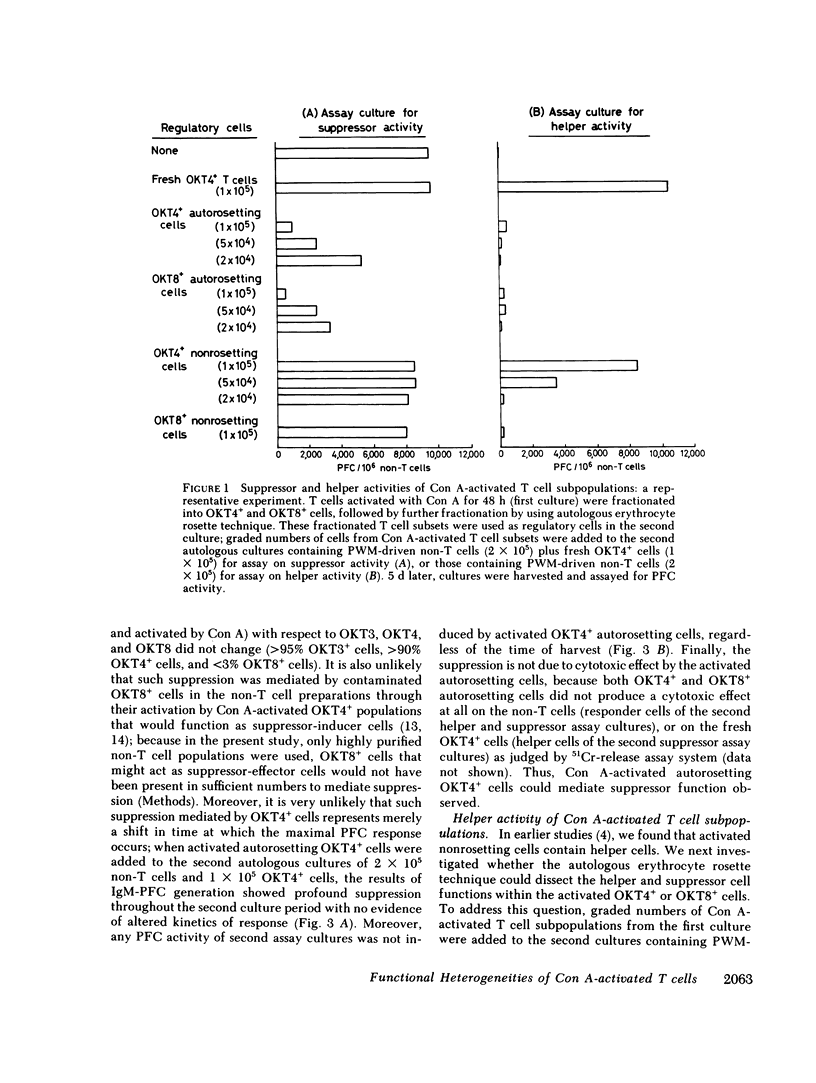
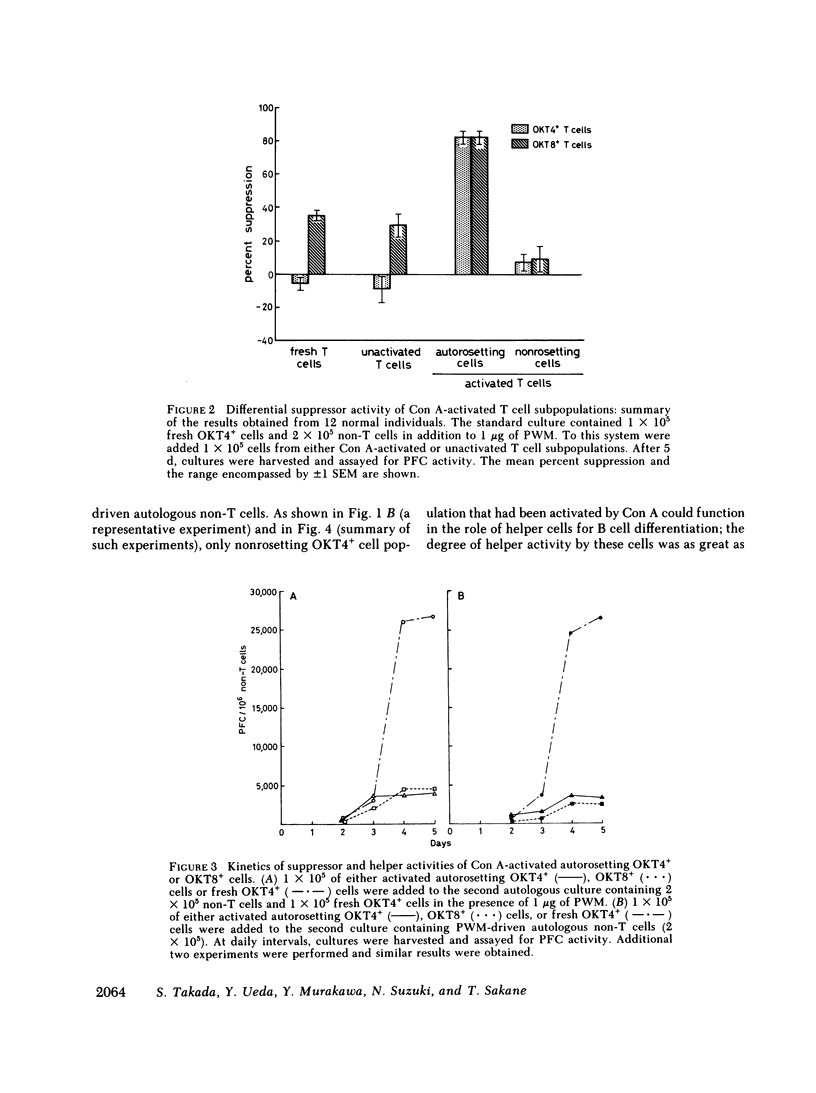
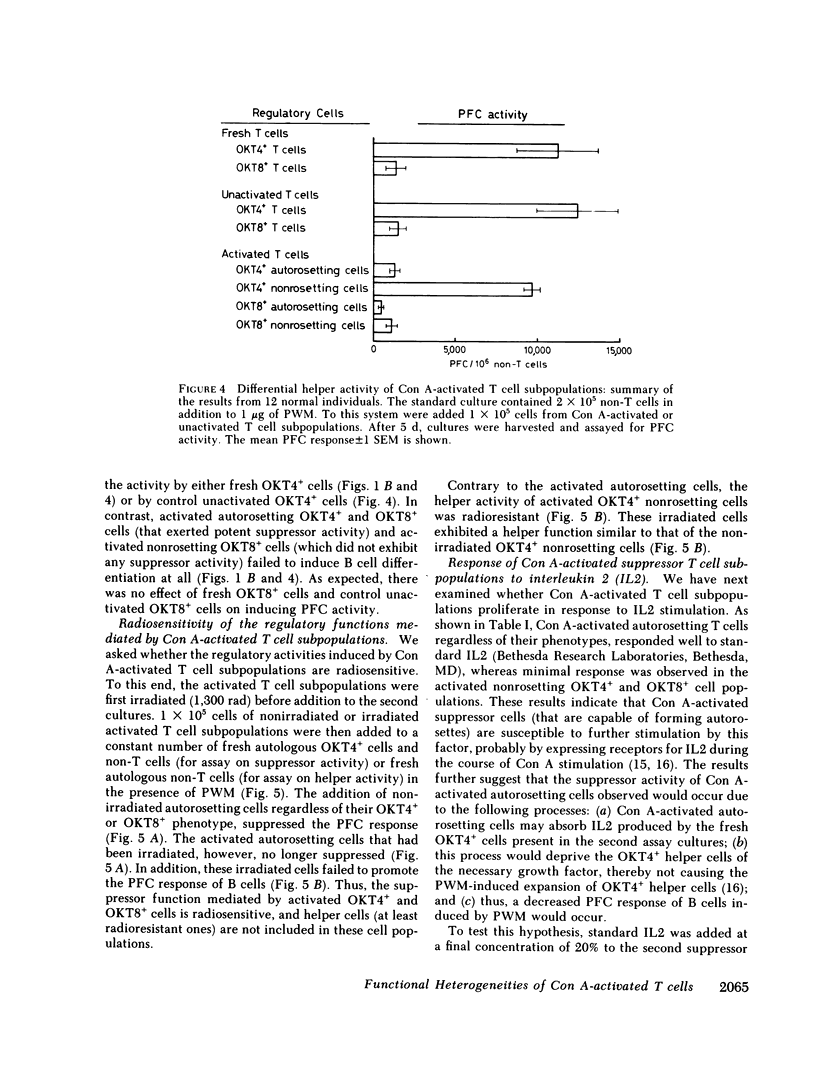
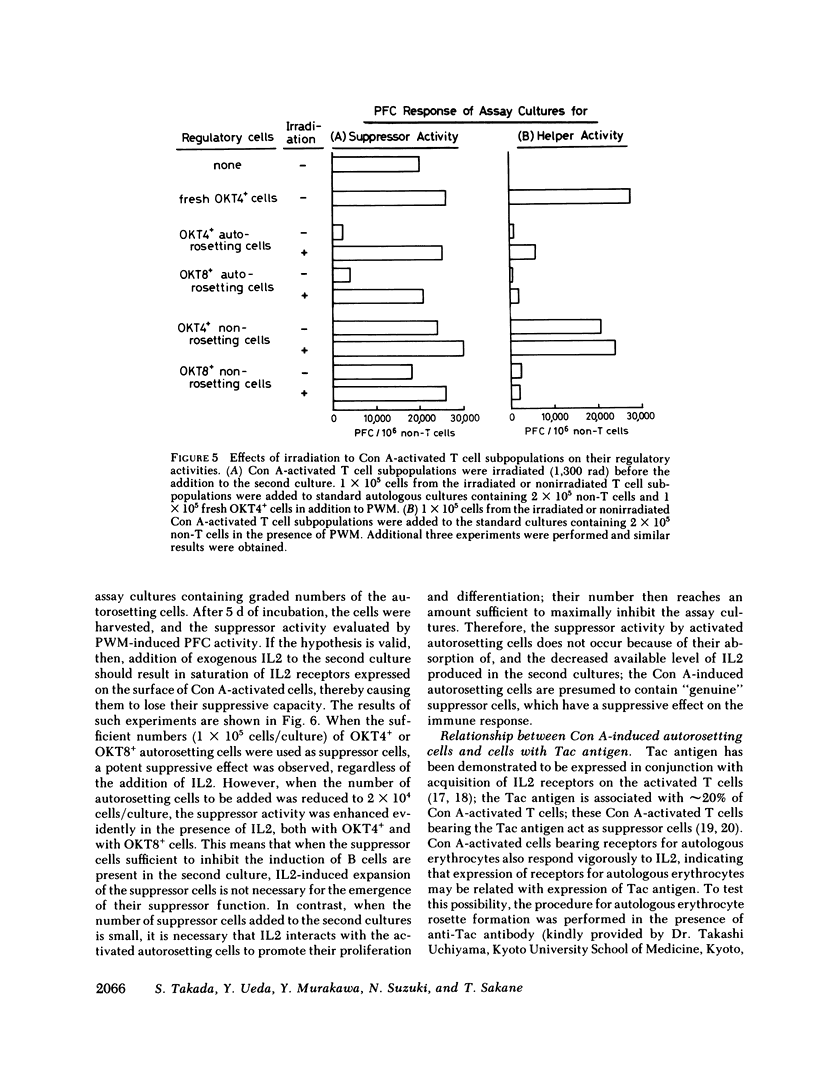
Abstract
Normal human peripheral blood T lymphocytes activated by concanavalin A (Con A) were fractionated into OKT4+ and OKT8+ populations by complement-dependent cell lysis using OKT8 and OKT4 antibodies, respectively. By using the preferential ability of some, but not all, Con A-activated T cells to form rosettes with autologous erythrocytes, each population was further divided into autorosetting cells and nonautorosetting cells, and thus Con A-activated OKT4+ autorosetting, OKT4+ nonautorosetting, OKT8+ autorosetting, and OKT8+ nonautorosetting cells were obtained. The immune regulatory function of these populations was then investigated using a pokeweed mitogen-driven B cell plaque-forming cell system. These studies demonstrated that (a) autorosetting cells can exert potent suppressor activity regardless of their phenotypes of OKT4+ and OKT8+ antigens, and fail to help B cell differentiation; suppressor function mediated by these cells is radiosensitive; moreover, receptors for autologous erythrocytes may constitute either the interleukin 2 (IL2) receptors themselves or a component of an IL2 receptor-effector complex involved in modulating the growth signal that IL2 transmits to T cells; (b) OKT4+ nonrosetting cells serve adequately as radioresistant helper cells, but are devoid of suppressor cells; and (c) OKT8+ nonrosetting cells are found to lack either suppressor or helper activity, suggesting that they may belong to a T lymphocyte subset distinct from the subsets related to immune regulation. The results lead us, therefore, to the conclusion that there may exist functional heterogeneities among both the OKT4+ and OKT8+ populations; these heterogeneities can be dissected by virtue of the autologous erythrocyte rosette technique.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Sagawa A., Pascual E., Hebert J., Sadeghee S. Suppressor T-cell abnormality in idiopathic systemic lupus erythematosus. Clin Immunol Immunopathol. 1976 Sep;6(2):192–199. doi: 10.1016/0090-1229(76)90110-0. [DOI] [PubMed] [Google Scholar]
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatenoud L., Baudrihaye M. F., Kreis H., Goldstein G., Schindler J., Bach J. F. Human in vivo antigenic modulation induced by the anti-T cell OKT3 monoclonal antibody. Eur J Immunol. 1982 Nov;12(11):979–982. doi: 10.1002/eji.1830121116. [DOI] [PubMed] [Google Scholar]
- Corte G., Moretta L., Damiani G., Mingari M. C., Bargellesi A. Surface antigens specifically expressed by activated T cells in humans. Eur J Immunol. 1981 Feb;11(2):162–164. doi: 10.1002/eji.1830110220. [DOI] [PubMed] [Google Scholar]
- Cosimi A. B., Colvin R. B., Burton R. C., Rubin R. H., Goldstein G., Kung P. C., Hansen W. P., Delmonico F. L., Russell P. S. Use of monoclonal antibodies to T-cell subsets for immunologic monitoring and treatment in recipients of renal allografts. N Engl J Med. 1981 Aug 6;305(6):308–314. doi: 10.1056/NEJM198108063050603. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Gupta S. Heterogeneity of concanavalin A-induced suppressor T cells in man defined with monoclonal antibodies. Clin Exp Immunol. 1982 Jun;48(3):581–588. [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Steinberg A. D., Haynes B. F., Whalen G. Immunoregulatory aberrations in systemic lupus erythematosus. J Immunol. 1978 Oct;121(4):1473–1479. [PubMed] [Google Scholar]
- Fournier C., Charreire J. Activation of a human T cell subpopulation bearing receptors for autologous erythrocytes by concanavalin A. J Immunol. 1978 Aug;121(2):771–776. [PubMed] [Google Scholar]
- Friedman S. M., Hunter S. B., Irigoyen O. H., Kung P. C., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. II. Collaborative T-T interactions in the generation of TNP-altered-self-reactive cytotoxic T lymphocytes. J Immunol. 1981 May;126(5):1702–1705. [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Horowitz S., Borcherding W., Moorthy A. V., Chesney R., Schulte-Wisserman H., Hong R. Induction of suppressor T cells in systemic lupus erythematosus by thymosin and cultured thymic epithelium. Science. 1977 Sep 2;197(4307):999–1001. doi: 10.1126/science.302032. [DOI] [PubMed] [Google Scholar]
- Kimura A. K., Wigzell H. Cell surface glycoproteins of murine cytotoxic T lymphocytes. I. T 145, a new cell surface glycoprotein selectively expressed on Ly 1-2+ cytotoxic T lymphocytes. J Exp Med. 1978 May 1;147(5):1418–1434. doi: 10.1084/jem.147.5.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krensky A. M., Reiss C. S., Mier J. W., Strominger J. L., Burakoff S. J. Long-term human cytolytic T-cell lines allospecific for HLA-DR6 antigen are OKT4+. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2365–2369. doi: 10.1073/pnas.79.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson E. L., Coutinho A. The role of mitogenic lectins in T-cell triggering. Nature. 1979 Jul 19;280(5719):239–241. doi: 10.1038/280239a0. [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Yachie A., Uwadana N., Ohzeki S., Nagaoki T., Taniguchi N. Functional significance of Tac antigen expressed on activated human T lymphocytes: Tac antigen interacts with T cell growth factor in cellular proliferation. J Immunol. 1982 Dec;129(6):2474–2478. [PubMed] [Google Scholar]
- Moretta A., Mingari M. C., Haynes B. F., Sekaly R. P., Moretta L., Fauci A. S. Phenotypic characterization of human cytolytic T lymphocytes in mixed lymphocyte culture. J Exp Med. 1981 Jan 1;153(1):213–218. doi: 10.1084/jem.153.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Distaso J. A., Borel Y., Schlossman S. F., Reinherz E. L. Communicative interactions between subpopulations of human T lymphocytes required for generation of suppressor effector function in a primary antibody response. J Immunol. 1982 Apr;128(4):1645–1650. [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Abe T., Homma M., Schlossman S. F. Characteristics of anti-T-cell antibodies in systemic lupus erythematosus: evidence for selective reactivity with normal suppressor cells defined by monoclonal antibodies. Clin Immunol Immunopathol. 1980 Aug;16(4):474–484. doi: 10.1016/0090-1229(80)90189-0. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Borel Y., Mantzouranis E., Steinberg A. D., Schlossman S. F. Autoantibody to an immunoregulatory inducer population in patients with juvenile rheumatoid arthritis. J Clin Invest. 1981 Mar;67(3):753–761. doi: 10.1172/JCI110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Reinherz E. L., Schlossman S. F., Schur P. H., Mills J. A., Steinberg A. D. Alterations in immunoregulatory T cell subsets in active systemic lupus erythematosus. J Clin Invest. 1980 Nov;66(5):1171–1174. doi: 10.1172/JCI109948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraguchi A., Butler J. L., Kehrl J. H., Fauci A. S. Differential sensitivity of human B cell subsets to activation signals delivered by anti-mu antibody and proliferative signals delivered by a monoclonal B cell growth factor. J Exp Med. 1983 Feb 1;157(2):530–546. doi: 10.1084/jem.157.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Llorente L., Alarcón-Segovia D., Ruíz-Arguelles A., Díaz-Jouanen E. Autologous rosette-forming T cells as the responding cells in human autologous mixed-lymphocyte reaction. J Clin Invest. 1980 Jun;65(6):1527–1530. doi: 10.1172/JCI109819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Möller G. T cell growth factor abrogates concanavalin A-induced suppressor cell function. J Exp Med. 1981 May 1;153(5):1360–1365. doi: 10.1084/jem.153.5.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Current concepts in immunology: Regulation of the immune response--inducer and suppressor T-lymphocyte subsets in human beings. N Engl J Med. 1980 Aug 14;303(7):370–373. doi: 10.1056/NEJM198008143030704. [DOI] [PubMed] [Google Scholar]
- Sagawa A., Abdou N. I. Suppressor-cell dysfunction in systemic lupus erythematosus. Cells involved and in vitro correction. J Clin Invest. 1978 Oct;62(4):789–796. doi: 10.1172/JCI109190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Green I. Protein A from Staphylococcus aureus-a mitogen for human T lymphocytes and B lymphocytes but not L lymphocytes. J Immunol. 1978 Jan;120(1):302–311. [PubMed] [Google Scholar]
- Sakane T., Honda M., Taniguchi Y., Kotani H. Separation of concanavalin A-induced human suppressor and helper T cells by the autologous erythrocyte rosette technique. J Clin Invest. 1981 Aug;68(2):447–453. doi: 10.1172/JCI110274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Kotani H., Takada S., Murakawa Y., Ueda Y. A defect in the suppressor circuits among OKT4+ cell populations in patients with systemic lupus erythematosus occurs independently of a defect in the OKT8+ suppressor T cell function. J Immunol. 1983 Aug;131(2):753–761. [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Studies of immune functions of patients with systemic lupus erythematosus. I. Dysfunction of suppressor T-cell activity related to impaired generation of, rather than response to, suppressor cells. Arthritis Rheum. 1978 Jul-Aug;21(6):657–664. doi: 10.1002/art.1780210608. [DOI] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Studies of immune functions of patients with systemic lupus erythematosus. V. T cell suppressor function and autologous mixed lymphocyte reaction during active and inactive phases of disease. Arthritis Rheum. 1980 Feb;23(2):225–231. doi: 10.1002/art.1780230214. [DOI] [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Reeves J. P., Green I. Studies of immune functions of patients with systemic lupus erythematosus. Complement-dependent immunoglobulin M anti-thymus-derived cell antibodies preferentially inactivate suppressor cells. J Clin Invest. 1979 May;63(5):954–965. doi: 10.1172/JCI109396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane T., Takada S., Kotani H., Tsunematsu T. Effects of methyl-B12 on the in vitro immune functions of human T lymphocytes. J Clin Immunol. 1982 Apr;2(2):101–109. doi: 10.1007/BF00916893. [DOI] [PubMed] [Google Scholar]
- Stastny P., Ziff M. Antibodies against cell membrane constituents in systemic lupus erythematosus and related diseases. I. Cytotoxic effect of serum from patients with systemic lupus erythematosus (SLE) for allogeneic and for autologous lymphocytes. Clin Exp Immunol. 1971 Apr;8(4):543–550. [PMC free article] [PubMed] [Google Scholar]
- Terasaki P. I., Mottironi V. D., Barnett E. V. Cytotoxins in disease. Autocytotoxins in lupus. N Engl J Med. 1970 Oct 1;283(14):724–728. doi: 10.1056/NEJM197010012831403. [DOI] [PubMed] [Google Scholar]
- Thomas Y., Rogozinski L., Irigoyen O. H., Friedman S. M., Kung P. C., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. IV. Induction of suppressor cells within the OKT4+ population. J Exp Med. 1981 Aug 1;154(2):459–467. doi: 10.1084/jem.154.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas Y., Rogozinski L., Irigoyen O. H., Shen H. H., Talle M. A., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. V. Suppressor cells within the activated OKT4+ population belong to a distinct subset. J Immunol. 1982 Mar;128(3):1386–1390. [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Uchiyama T., Nelson D. L., Fleisher T. A., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. II. Expression of Tac antigen on activated cytotoxic killer T cells, suppressor cells, and on one of two types of helper T cells. J Immunol. 1981 Apr;126(4):1398–1403. [PubMed] [Google Scholar]


