Abstract
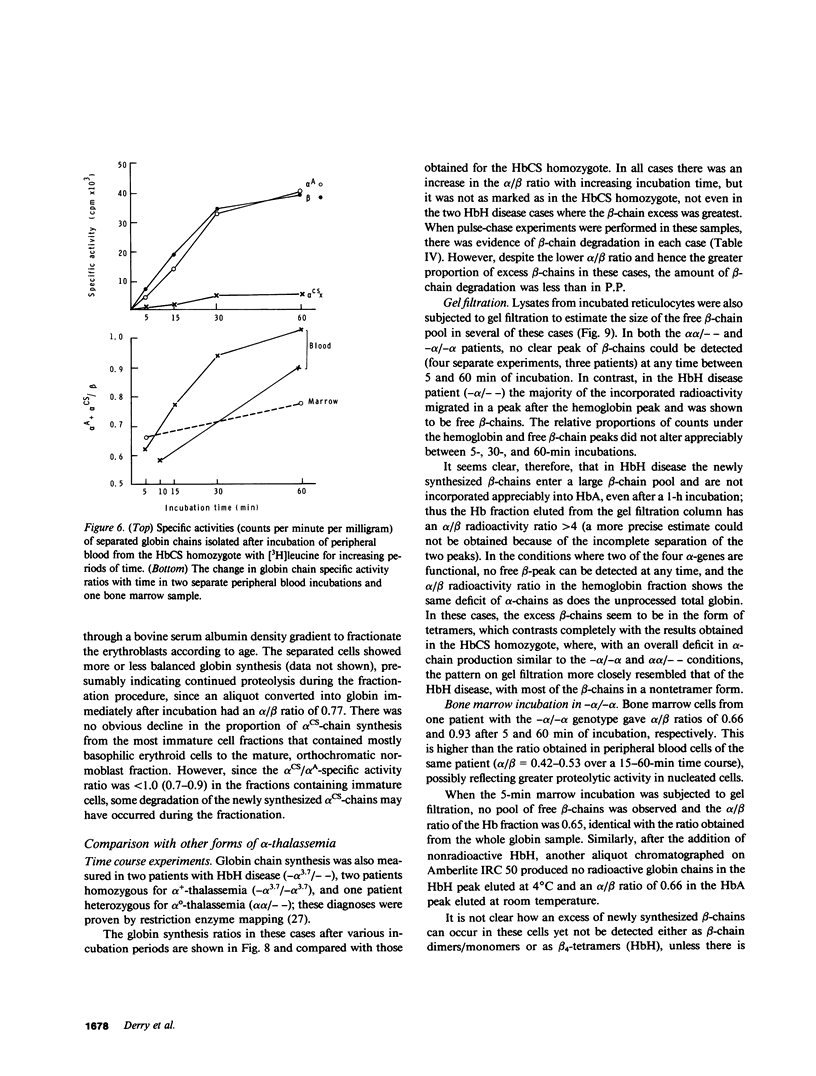
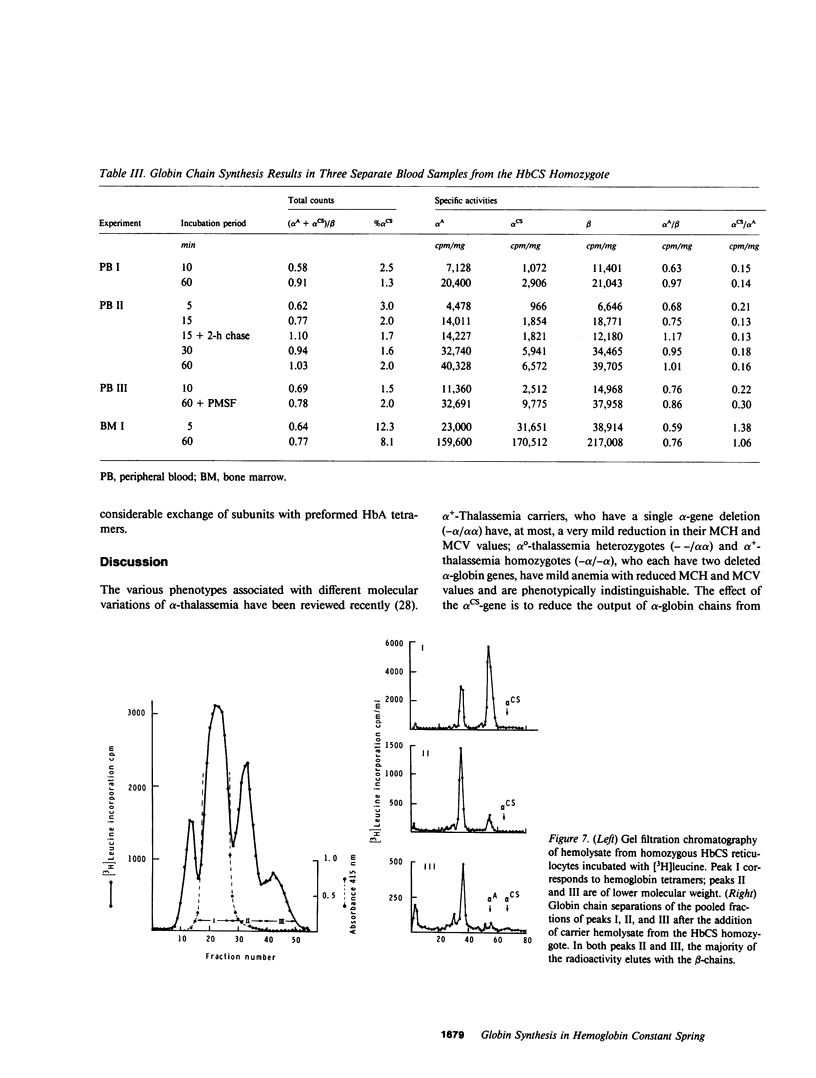
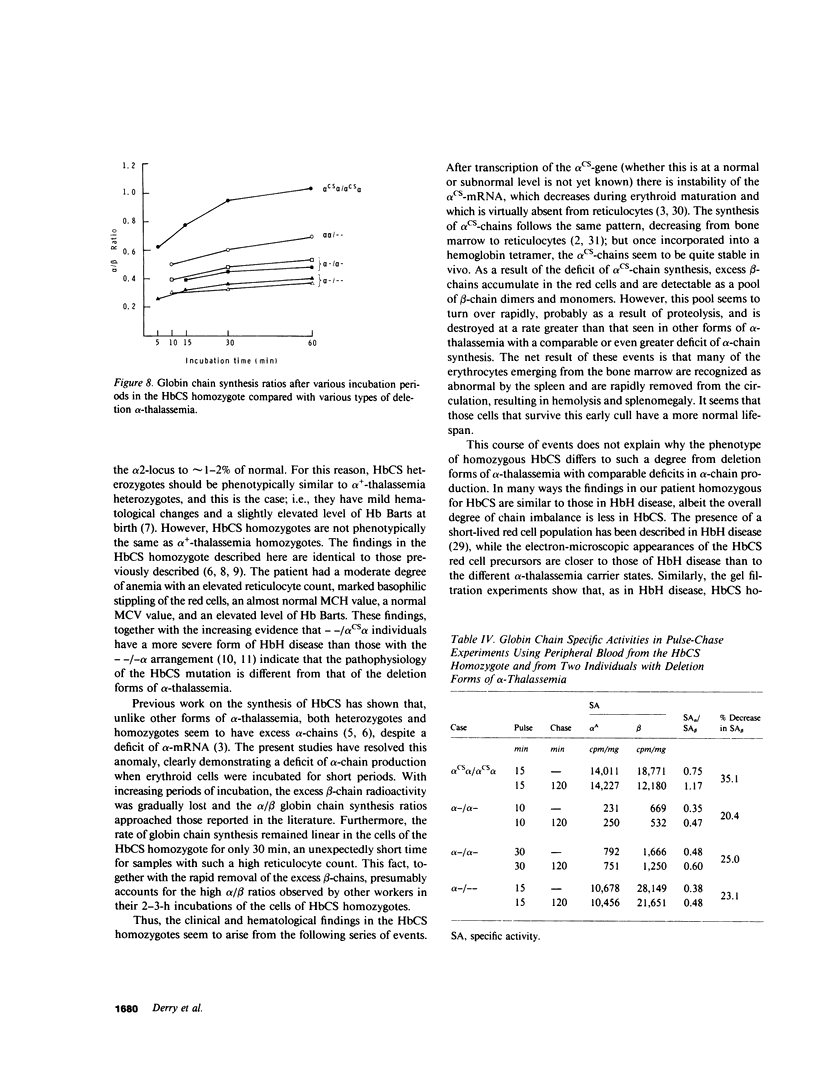
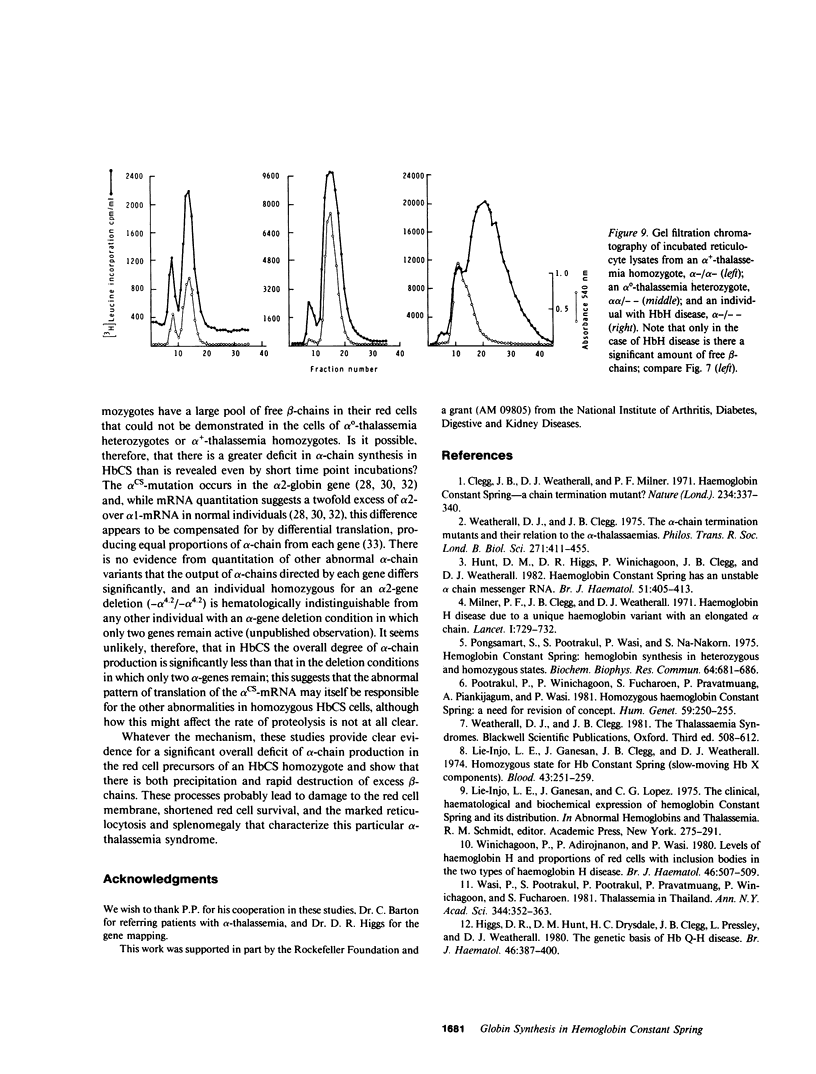
The elongated alpha-globin chains of hemoglobin Constant Spring (alpha cs chain of HbCS ) are produced in low amounts such that the alpha cs-gene acts as a form of alpha-thalassemia; yet in the homozygous state the pathophysiological effects of this mutant are more severe than in the corresponding conditions that result from alpha-globin gene deletions. In studies designed to examine this discrepancy, we have demonstrated that a significant proportion of red cells produced in an HbCS homozygote has a much reduced red cell life span. Contrary to previous reports, we have been able to demonstrate the expected deficit in alpha-chain production in this condition and have shown that both the cessation of globin chain synthesis in vitro and the destruction of the excess beta-chains occur unusually rapidly. Comparison with various deletion forms of alpha-thalassemia suggests that, in terms of intracellular globin chain precipitates and free beta-chain pool, homozygous HbCS red cells more closely resemble those of HbH disease, with three of the four alpha-genes inactivated, than they do the more comparable alpha-thalassemia carriers with only two genes deleted.
Full text
PDF


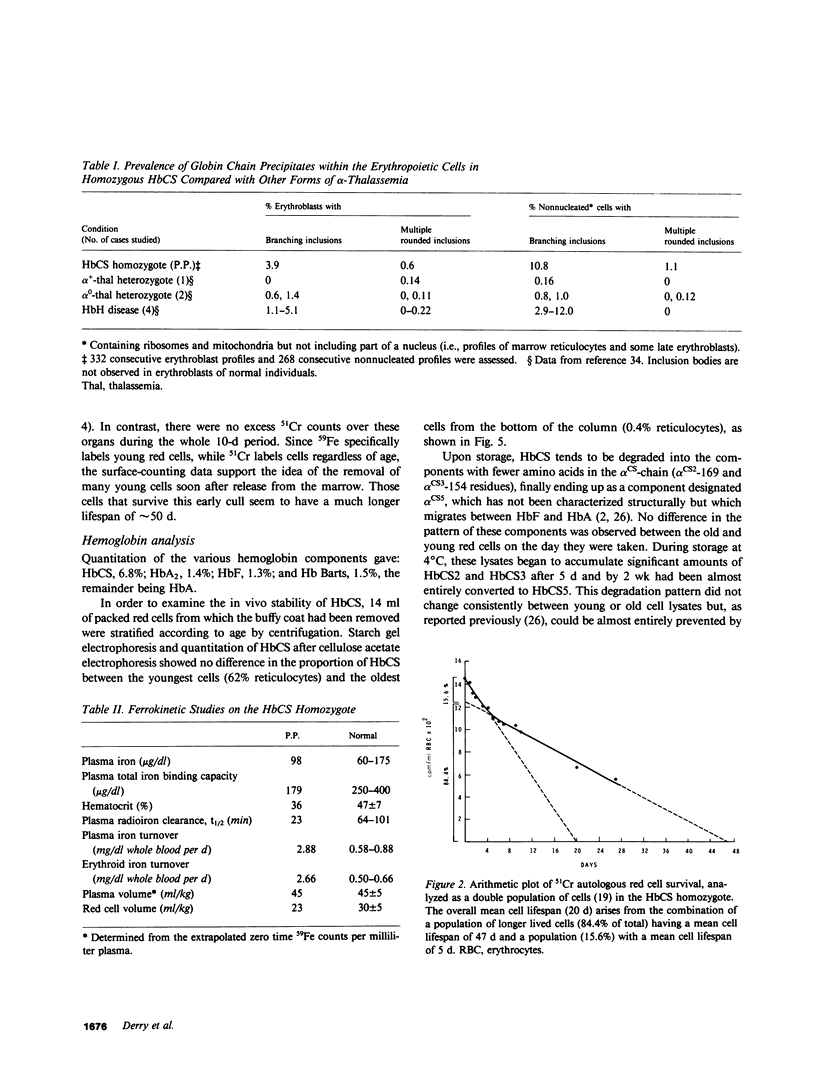
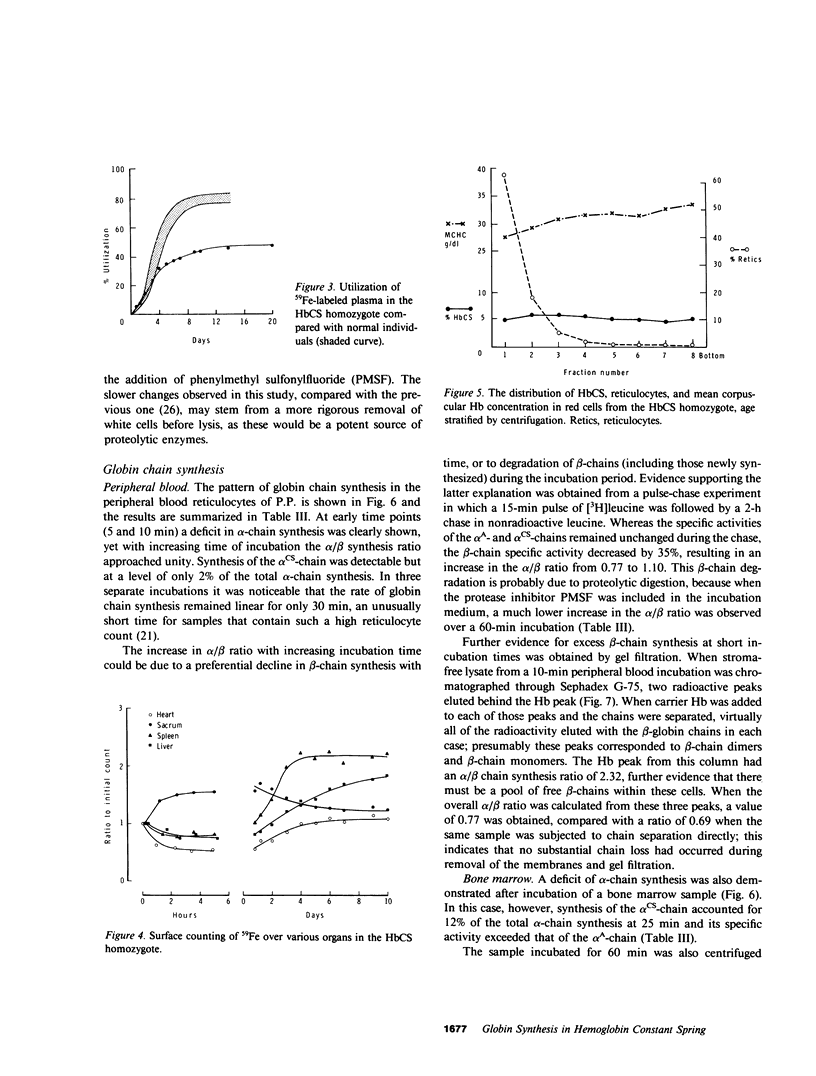

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adirojnanon P., Wasi P. Levels of haemoglobin H and proportions of red cells with inclusion bodies in the two types of haemoglobin H disease. Br J Haematol. 1980 Nov;46(3):507–509. doi: 10.1111/j.1365-2141.1980.tb06002.x. [DOI] [PubMed] [Google Scholar]
- Barosi G., Baraldi A., Bonomi F., Cazzola M., Dacco M., Spriano P., Stefanelli M. Competing models for the analysis of red cell survival obtained with 51Cr labelling technique. Scand J Haematol. 1983 Oct;31(4):381–388. doi: 10.1111/j.1600-0609.1983.tb00666.x. [DOI] [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J. Haemoglobin synthesis in alpha-thalassaemia (haemoglobin H disease). Nature. 1967 Sep 16;215(5107):1241–1243. doi: 10.1038/2151241a0. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J., Milner P. F. Haemoglobin Constant Spring--a chain termination mutant? Nature. 1971 Dec 10;234(5328):337–340. doi: 10.1038/234337a0. [DOI] [PubMed] [Google Scholar]
- Higgs D. R., Hunt D. M., Drysdale H. C., Clegg J. B., Pressley L., Weatherall D. J. The genetic basis of Hb Q-H disease. Br J Haematol. 1980 Nov;46(3):387–400. doi: 10.1111/j.1365-2141.1980.tb05985.x. [DOI] [PubMed] [Google Scholar]
- Higgs D. R., Weatherall D. J. Alpha-thalassemia. Curr Top Hematol. 1983;4:37–97. [PubMed] [Google Scholar]
- Hunt D. M., Higgs D. R., Winichagoon P., Clegg J. B., Weatherall D. J. Haemoglobin Constant Spring has an unstable alpha chain messenger RNA. Br J Haematol. 1982 Jul;51(3):405–413. doi: 10.1111/j.1365-2141.1982.tb02796.x. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Todd D., Dozy A. M. Haemoglobin Constant Spring synthesis in red cell precursors. Br J Haematol. 1974 Sep;28(1):103–107. doi: 10.1111/j.1365-2141.1974.tb06643.x. [DOI] [PubMed] [Google Scholar]
- Lie-Injo L. E., Ganesan J., Clegg J. B., Weatherall D. J. Homozygous state for Hb Constant Spring (slow-moving Hb X components). Blood. 1974 Feb;43(2):251–259. [PubMed] [Google Scholar]
- Liebhaber S. A., Kan Y. W. Different rates of mRNA translation balance the expression of the two human alpha-globin loci. J Biol Chem. 1982 Oct 25;257(20):11852–11855. [PubMed] [Google Scholar]
- Liebhaber S. A., Kan Y. W. Differentiation of the mRNA transcripts originating from the alpha 1- and alpha 2-globin loci in normals and alpha-thalassemics. J Clin Invest. 1981 Aug;68(2):439–446. doi: 10.1172/JCI110273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner P. F., Clegg J. B., Weatherall D. J. Haemoglobin-H disease due to a unique haemoglobin variant with an elongated alpha-chain. Lancet. 1971 Apr 10;1(7702):729–732. doi: 10.1016/s0140-6736(71)91992-1. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goff S. C. The duplicated human alpha-globin genes: their relative expression as measured by RNA analysis. Cell. 1981 May;24(2):345–351. doi: 10.1016/0092-8674(81)90324-x. [DOI] [PubMed] [Google Scholar]
- Pongsamart S., Pootrakul S., Wasi P., Na-Nakorn S. Hemoglobin Constant Spring: hemoglobin synthesis in heterozygous and homozygous states. Biochem Biophys Res Commun. 1975 May 19;64(2):681–686. doi: 10.1016/0006-291x(75)90374-5. [DOI] [PubMed] [Google Scholar]
- Pootrakul P., Winichagoon P., Fucharoen S., Pravatmuang P., Piankijagum A., Wasi P. Homozygous haemoglobin Constant Spring: a need for revision of concept. Hum Genet. 1981;59(3):250–255. doi: 10.1007/BF00283674. [DOI] [PubMed] [Google Scholar]
- Wasi P., Pootrakul S., Pootrakul P., Pravatmuang P., Winichagoon P., Fucharoen S. Thalassemia in Thailand. Ann N Y Acad Sci. 1980;344:352–363. doi: 10.1111/j.1749-6632.1980.tb33674.x. [DOI] [PubMed] [Google Scholar]
- Weatherall D. J., Clegg J. B. The alpha-chain-termination mutants and their relation to the alpha-thalassaemias. Philos Trans R Soc Lond B Biol Sci. 1975 Aug 7;271(913):411–455. doi: 10.1098/rstb.1975.0061. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe S. N., Hughes M., Fucharoen S., Wasi P. The fate of excess beta-globin chains within erythropoietic cells in alpha-thalassaemia 2 trait, alpha-thalassaemia 1 trait, haemoglobin H disease and haemoglobin Q-H disease: an electron microscope study. Br J Haematol. 1984 Mar;56(3):473–482. doi: 10.1111/j.1365-2141.1984.tb03977.x. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe S. N., Hughes M., Hollán S. R., Horányi M., Szelényi J. Electron microscope and high resolution autoradiographic studies of the erythroblasts in haemoglobin H disease. Br J Haematol. 1980 Jul;45(3):401–404. doi: 10.1111/j.1365-2141.1980.tb07160.x. [DOI] [PubMed] [Google Scholar]
- Wood W. G., Stamatoyannopoulos G. Globin synthesis in fractionated Normoblasts of beta-thalassemia heterozygotes. J Clin Invest. 1975 Mar;55(3):567–578. doi: 10.1172/JCI107964. [DOI] [PMC free article] [PubMed] [Google Scholar]