOVA with post hoc adjustment for multiple comparisons using the Bonferroni correction. Simple Pearson correlations were performed to test the associations among parameters. Linear regression models were used to assess the determinants of oGE and the impact of oGE on glucose tolerance dynamics. P < 0.05 was considered to be statistically significant. The analysis was performed using SPSS version 19.0 for Windows.
Results
Validation of the OGTT-Derived Surrogate of GE
Ninety-eight subjects (39 males, 59 females, 78 African Americans, 11 whites, 6 of more than one race, and 3 of unknown race or with race not reported) participated in the validation analyses. There were 82 participants in the NGT category, 10 participants in the IGT/IFG category, and 6 participants in the T2DM category. GE values derived from the FSIVGTT data were 0.021, 0.014, and 0.011 min−1 for the NGT, IGT/IFG, and T2DM categories, respectively, while GE values calculated from the OGTT data were 2.14, 1.71, and 1.20 mg/dL/min, respectively. The Pearson correlation coefficients of GE values derived from the IVGTT and of the oGE were r = 0.35 (P < 0.001) for the entire group and r = 0.51 (P < 0.001) for the NGT participants.
Relation of the oGE With Anthropometric and Metabolic Parameters
A total of 1,418 children and adolescents from the Yale cohort participated in this analysis. They were divided according to the degree of obesity categories (lean, overweight, moderately obese, and severely obese) based on their BMI z scores. As shown in Table 1, the mean age of participants was slightly lower in the severely obese group. Sex distribution was characterized by more males in the severely obese group. African Americans were overrepresented in the severely obese group. Weight, BMI, and BMI z score were obviously significantly different between each category.
Table 1.
Study participants by weight categories
| Lean (n = 87) | Overweight (n = 131) | Moderately obese (n = 621) | Severely obese (n = 579) | P* | |
|---|---|---|---|---|---|
| Age (years) | 13.8 ± 2.9 | 13.4 ± 2.8 | 13.4 ± 2.8 | 12.6 ± 3.1 | <0.001 |
| Sex | <0.001† | ||||
| Male | 34 | 39 | 229 | 285 | |
| Female | 53 | 92 | 392 | 294 | |
| Ethnicity | <0.001† | ||||
| Caucasian | 39 | 72 | 269 | 206 | |
| African American | 24 | 35 | 223 | 250 | |
| Hispanic | 24 | 24 | 129 | 123 | |
| Height (cm) | 158 ± 13 | 158 ± 11 | 159 ± 12 | 158 ± 14 | <0.001 |
| Weight (kg) | 59.6 ± 15.9 | 71.0 ± 14.6 | 84.6 ± 19.5 | 104.6 ± 31.6 | <0.001 |
| BMI | |||||
| kg/m2 | 23.19 ± 3.31 | 27.95 ± 2.64 | 32.97 ± 4.30 | 40.52 ± 7.38 | <0.001 |
| z score | 1.01 ± 0.73 | 1.86 ± 0.09 | 2.28 ± 0.13 | 2.72 ± 0.19 | <0.001 |
| NGT | 87 | 124 | 477 | 440 | <0.001† |
| IFG/IGT | 0 | 7 | 126 | 125 | |
| T2DM | 0 | 0 | 18 | 13 | |
| Metabolic parameters | |||||
| Fasting glucose level (mg/dL) | 90 ± 8 | 90 ± 7 | 93 ± 9 | 94 ± 10 | <0.001 |
| 2-h glucose level (mg/dL) | 112 ± 14 | 113 ± 17 | 124 ± 29 | 125 ± 29 | <0.001 |
| Fasting insulin level (µU/mL) | 18 ± 9 | 25 ± 10 | 34 ± 23 | 40 ± 22 | <0.001 |
| Insulinogenic index | 3.50 ± 3.24 | 4.10 ± 3.52 | 5.14 ± 6.21 | 5.50 ± 4.51 | <0.001 |
| Matsuda index | 3.17 ± 1.81 | 2.25 ± 1.12 | 1.80 ± 1.00 | 1.56 ± 0.96 | <0.001 |
| oDI | 8.26 ± 4.97 | 7.81 ± 5.51 | 8.50 ± 7.62 | 7.82 ± 9.43 | 0.73 |
| GE (mg/dL/min) | 3.86 ± 1.24 | 3.05 ± 0.77 | 2.46 ± 0.89 | 1.99 ± 1.01 | <0.001 |
Data are reported as the mean ± SD or n, unless otherwise indicated.
P values were determined by ANOVA, unless otherwise indicated.
χ2 test.
We evaluated fasting and OGTT-derived parameters in relation to increasing degree of obesity category. Fasting and 2-h glucose levels as well as fasting insulin levels increased with the degree of obesity (P < 0.001 for all). The insulinogenic index values increased and insulin sensitivity decreased with the degree of obesity (P < 0.001 for both). Of note, the oGE values significantly decreased with rising degree of obesity (P < 0.001). In the analysis of oGE, group comparisons indicated that each BMI category was significantly different from all of the three other categories (P < 0.001 for all comparisons performed).
Upon evaluation of oGE as a dependent variable in a linear regression with age, sex, ethnicity, weight category, glucose tolerance, and oDI as independent variables, the degree of obesity remained a significant predictor of oGE (P < 0.001, for each degree of obesity compared with the others).
Relation of oGE, the oDI, and 2-h Glucose Level
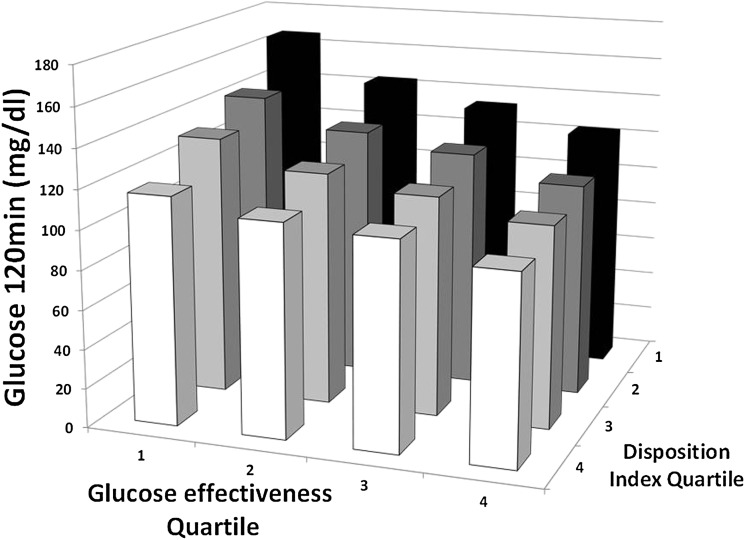
We evaluated the 2-h glucose level as a dependent variable and used age, sex, ethnicity, degree of obesity, fasting glucose level, and quartiles of the oDI and oGE as independent variables. As shown in Fig. 1, both quartiles of the oDI and oGE were significantly associated with 2-h glucose levels (P < 0.001, for both) as was the interaction between them (P < 0.001). Thus, while controlling for potential confounders, low oDI and low oGE values were independently and synergistically associated with higher 2-h glucose levels on the OGTT results.
Figure 1.
Relation of the oDI, GE, and 2-h glucose levels on the OGTT results. Quartiles of the oDI and of GE are significantly associated with 2-h glucose levels (P < 0.001 for both), as is the interaction between them (P < 0.001).
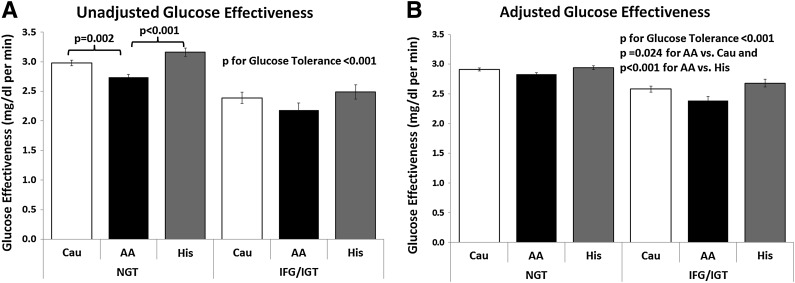
Upon comparing degrees of glucose tolerance, we analyzed only subjects with NGT and IFG/IGT due to the modest T2DM group. Subjects with NGT had greater oGE values than those with IFG/IGT (2.92 ± 1.07 vs. 2.36 ± 0.91 mg/dL/min for NGT and IGT/IFG, respectively, P < 0.001) (Fig. 2A). Among the NGT participants, African Americans had significantly lower oGE values compared with their Caucasian and Hispanic counterparts (P = 0.002 and P < 0.001, respectively), while no such differences were observed within the IFG/IGT group. After adjustment for age, sex, race, BMI z score, and oDI values using a linear regression model, these differences remained unattenuated (Fig. 2B) and highly significant (P < 0.001 for glucose tolerance category comparison). Of note, in this model, which had an adjusted R2 of 0.75, African Americans had significantly lower oGE values in comparison with Caucasians and Hispanics (P = 0.024 vs. Caucasians and P < 0.001 vs. Hispanics).
Figure 2.
Unadjusted and adjusted GE in relation to glucose tolerance and ethnicity. A: Unadjusted GE is significantly and negatively associated with altered glucose tolerance and is lower in African Americans with NGT. B: Adjusted GE is significantly and negatively associated with altered glucose tolerance and is significantly lower in obese African American children in comparison with their Caucasian and Hispanic counterparts. AA, African American; Cau, Caucasian; His, Hispanic.
Anthropometric and oGE Changes in Relation to 2-h Glucose Dynamics
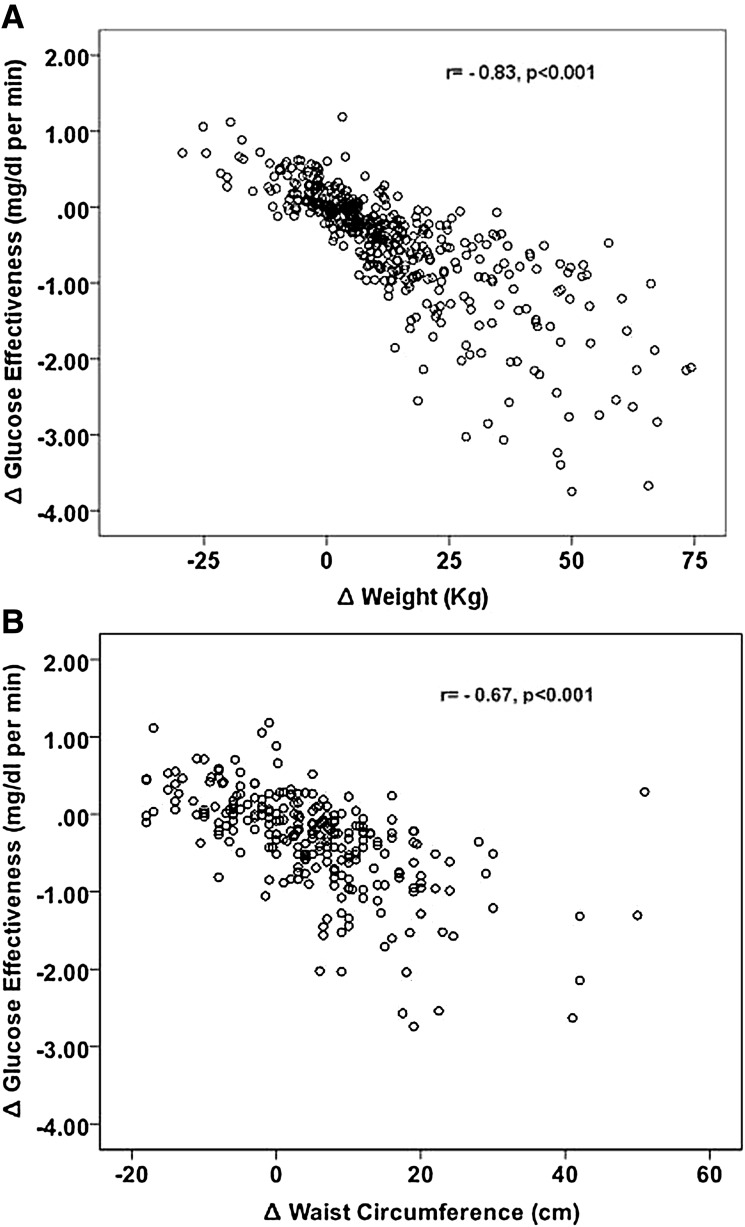
Four hundred twenty-one (157 males and 264 females) nondiabetic obese subjects (276 NGT subjects and 145 IGT subjects) repeated their OGTT after a mean duration of 28 ± 16 months. As shown in Fig. 3, using simple correlations we show that weight and waist circumference changes were negatively and significantly correlated with changes in oGE (r = −0.83 and r = −0.67, respectively, P < 0.001 for both). The change in oGE was negatively and more tightly associated with the change in the 2-h glucose value (r = −0.52, P < 0.001) than the change in BMI z score (r = 0.41, P < 0.001). Upon modeling the change in 2-h glucose levels as a dependent variable in a linear regression, while using baseline age, BMI z score, 2-h glucose levels, oDI, oGE, sex, and race along with the time between studies and the changes in BMI z score and oGE as independent factors, the adjusted R2 of the model was 0.56 (P < 0.001). Baseline oGE values along with the change in oGE values over time emerged as significant predictors of the change in 2-h glucose levels (standardized B = −0.76 and B = −0.98, respectively, P < 0.001 for both). Baseline 2-h glucose levels, BMI z scores, and their change also were significant predictors of changes in 2-h glucose levels in this model (standardized B = −0.54, B = −0.32, and B = −0.23, respectively, P < 0.001 for all), while baseline oDI values were not. Substituting the change in BMI z score with absolute weight change in this model did not attenuate the results (standardized B values for baseline oGE, change in oGE, and weight change were −0.72, −0.99, and −0.32, respectively, P < 0.001 for all).
Figure 3.
Relation of anthropometric changes with GE dynamics. Both weight gain (A) and increase in waist circumference (B) are negatively associated with the change in GE over time.
Of the 276 subjects with NGT at baseline, 40 converted to IGT at follow-up. In a logistic regression in which the development of IGT at follow-up was the dependent variable, and baseline age, BMI z score, race, sex, baseline 120-min glucose level, and oGE values were the independent variables, oGE values emerged as a significant predictor subjects converting from NGT to IGT (P = 0.04), along with the baseline 120-min glucose level (P = 0.01).
Conclusions
The ability of glucose to promote its own disposition by way of enhancing glucose uptake and suppressing glucose production, known as GE, plays a major role in glucose disposal during fasting (basal insulin concentrations), yet it has a significant effect on postabsorptive glucose uptake as well. In this analysis, we used an OGTT-derived surrogate of GE to study the relation of this important factor with anthropometric and metabolic factors. The increasing degree of obesity in childhood is associated with lower oGE values. We also demonstrated that, as expected among obese children, abnormal glucose tolerance is also associated with lower oGE values, and that obese African American youth have lower oGE values per given glucose tolerance level. Importantly, baseline oGE and its dynamics were independently associated with the change in 2-h glucose levels over time. The change in oGE values was tightly negatively associated with weight gain and increasing waist circumference.
Involvement of the brain in the regulation of blood glucose levels has been known since the mid-1800s (28). While islet and insulin-responsive peripheral tissue involvement in the pathogenesis of T2DM has been widely studied (29), the mechanisms governing brain-regulated glucose control and its role in the pathogenesis of diabetes are mostly unknown. The brain has been shown to have the capacity to normalize diabetic hyperglycemia in rodents in the presence of sufficient amounts of central nervous system leptin (30). It is estimated that the contribution of insulin-independent glucose disposal to overall glucose homeostasis is similar to that of insulin (2). The hypothalamic inhibitory response to oral glucose has been shown to be delayed and blunted in obese humans compared with lean humans, suggesting a functional impact of obesity on homeostatic neural pathways (31). In order for glucose tolerance to deteriorate, in addition to the typical β-cell defect that fails to compensate appropriately for peripheral insulin resistance, GE may therefore also be impaired and unable to appropriately compensate by reducing blood glucose levels. Indeed, reduced GE has been shown to contribute to the development of hyperglycemia in patients with T2DM (32). In line with this, we show that worsening glucose tolerance is associated with lower oGE values in obese youth. We also show that baseline values and dynamics of the oGE are significant independent predictors of changes in 2-h glucose levels over time, highlighting the role of this factor in the development of altered glucose metabolism.
We show that increased obesity is associated with reduced oGE values. This finding seems to be in contrast to that of Hoffman and Armstrong (14), yet these differences may be the result of different definitions of obesity (BMI <75th percentile), modest sample size, and lack of adjustment for potential confounders in that study. Using our large sample, we furthermore show that changes in anthropometric indices, whether weight gain or increase in waist circumference (as a surrogate of abdominal obesity) are tightly associated with reduction in oGE values. Human as well as rodent studies (33,34) have recently demonstrated that obesity is associated with enhanced gliosis in hypothalamic loci. It is possible that such scarring of brain tissue may be the underlying mechanism involved in impaired brain-mediated glucose regulation. We have previously characterized the reduction in peripheral insulin sensitivity along with the progressive dysfunction of β-cells associated with the deterioration of glucose metabolism in obese children (23,35,36). This analysis provides an additional piece of the complex puzzle of the pathophysiological process of glucose metabolism deterioration in obese youth, by showing that, in parallel with the peripheral core defects of T2DM that are present prior to the appearance of overt disease in obese children, an additional defect in GE is already evident. The finding that lower GE is associated with the deterioration of glucose tolerance over time has been shown in nondiabetic offspring of subjects with T2DM (37) and indicates the agreement between the intravenously and orally derived indices of GE in this regard. We speculate, based on the fact that hepatic glucose production is not significantly increased in obese children with prediabetes (35), that the impact of reduced baseline GE and further reduction of GE over time on deteriorating glucose tolerance is mainly mediated by altered non–insulin-dependent peripheral glucose uptake mechanisms.
A surprising finding in this analysis is that, per the given degree of glucose tolerance, African American obese youth have lower oGE values, even after adjustment for potential confounders. We do not have a mechanistic explanation for this observation, yet the majority of the difference seems to be at the NGT stage, while in those with IFG/IGT this difference is modest. However, taking into consideration the high prevalence of T2DM in severely obese African American adolescents in comparison with Caucasians and Hispanics in the U.S., this finding may provide an additional mechanism by which to explain this phenomenon.
This analysis has several limitations. First, our validation cohort included only a modest number of children with IGT (n = 10) or T2DM (n = 6). Validation of the oGE in obese children within these glucose tolerance categories will need to be replicated in larger cohorts in the future. Second, the insulin dose used in IVGTT studies has been shown to be inversely correlated with insulin sensitivity derived from the study (38). It is worthy of testing whether different insulin doses also affect the GE calculation derived from the IVGTT. Third, we used the Matsuda index as a surrogate of whole-body insulin sensitivity and included it in calculations of the oDI. Despite being a surrogate, the Matsuda index has been shown to correlate well with the hyperinsulinemic-euglycemic clamp in this population specifically (39). Despite its limitations and high dependency on insulin dynamics during the OGTT, it has been shown to be useful as a surrogate of insulin sensitivity and as part of the oDI calculation (26).
Measurement of GE by “gold standard” methodology involves the performance of a graded hyperglycemic clamp with infusion of somatostatin, insulin, and glucagon in order to resemble the fasting hormonal milieu in the face of increasing glucose levels. This is impractical in large-scale studies, especially in children. An alternative is to calculate the GE from a model using the FSIVGTT, which is also a sophisticated protocol, limiting its applicability to large-scale epidemiological studies. The contribution of Nagasaka et al. (13) is the development and validation of a methodology for calculating an oGE. While the parameters achieved for obese children using this methodology were slightly different than those originally described, this may be expected because of the different age groups and ethnic backgrounds of subjects in the study sample. In addition, an oral glucose load (in contrast with an intravenous glucose challenge) involves incretin secretion, which significantly enhances the insulin profile and may thus create a different balance between insulin-dependent and insulin-independent whole-body glucose uptake. The discrepancy in insulin secretion profiles between these studies implicates that an optimal correlation between the indices of GE should not be expected. The correlation of the surrogate with the GE derived from an IVGTT was slightly stronger than that described by Nagasaka et al. (13) for the entire cohort and significantly stronger in participants with NGT. The ability of this novel surrogate to predict the development of T2DM in this population in combination with other clinical and metabolic parameters may help to identify obese children and adolescents who are at the greatest risk for the disease.
Supplementary Material
Article Information
Acknowledgments. The authors thank the patients and their families as well as the Hospital Research Unit personnel.
Funding. R.W. is funded by the Stephen Morse Diabetes Research Foundation. N.S. is funded by the American Heart Association (13SDG14640038). C.G. is funded by the European Society of Paediatric Endocrinology (ESPE Long-Term Research Fellowship 2011). S.C. is funded by the National Institutes of Health (NIH) (grants R01-HD-40787 and R01-HD-28016) and the American Diabetes Association (Distinguished Clinical Scientist Award DK-49230). S.N.M. is supported by the NIH (grant K23 PA05143), Patient-Oriented Research Career Development Award, and the Clinical and Translational Research Center of the National Center for Research Resources (UL1-RR-024134). This work was supported by Clinical and Translational Science Awards grant UL1-RR-024139 from the National Center for Advancing Translational Sciences; grant DK-045735 to the Yale Diabetes Endocrinology Research Center, a component of the NIH; and the NIH Roadmap for Medical Research.
The contents of this scientific contribution are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.W. and S.C. designed the study, collected and analyzed the data, and wrote the manuscript. S.N.M., N.S., C.G., R.B., and T.H. collected and analyzed the data and reviewed the manuscript. M.S. and E.D. collected the data, took part in clinical care, and provided critical inputs to the text. K.J.H. analyzed the data and reviewed the manuscript. All authors approved the manuscript in its final version. R.W. and S.C. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-2183/-/DC1.
References
- 1.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev 1985;6:45–86 [DOI] [PubMed] [Google Scholar]
- 2.Best JD, Kahn SE, Ader M, Watanabe RM, Ni TC, Bergman RN. Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care 1996;19:1018–1030 [DOI] [PubMed] [Google Scholar]
- 3.Nagasaka S, Tokuyama K, Kusaka I, et al. Endogenous glucose production and glucose effectiveness in type 2 diabetic subjects derived from stable-labeled minimal model approach. Diabetes 1999;48:1054–1060 [DOI] [PubMed] [Google Scholar]
- 4.Basu A, Caumo A, Bettini F, et al. Impaired basal glucose effectiveness in NIDDM: contribution of defects in glucose disappearance and production, measured using an optimized minimal model independent protocol. Diabetes 1997;46:421–432 [DOI] [PubMed] [Google Scholar]
- 5.Doi K, Taniguchi A, Nakai Y, et al. Decreased glucose effectiveness but not insulin resistance in glucose-tolerant offspring of Japanese non-insulin-dependent diabetic patients: a minimal-model analysis. Metabolism 1997;46:880–883 [DOI] [PubMed] [Google Scholar]
- 6.Taniguchi A, Nakai Y, Fukushima M, et al. Insulin sensitivity, insulin secretion, and glucose effectiveness in subjects with impaired glucose tolerance: a minimal model analysis. Metabolism 1994;43:714–718 [DOI] [PubMed] [Google Scholar]
- 7.Tonelli J, Kishore P, Lee DE, Hawkins M. The regulation of glucose effectiveness: how glucose modulates its own production. Curr Opin Clin Nutr Metab Care 2005;8:450–456 [DOI] [PubMed] [Google Scholar]
- 8.Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 2009;297:E578–E591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vahl TP, Paty BW, Fuller BD, Prigeon RL, D’Alessio DA. Effects of GLP-1-(7-36)NH2, GLP-1-(7-37), and GLP-1- (9-36)NH2 on intravenous glucose tolerance and glucose-induced insulin secretion in healthy humans. J Clin Endocrinol Metab 2003;88:1772–1779 [DOI] [PubMed] [Google Scholar]
- 10.D’Alessio DA, Kahn SE, Leusner CR, Ensinck JW. Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-independent glucose disposal. J Clin Invest 1994;93:2263–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawkins M, Tonelli J, Kishore P, et al. Contribution of elevated free fatty acid levels to the lack of glucose effectiveness in type 2 diabetes. Diabetes 2003;52:2748–2758 [DOI] [PubMed] [Google Scholar]
- 12.Schwartz MW, Seeley RJ, Tschöp MH, et al. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 2013;503:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagasaka S, Kusaka I, Yamashita K, et al. Index of glucose effectiveness derived from oral glucose tolerance test. Acta Diabetol 2012;49(Suppl. 1):S195–S204 [DOI] [PubMed] [Google Scholar]
- 14.Hoffman RP, Armstrong PT. Glucose effectiveness, peripheral and hepatic insulin sensitivity, in obese and lean prepubertal children. Int J Obes Relat Metab Disord 1996;20:521–525 [PubMed] [Google Scholar]
- 15.Hasson RE, Adam TC, Davis JN, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab 2010;95:4048–4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Suppl. 1):S81–S90 [DOI] [PubMed] [Google Scholar]
- 17.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002;109:45–60 [DOI] [PubMed] [Google Scholar]
- 18.Rickels MR, Naji A, Teff KL. Insulin sensitivity, glucose effectiveness, and free fatty acid dynamics after human islet transplantation for type 1 diabetes. J Clin Endocrinol Metab 2006;91:2138–2144 [DOI] [PubMed] [Google Scholar]
- 19.Mari A. Assessment of insulin sensitivity and secretion with the labelled intravenous glucose tolerance test: improved modelling analysis. Diabetologia 1998;41:1029–1039 [DOI] [PubMed] [Google Scholar]
- 20.Ward GM, Walters JM, Barton J, Alford FP, Boston RC. Physiologic modeling of the intravenous glucose tolerance test in type 2 diabetes: a new approach to the insulin compartment. Metabolism 2001;50:512–519 [DOI] [PubMed] [Google Scholar]
- 21.Kekäläinen P, Sarlund H, Laakso M. Long-term association of cardiovascular risk factors with impaired insulin secretion and insulin resistance. Metabolism 2000;49:1247–1254 [DOI] [PubMed] [Google Scholar]
- 22.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed 1986;23:113–122 [DOI] [PubMed] [Google Scholar]
- 23.Weiss R, Dziura J, Burgert TS, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–2374 [DOI] [PubMed] [Google Scholar]
- 24.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 25.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med 1994;11:286–292 [DOI] [PubMed] [Google Scholar]
- 26.Weiss R, Dziura JD, Burgert TS, Taksali SE, Tamborlane WV, Caprio S. Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia 2006;49:571–579 [DOI] [PubMed] [Google Scholar]
- 27.Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009;32:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernard C. Lecons de ohphisiologie experimentale appliques a la medicine. Paris, J.B. Bailliere, 1854
- 29.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.German JP, Thaler JP, Wisse BE, et al. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology 2011;152:394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuda M, Liu Y, Mahankali S, et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 1999;48:1801–1806 [DOI] [PubMed] [Google Scholar]
- 32.Lorenzo C, Wagenknecht LE, Rewers MJ, et al. Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2010;33:2098–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horvath TL, Sarman B, García-Cáceres C, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A 2010;107:14875–14880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012;122:153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiss R, Dufour S, Taksali SE, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 2003;362:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss R, Cali AM, Dziura J, Burgert TS, Tamborlane WV, Caprio S. Degree of obesity and glucose allostasis are major effectors of glucose tolerance dynamics in obese youth. Diabetes Care 2007;30:1845–1850 [DOI] [PubMed] [Google Scholar]
- 37.Egede MB, Henriksen JE, Durck TT, et al. Glucose effectiveness in nondiabetic relatives: dysglycemia and β-cell function at 10 years. J Clin Endocrinol Metab 2014;99:1420–1424 [DOI] [PubMed] [Google Scholar]
- 38.Prigeon RL, Røder ME, Porte D Jr, Kahn SE. The effect of insulin dose on the measurement of insulin sensitivity by the minimal model technique. Evidence for saturable insulin transport in humans. J Clin Invest 1996;97:501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeckel CW, Weiss R, Dziura J, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab 2004;89:1096–1101 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.