Abstract
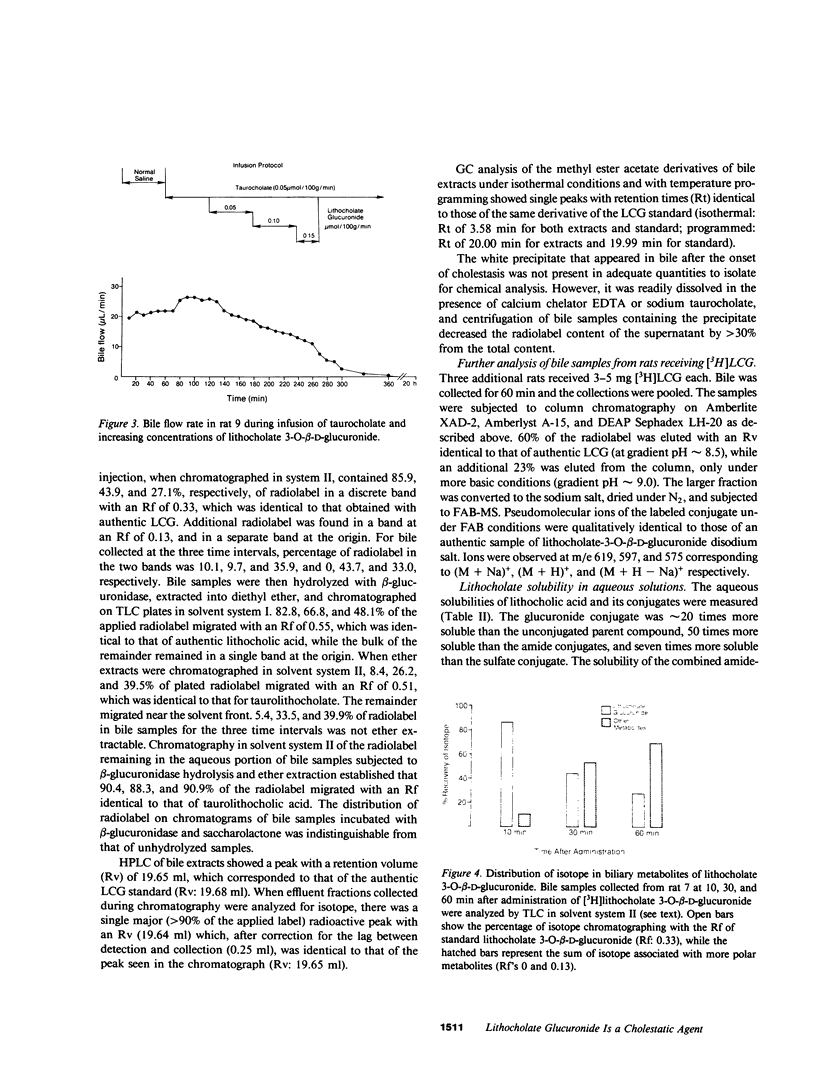
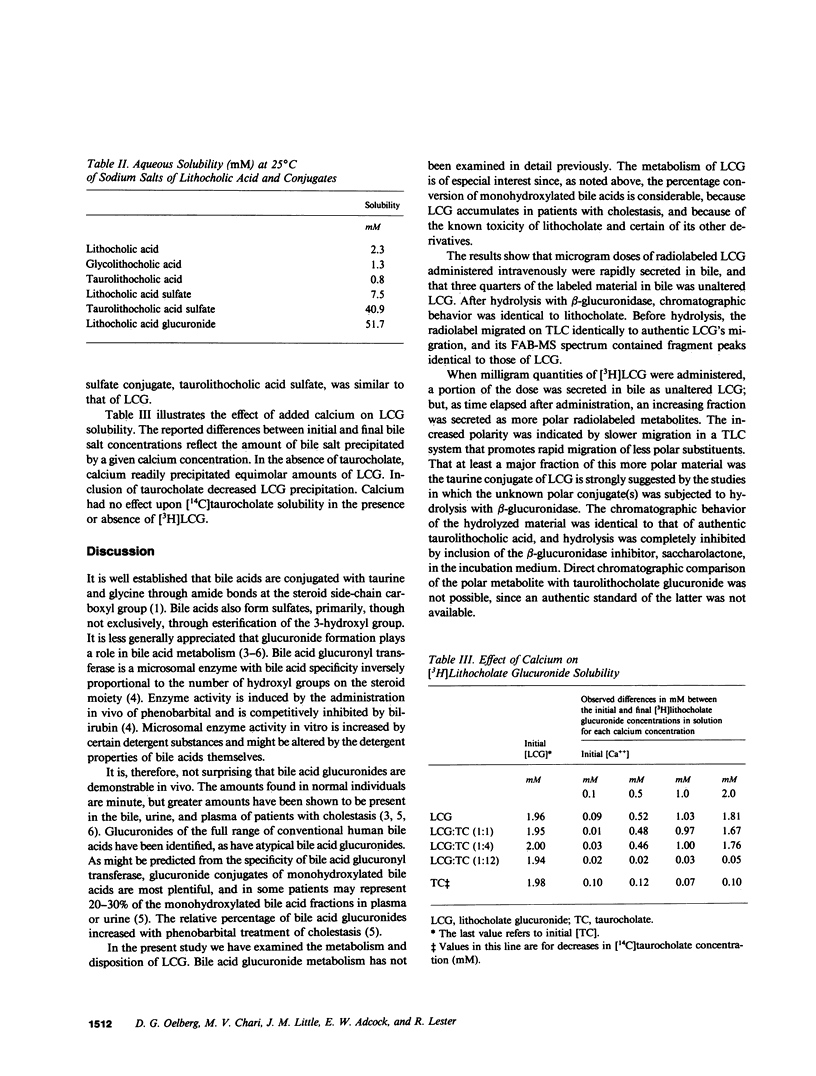
Lithocholic acid and its taurine, glycine, and sulfate derivatives are potent cholestatic agents. Lithocholate glucuronide is present in the plasma and urine of patients with cholestatic syndromes, but little is known of its metabolism, excretion, and cholestatic potential. [3 beta-3H]lithocholate 3-O-beta-D-glucuronide was synthesized, and chemical and radiochemical purity were established. The aqueous solubility of lithocholate glucuronide was determined and found to be greater than that of lithocholic acid or several of its derivatives. In the range of concentrations examined, calcium ions precipitated lithocholate glucuronide stoichiometrically. The material was administered to rats prepared with an external biliary fistula. When 17-25 micrograms quantities were administered, 89.1 +/- 4.5% (mean +/- SEM) of the radiolabel was secreted in bile within the first 20 h after administration, the major fraction being secreted in less than 20 min. Four-fifths of the radiolabeled material in bile was the administered unaltered parent compound, while a minor fraction consisted of a more polar derivative(s). We showed that increasing biliary concentrations of more polar derivatives were observed with milligram doses of [3H]lithocholate glucuronide, and with time after the administration of these loading doses. Milligram doses of [3H]lithocholate glucuronide resulted in partial or complete cholestasis. When induced cholestasis was partial, secretion in bile remained the primary excretory route (82.5-105.6% recovery in bile), while, when complete cholestasis was induced, wide tissue distribution of radiolabel was observed. Cholestasis developed rapidly during infusion of [3H]lithocholate glucuronide. Bile flow was diminished within 10-20 min of the start of an infusion of 0.05 mumol, 100 g-1 body weight, minute-1, administered concomitantly with an equimolar infusion of taurocholate. The results establish that lithocholate glucuronide exerts cholestatic effects comparable to those exerted by unconjugated lithocholic acid.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almé B., Bremmelgaard A., Sjövall J., Thomassen P. Analysis of metabolic profiles of bile acids in urine using a lipophilic anion exchanger and computerized gas-liquid chromatorgaphy-mass spectrometry. J Lipid Res. 1977 May;18(3):339–362. [PubMed] [Google Scholar]
- Back P., Bowen D. V. Bile acid glucuronides, III[1, 2]. Chemical synthesis and characterization of glucuronic acid coupled mono-, di- and trihydroxy bile acids. Hoppe Seylers Z Physiol Chem. 1976 Feb;357(2):219–224. doi: 10.1515/bchm2.1976.357.1.219. [DOI] [PubMed] [Google Scholar]
- Balistreri W. F., Leslie M. H., Cooper R. A. Increased cholesterol and decreased fluidity of red cell membranes (spur cell anemia) in progressive intrahepatic cholestasis. Pediatrics. 1981 Apr;67(4):461–466. [PubMed] [Google Scholar]
- Balistreri W. F., Suchy F. J., Farrell M. K., Heubi J. E. Pathologic versus physiologic cholestasis: elevated serum concentration of a secondary bile acid in the presence of hepatobiliary disease. J Pediatr. 1981 Mar;98(3):399–402. doi: 10.1016/s0022-3476(81)80702-0. [DOI] [PubMed] [Google Scholar]
- Bremmelgaard A., Almé B. Analysis of plasma bile acid profiles in patients with liver diseases associated with cholestasis. Scand J Gastroenterol. 1980;15(5):593–600. doi: 10.3109/00365528009182221. [DOI] [PubMed] [Google Scholar]
- Cowen A. E., Korman M. G., Hofmann A. F., Cass O. W., Coffin S. B. Metabolism of lithocholate in healthy man. II. Enterohepatic circulation. Gastroenterology. 1975 Jul;69(1):67–76. [PubMed] [Google Scholar]
- Drew R., Priestly B. G. Choleretic and cholestatic effects of infused bile salts in the rat. Experientia. 1979 Jun 15;35(6):809–811. doi: 10.1007/BF01968265. [DOI] [PubMed] [Google Scholar]
- Fröhling W., Stiehl A. Bile salt glucuronides: identification and quantitative analysis in the urine of patients with cholestasis. Eur J Clin Invest. 1976 Jan 30;6(1):67–74. doi: 10.1111/j.1365-2362.1976.tb00495.x. [DOI] [PubMed] [Google Scholar]
- Javitt N. B., Emerman S. Effect of sodium taurolithocholate on bile flow and bile acid exeretion. J Clin Invest. 1968 May;47(5):1002–1014. doi: 10.1172/JCI105790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakis G., Phillips M. J., Yousef I. M. The respective roles of membrane cholesterol and of sodium potassium adenosine triphosphatase in the pathogenesis of lithocholate-induced cholestasis. Lab Invest. 1980 Jul;43(1):73–81. [PubMed] [Google Scholar]
- Kakis G., Yousef I. M. Pathogenesis of lithocholate- and taurolithocholate-induced intrahepatic cholestasis in rats. Gastroenterology. 1978 Oct;75(4):595–607. [PubMed] [Google Scholar]
- Marks J. W., Sue S. O., Pearlman B. J., Bonorris G. G., Varady P., Lachin J. M., Schoenfield L. J. Sulfation of lithocholate as a possible modifier of chenodeoxycholic acid-induced elevations of serum transaminase in patients with gallstones. J Clin Invest. 1981 Nov;68(5):1190–1196. doi: 10.1172/JCI110364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulos K. A., Myant N. B. The formation of lithocholic acid, chenodeoxycholic acid and alpha- and beta-muricholic acids from cholesterol incubated with rat-liver mitochondria. Biochem J. 1967 May;103(2):472–479. doi: 10.1042/bj1030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- St Pyrek J., Lester R., Adcock E. W., Sanghvi A. T. Constituents of human meconium--I. Identification of 3-hydroxy-etianic acids. J Steroid Biochem. 1983 Mar;18(3):341–351. doi: 10.1016/0022-4731(83)90113-9. [DOI] [PubMed] [Google Scholar]
- Stiehl A., Becker M., Czygan P., Fröhling W., Kommerell B., Rotthauwe H. W., Senn M. Bile acids and their sulphated and glucuronidated derivatives in bile, plasma, and urine of children with intrahepatic cholestasis: effects of phenobarbital treatment. Eur J Clin Invest. 1980 Aug;10(4):307–316. doi: 10.1111/j.1365-2362.1980.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Stiehl A., Raedsch R., Rudolph G., Czygan P., Walker S. Analysis of bile acid glucuronides in urine: group separation on a lipophilic anion exchanger. Clin Chim Acta. 1982 Aug 18;123(3):275–285. doi: 10.1016/0009-8981(82)90172-3. [DOI] [PubMed] [Google Scholar]
- Yousef I. M., Tuchweber B., Vonk R. J., Massé D., Audet M., Roy C. C. Lithocholate cholestasis--sulfated glycolithocholate-induced intrahepatic cholestasis in rats. Gastroenterology. 1981 Feb;80(2):233–241. [PubMed] [Google Scholar]



