Abstract
Obesity and diabetes represent a significant healthcare concern. In contrast to genome-wide association studies that, some exceptions notwithstanding, have offered modest clues about pathomechanism, the dissection of rare disorders in which obesity represents a core feature have highlighted key molecules and structures critical to energy regulation. Here we focus on the primary cilium, an organelle whose roles in energy homeostasis have been underscored by the high incidence of obesity and type II diabetes in patients and mouse mutants with compromised ciliary function. We discuss recent evidence linking ciliary dysfunction to metabolic defects and we explore the contribution of neuronal and non-neuronal cilia to these phenotypes.
Introduction
Obesity and diabetes are public health concerns that affect up to 15% of the world population (Chen et al., 2012). The observed high heritability of these disorders (BMI heritability between 40 and 70% (Elks et al., 2012)) have suggested that genetic approaches may represent an opportunity to understand pathomechanism and to develop better therapeutic paradigms. Driven by a common disease/common allele hypothesis, single nucleotide polymorphisms (SNPs) in over 100 loci (Vattikuti et al., 2012), a variety of endophenotypes such as waist-to-hip circumference (Vattikuti et al., 2012), or discrete lipid traits (Wang et al., 2009) have been associated with BMI. However, despite these exciting advances, not only has each locus been found to contribute modestly to BMI (Gusev et al., 2013), but the persisting dearth of causal genes, alleles, and direction of effect have frustrated the elucidation of molecular mechanisms and targetable pathways.
Although the field is optimistic that the accrual of genomic data from increasingly-expanding cohorts will improve common allele-driven studies, an alternative approach, based on a rare disease/rare allele hypothesis, has focused on rare, familial, typically penetrant, genetic disorders whose phenotypes include obesity and diabetes. Such studies have made significant progress in extending our understanding of energy regulation derived from the study of model organisms or have potentiated the identification of new key molecules altogether. One exemplar is melanocortin and its receptors, mutations in some of which cause familial morbid obesity (Vaisse et al., 1998; Yeo et al., 1998); the genetic study of human populations coupled with functional analysis of MC4R signaling revealed a contribution of this molecule (and pathway) in more common forms of obesity as well (Loos et al., 2008). Similarly, loss of function mutations in leptin and its receptor have been correlated with perpetual starvation in mice and profound obesity and type II diabetes consistent with morbid obesity in humans (Chen et al., 1996; Zhang et al., 1994). The concurrent identification of human mutations in the ligand-receptor complex (Clement et al., 1998; Montague et al., 1997) has catalyzed a new wave of biochemical and pharmacological efforts to characterize molecular targets and signaling pathways relevant to the treatment of obesity.
Here we will focus on the primary cilium, a structure that has garnered significant attention recently with regard to its roles in energy homeostasis; this is largely because mutations in genes that compromise ciliary structure and function, in either humans or model organisms, cause monogenic or oligogenic forms of obesity, type II diabetes, and a host of metabolic defects. We provide a framework to evaluate the role of proteins that localize to primary cilium, or are necessary for its function in interpreting the extracellular environment and energy homeostasis in the central nervous system (CNS) and other sites of metabolic regulation.
A brief overview of cilia and ciliopathies
Once considered a vestigial organelle, the primary cilium serves a critical role in regulating diverse signaling pathways during vertebrate development and disease (Hildebrandt et al., 2011; Oh and Katsanis, 2012). Emanating from the basal body of most vertebrate cells, cilia are classified broadly based on the arrangement of the microtubule network as motile (containing nine microtubule doublets forming a ring around a central pair of single microtubules; 9+2 array) or non-motile (nine microtubule doublets forming a ring without a central pair of microtubules; 9+0 array) with some exceptions to this rule (Baldari and Rosenbaum, 2010; Gerdes et al., 2009; Kramer-Zucker et al., 2005; Nonaka et al., 1998; Reese, 1965). Protein synthesis is not thought to occur in the cilium; proteins are transported through a series of highly regulated processes that include the translocation of proteins from the cytoplasm to the axoneme through the transition zone (Czarnecki and Shah, 2012), followed by transport across the axonemal microtubules, a process termed intraflagellar transport (IFT) (Kozminski et al., 1993).
In the context of disease burden in humans, historical emphasis had been placed on structural abnormalities of motile cilia that give rise to defects in left-right axis determination, hydrocephalus, respiratory defects and infertility (reviewed in (Yoshiba and Hamada, 2014)). However, the discovery of a hypomorphic mutation in Ift88 in the orpk cystic renal mouse model (Pazour et al., 2000) highlighted the critical importance of primary cilia in the kidney; the subsequent identification of mutations in basal body proteins in patients with Bardet-Biedl sydrome (BBS) showed that dysfuncion in primary cilia can cause a host of phenotypes across body organs and, for the first time, suggested a direct link between cilia and energy homeostasis (Ansley et al., 2003). Since then, more than 50 causal loci have been associated with >15 human genetic disorders, including Nephronophthisis (NPHP), Joubert Syndrome (JBTS), BBS, Meckel-Gruber Syndrome (MKS), Alstrom Syndrome (ALMS), Jeune Asphyxiating Thoracic Dystrophy (JATD) (Valente et al., 2014) and others (Davis and Katsanis, 2012). Such disorders are now classified under an umbrella term of ciliopathies (Badano et al., 2006) which, although individually rare, have a combined frequency of as much as 1:1000 live births (Zaghloul and Katsanis, 2009).
As widely studied models of ciliopathies, both BBS and ALMS share common overlapping features such as obesity, rod-cone dystrophy, infertility and less penetrant phenotypes including type II diabetes and insulin resistance (the latter manifesting early in life in ALMS patients but late, if ever, in BBS patients (Feuillan et al., 2011)). Point mutations and deletions in 20 published genes have been linked to BBS {(Lindstrand et al., 2014) and references within}, while mutations in ALMS1 account for Alstrom syndrome (Marshall et al., 2011). Unlike IFT88, and other axonemal proteins, loss of function mutations in ALMS1 and most BBS genes do not lead to the complete structural loss of the cilium in most cell-types. Instead, they disrupt the function of the organelle, most prominently by perturbing the homeostasis of a number of paracrine signaling cascades (Oh and Katsanis, 2012). In the context of energy metabolism, mutant mice for Alms1 and almost all Bbs genes (Bbs3 mutant mice are not hyperphagic nor obese (Zhang et al., 2011)) generated to date recapitulate several clinical features seen in patients, albeit with variable penetrance and expressivity (Oh and Katsanis, 2012). These include obesity in both Alms1 and Bbs mutant mice and diabetes in Alms1 mutant mice (Arsov et al., 2006; Collin et al., 2005). In parallel, cilia have been found in orexigenic and anorexigenic neurons in the hypothalamus, suggesting a direct link between these sites and obesity. At the same time, cilia have also been detected in a number of distal sites, including adipocytes and muscle progenitors, with concomitant defects in adipocyte differentiation (Marion et al., 2009; Przybylski, 1971), suggesting that ciliary defects in the CNS might not be the sole driver of the observed metabolic pathologies.
Establishing a role for the cilium in the hypothalamus
Located adjacent to the floor of the third ventricle in the hypothalamus, the arcuate nucleus controls a range of neuroendocrine functions in the brain that include energy balance and the regulation of food intake. The arcuate nucleus is comprised of two distinct populations of ciliated neurons: the orexigenic class of neurons promote food intake and co-express both Neuropeptide Y (NpY) and Agouti Related Peptide (AgRP) (Broberger et al., 1998; Hahn et al., 1998); the anorexigenic neurons attenuate food intake and coexpress pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) neuropeptides (Elias et al., 1998). NpY and POMC neurons are responsive to the adipocyte hormone leptin and the pancreatic hormone insulin. Leptin binds to the leptin receptor (LepR) (Ebihara et al., 1999; Elias et al., 1998; Huang et al., 1998) and inhibits NpY neurons while activating POMC neurons. Of the five known leptin receptor isoforms, the signaling capabilities reside primarily with LepR-b which, upon stimulation, results in the activation of Akt and STAT3 (Friedman and Halaas, 1998; Vaisse et al., 1996). The phosphorylation and activation of STAT3 has opposing transcriptional effects on the pomc and agrp promoters, leading to increased expression of POMC and the inhibition of NpY (Ernst et al., 2009; Mesaros et al., 2008). As a common molecular switch, STAT3 is also activated in response to other satiety signals, such as insulin (Varela and Horvath, 2012).
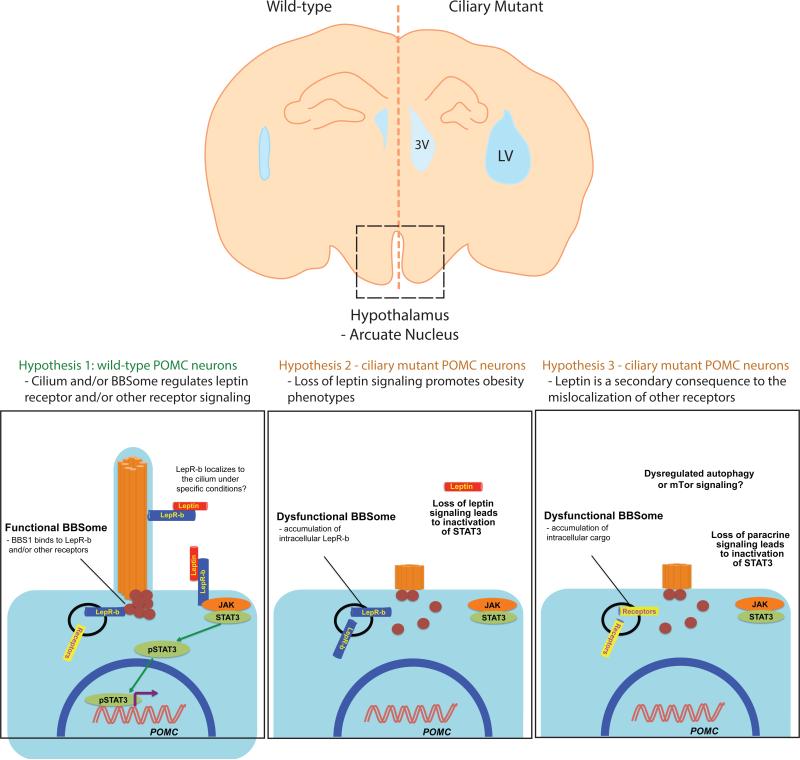
The observation of cilia in hypothalamic neurons stimulated a major effort to establish whether ciliary proteins regulate energy homeostasis (Davenport et al., 2007). Global ablation of cilia is not compatible with life; therefore, a first undertaking to query a physiological role for the cilium was to delete IFT genes using tamoxifen-inducible conditional Ift88 or Kif3a knockout mice. Systemic ablation of Ift88 or Kif3a in adult mice resulted in hyperphagia-induced obesity with increased leptin, glucose, and insulin levels (Davenport et al., 2007). Pair-fed mutant Kif3a mice did not develop obesity or show elevated fasting serum glucose and insulin suggesting that defective cilia may be result in defective feeding behaviors. To test whether neuronal cilia may be driving disease phenotypes, both Kif3a and Ift88-floxed mouse lines were bred to a Synapsin I-Cre driver to remove cilia exclusively in neurons (Davenport et al., 2007). Similar to the tamoxifen-inducible lines, hyperphagia-induced obesity was observed, suggesting that neuronal cilia can participate directly in the regulation of energy homeostasis. Further studies characterizing a POMC-dependent deletion of Kif3a led to the observation of significant weight gain in adult mice (Figure 1), reinforcing the potential role of hypothalamic neuronal cilia in the observed phenotypes (Davenport et al., 2007). Taken together, these studies do support a role of ciliary proteins in obesity. However, given that both KIF3A and IFT88 have roles in cytoplasmic microtubule-based transport (Finetti et al., 2009; Muresan et al., 1999), direct proof that defects of the ciliary structure per se is necessary and sufficient to explained the observed pathologies is lacking. Nonetheless, additional indirect evidence has become available; a variety of proteins involved in ciliary and basal body function (but not cytoplasmic transport) have now been associated with syndromic obesity in humans, while a number of key signaling components known to regulate energy metabolism have also been observed in the neuronal primary cilium.
Figure 1. Hypothalamic neuronal cilia regulate energy metabolism.
Neuronal cilia emanating from POMC-expressing neurons regulate obesity phenotypes in the ciliopathies. Under normal conditions, neuronal cilia and the BBSome complex may serve to modulate signaling from the leptin receptor and/or other receptor complexes. Upon perturbation of the cilium, mistrafficking of receptor complexes leads to the inactivation of STAT3 and to dysfunctional cellular signaling.
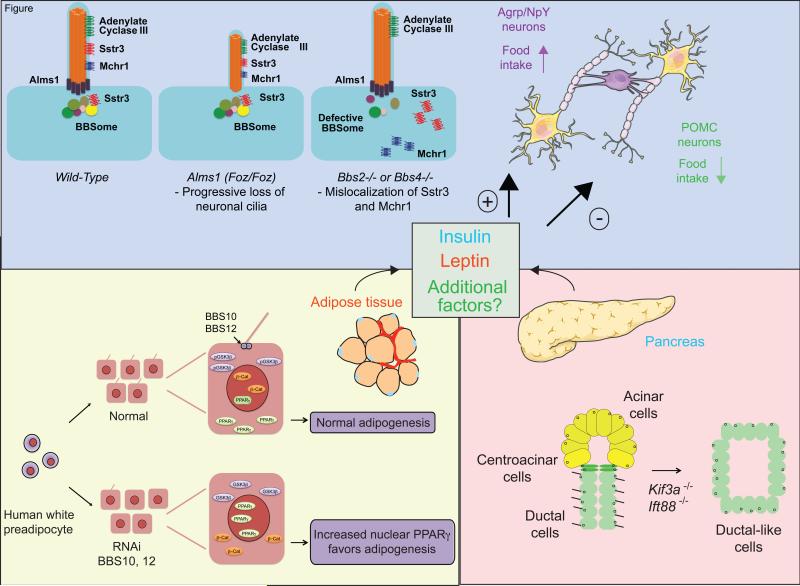
Somatostatin receptor 3 (SSTR3) and melanin-concentrating hormone receptor 1 (MHCR1) contain a ciliary targeting signal that promotes localization to the neuronal cilium in the hypothalamus (Berbari et al., 2008). Binding of the somatostatin and melanin-concentrating hormone G-protein-coupled receptors (GPCRs) inhibits the release of glucagon and insulin in postprandial states. Importantly, SSTR3- and MHCR1-expressing neurons are also leptin-responsive (Stepanyan et al., 2003). Genetic ablation of Bbs2 and Bbs4 in the mouse was shown to result in the loss of Sstr3 and Mhcr1 localization to primary cilia (Berbari et al., 2008). Given the observation of hyperphagic-induced obesity in Bbs2−/− and Bbs4−/− mice, perturbation of Sstr3 and Mchr1 ciliary targeting was thus correlated with the regulation of satiety signals (Berbari et al., 2008). The role of Sstr3 in the brain has also been studied in foz/foz mice, where truncated Alms1 protein fails to localize to the cilium in hypothalamic neurons. Unlike Bbs mouse mutants, expression of truncated Alms1 leads to fewer ciliated neurons in the hypothalamus (Heydet et al., 2013). Surprisingly, no significant difference in Sstr3 and Mhcr1 ciliary localization was observed in foz/foz mice (Heydet et al., 2013), arguing that perturbation of ciliary targeting of GPCRs to the cilium is not always necessary to give rise to obesity.
Leptin plays a central role in regulating energy intake through the activation of anorexigenic pathways in the hypothalamus (Figure 2); however, it is unclear whether leptin resistance is a primary or secondary cause of obesity. Leptin resistance is defined as the inability of elevated leptin levels to curb adiposity in common obesity. Such phenomena have been attributed to obesity phenotypes observed in Bbs2−/−, Bbs4−/−, and Bbs6−/− mutant mice, all of which have been reported to be hyperleptinemic (Rahmouni et al., 2008). Additionally, attenuated POMC transcript levels in the hypothalamus of these mutants suggested that circulating leptin cannot stimulate transcriptional activation of POMC expression (Rahmouni et al., 2008). Defective LepR-b trafficking in Bbs mutant mice further bolstered the hypothesis that leptin resistance might contribute to obesity in BBS. The mislocalization of LepR-b to large intracellular vesicles upon loss of either Bbs1 or Bbs2 and a biochemical interaction of LepR-b with Bbs1 argue that the leptin receptor localizes possibly to the primary cilia in a manner dependent on the secretory pathway and the BBSome (Seo et al., 2009).
Figure 2. Evaluation of the role of cilia during energy homeostasis.
Several covariates drive obesity phenotypes in the ciliopathies. In the adipose tissue, depletion of BBS proteins leads to the loss of cilia from preadipocytes; the failure to respond to environmental cues through the primary cilium results in the perturbation of adipogenesis. In the pancreas, depletion of IFT proteins results in the transformation of centroacinar cells to ductal-like cells. Insulin, leptin and potentially other, uncharacterized factors regulate neurons in the hypothalamus which are also sensitive to the loss of cilia.
Does leptin resistance contribute to obesity in ciliopathies?
Although data from conditional Ift mouse mutants outline a requirement of primary cilia in the hypothalamus for energy homeostasis, the function of neuronal cilia in regulating satiety signals through LepR-b remains unanswered. Recent studies utilizing tamoxifen induced ablation of Ift88 (Ift88Δ/Δ) in adult mice (eight weeks) examined the role(s) of cilia and leptin signaling in obesity (Berbari et al., 2013). Following tamoxifen administration at eight weeks, a lean phase persisted for about three weeks, where the body weight of mutant mice did not differ significantly from that of littermates. While ad libitum feeding resulted in obesity at three months of age, caloric restriction of Ift88Δ/Δ mice led to a recovery in body weight to wildtype levels from the obese phase at five months of age. Mice were categorized into three chronological phases: lean; obese; and lean based on their body weight post tamoxifen administration. Ift88Δ/Δ mutants exhibited elevated serum leptin with respect to body fat and peaked during the obese phase. A striking feature of these analyses was the return of serum leptin levels to wildtype levels in the late lean phase (five months). Furthermore, activation of p-STAT3 in the arcuate nucleus upon leptin administration during the initial lean phase in Ift88Δ/Δ mice suggested that loss of cilia does not perturb leptin signaling in the hypothalamus. Similarly, leptin administration in pre-obese Bbs4−/− mice results in anorexia (Berbari et al., 2013). These observations differ from earlier studies carried out in Bbs2−/−, Bbs4−/−, and Bbs6−/− mice, in which serum leptin levels at 5-6 weeks of age was significantly higher in all three Bbs null mice (Rahmouni et al., 2008). Moreover, a four-day regimen of leptin administration failed to reduce the body weight in Bbs null mice (Rahmouni et al., 2008), whereas a similar experiment resulted in reduced food intake in pre-obese Bbs4−/− and Ift88Δ/Δ mice (Berbari et al., 2013). While it is unclear how to reconcile these differences, the previous studies were performed on mice from different background strains; such variables have been reported exhaustively to sensitize experimental parameters affecting pre-obese and obese phases (Ewart-Toland et al., 1999) and warrant further investigation. More recently, an alternate hypothesis has been proposed that the mislocalization of other GPCRs to the cilium may be responsible for hyperphagia-induced obesity in BBS (Loktev and Jackson, 2013). A screen for novel ciliary GPCRs led to the identification of neuropeptide Y receptor NPY2R as a candidate anorexigenic receptor defective in hypothalamic signaling in BBS and tubby (a ciliary protein required for the trafficking of GPCRs to neuronal cilia; mouse mutants show retinal degeneration and obesity phenotypes (Mukhopadhyay et al., 2010; Sun et al., 2012)) mutant mice. Importantly, mutant BBS mice were non-responsive to treatment with PYY3-36, an endogenous ligand to NPY2R, suggesting that ciliary targeting of additional GPCRs (Figure 1) may either drive or modulate the obesity phenotypes observed in ciliopathies (Loktev and Jackson, 2013).
To rationalize the role of the cilium and leptin in energy metabolism, the following observations should be considered; a) obesity resulting from systemic and tissue-specific ablation of Kif3a and Tg737 in POMC neurons; b) increased weight gain in Bbs null mice coupled with reduced p-STAT3 levels in the hypothalamus; c) mislocalization of LepR from cilia of ARPE cells upon ablation of BBS1 and BBS2; and d) a biochemical interaction between LepR-b and basal body proteins such as BBS1 and RPGRIP1L (Stratigopoulos et al., 2014). However, the data that indicate that ciliary defects might be secondary to the onset of metabolic dysfunction are: a) a positive leptin response in Ift88Δ/Δ mice during the initial and late lean phase; b) increased p-STAT3 signal in the hypothalamus in response to leptin; c) additional GPCRs modulate obesity phenotypes in BBS: and d) localization of LepR throughout the plasma membrane in ARPE cells. These observations suggest that, irrespective of whether leptin signaling is driven by ciliary mechanisms, loss of cilia in POMC neurons leads to obesity in mice. If activation of p-STAT3 is deterministic for leptin signaling, then it can be postulated that primary cilia are not required for leptin signaling in the arcuate nucleus, due to a positive leptin response in Ift88Δ/Δ mice. Taken together, the signal output from leptin-dependent activation appears to be modulated by ciliary and non-ciliary mechanisms which in toto appear to regulate energy homeostasis.
Cilia biology in adipocytes and the pancreas
Despite the functional relevance of cilia to hypothalamic neurons, a defective CNS is one of several covariates that can drive obesity phenotypes in ciliopathies (Figure 2). The human adipose tissue contains adipocytes that are insulin-sensitive and regulate lipid storage and hormone secretion. Adipocytes are derivatives of the mesenchymal lineage of stem cells that differentiate as preadipocytes before maturation to terminal adipocytes (Pittenger et al., 1999). Mature adipocytes are unique vertebrate cells as they are not thought to be ciliated; however, a transient primary cilium has been described during the differentiation of preadipocytes (Marion et al., 2009). To evaluate the relevance of the transient cilium, transcriptomic analyses were conducted to ask whether ciliary proteins are expressed during adipogenesis. Expression analyses revealed a significant upregulation of BBS1-4, and BBS6-12 expression during the differentiation of preadipocytes (Forti et al., 2007; Marion et al., 2009). Next, BBS10 and BBS12 were suppressed in confluent primary preadipocytes and cultured in preadipocyte differentiation medium containing D-Glucose and insulin. Two days after such treatment, proadipogenic peroxisome proliferator-activated receptor γ (PPARγ) was detected in BBS10 and BBS12-depleted preadipocytes, suggesting that a functional cilium/basal body can negatively regulate adipogenesis. These results are in contrast with studies showing a down-regulation of PPARγ suppression of Alms1 and IFT proteins in transformed 3T3-L1 mouse preadipocytes(Huang-Doran and Semple, 2010). However, given that human preadipocytes can proceed to terminal differentiation without post-confluence mitosis, differences in PPARγ responsiveness may be attributed to cell line-specific variables. In addition, ciliopathy proteins localize to subcellular structures other than the cilium and basal body (Collin et al., 2012; Delaval et al., 2011) raising the possibility that these unique localization patterns may account for some of the discrepancies between studies on human preadipocytes and 3T3-L1 mouse preadipocytes.
The exocrine (enzyme secretion) and the endocrine glands (hormone secretion) constitute the pancreas. The islets of Langerhans are patches of endocrine tissue that contain α, β, γ, δ, and ε cells that secrete glucagon, insulin, pancreatic polypeptide, somatostatin, and ghrelin respectively (Collombat et al., 2010). Insulin is one of the central regulators of energy homeostasis and is secreted in response to glucose and leptin (Collombat et al., 2010). Pancreatic defects are penetrant phenotypes in ciliopathies; they manifest mostly as pancreatic cysts originating from ducts cells in ADPKD, ARPKD, von Hippel-Lindau disease and other ciliary disorders (Table 1). Both severe exocrine defects and milder endocrine defects, such as reduced glucose tolerance and lower fasting blood glucose levels, have been reported in the hypomorphic Tg737orpk mutant mice (Zhang et al., 2005). In addition, acinar-to-ductal metaplasia, fibrosis, and lipomatosis were observed in pancreas-specific Kif3a-deleted mice (Cano et al., 2006). To evaluate whether primary cilia are necessary for the development of pancreatic islets, Kif3a was ablated at E10 or at four weeks of age. Normal development of the pancreas was observed, suggesting that the regulation of hormonal levels and islet formation are mutually exclusive (Cano et al., 2006). Although most IFT models of ciliopathies studied to date lead to pancreatic ductal dilations and cyst formation, no changes in insulin secretion have been observed. However, insulin secretion and the ciliary apparatus were associated upon examination of mice lacking Regulatory factor X, 3 (RFX3) (Ait-Lounis et al., 2007) and Gli-similar proteins 3 (Glis3) (Kang et al., 2009). Both RFX3 and Glis3 are transcription factors necessary for normal ciliary function; deletion of either gene results in pancreatic ductal defects in mice and decreased insulin secretion with impaired glucose tolerance. Given that structural deficits in the cilium may not be linked causally to insulin secretion, the previous findings may be explained due to ciliary proteins functioning both at the cilium and other organelles, which together influence insulin secretion and energy regulation.
Table 1.
Pancreatic defects are penetrant phenotypes in the ciliopathies
| Gene | Species | Phenotype | Reference |
|---|---|---|---|
| Pkd1 truncation | Mouse | Pancreatic cysts | Lu et al., 1997 |
| Pkhd1 (PCK) | Rat | Pancreatic duct dilation | Lager et al., 2001 |
| Inv | Mouse | Pancreatic cysts | Morgan et al., 1998 |
| Ift88 (Tg737) | Mouse | Pancreatic duct dilation or elevated insulin upon POMC-specific deletion | Cano et al. 2004, Davenport et al., 2007 |
| Kif3A (pancreas specific) | Mouse | Cyst formation, aberrant ductal morphology, and extensive fibrosis | Cano et al., 2006 |
| Hnf6 | Mouse | PKD and pancreatic cysts | Pierreux et al., 2006 |
| Alms1 (Fat Aussie) | Mouse | Islet cysts | Arsov et al. 2006 |
| Rfx3 | Mouse | Altered pancreatic endocrine cell differentiation | Ait-Lounis et al., 2007 |
| Pkhd1 (del2/del2) | Mouse | Pancreatic cysts and some pancreatic enlargement | Woollard et al. 2007 |
| Kif3A (POMC-Cre) | Mouse | Elevated insulin | Davenport et al., 2007 |
| Pkhd1 (del4/del4) | Mouse | Pancreatic cysts | Gallagher et al., 2008 |
| NPHP3 | Human | Pancreatic cysts | Bergmann et al., 2008 |
| Pkhd1 | Rat | Pancreatic duct dilation | Yi et al., 2012 |
| VHL | Human | Pancreatic cysts and pancreatic neuroendocrine tumors | van Asselt et al., 2013 |
| NEK8/NPHP9 | Human | Enlarged pancreas | Frank et al., 2013 |
Analysis of mouse loss of function mutant models of ciliary genes reveal aberrations in pancreatic function.
Ciliary signaling paradigms and energy homeostasis
Primary cilia are signaling hubs for a host of paracrine signaling pathways. While over 15 distinct signaling pathways have been associated to the cilium, the relevance of Wnt and Sonic Hedgehog (Shh) signaling to the ciliary apparatus has been most intensively studied over the last decade (Oh and Katsanis, 2013). Wnt ligands comprise a class of secreted glycoproteins that regulate conserved functions ranging from the development of the body axis to the regulation of cell polarity (Willert and Nusse, 2012). Activation of the canonical pathway results in the stabilization of β-catenin through disruption of the destruction complex comprising of Axin, Casein Kinase, GSK3β, PP2A, and Adenomatous Polyposis Coli, while the non-canonical pathway influences processes such as calcium levels and actin modification. Cilia are thought to control the balance between canonical and non-canonical Wnt signaling and components of the Wnt signaling machinery (β-catenin, Adenomatous Polyposis Coli, and frizzled receptors) localize to the ciliary axoneme (Oh and Katsanis, 2012). Leptin activates Wnt signaling via GSK3β inhibition in at least 70% of NpY neurons suggesting that the catabolic action of leptin is transduced through hypothalamic WNT signaling (Benzler et al., 2013).
The process by which preadipocytes differentiate into mature adipocytes is controlled by Wnt proteins; inhibition of Wnt signaling promotes differentiation and activation terminates adipogenesis (Ross et al., 2000; Wright et al., 2007). In vivo support for this model was demonstrated when wild-type or Lepob/ob mice expressing Wnt10b under the control of the adipose-specific FABP4 promoter showed a 50% reduction in total body fat (Longo et al., 2004; Wright et al., 2007). As might be predicted, in reciprocal studies using mice lacking Wnt10b, an increased adipogenic potential was observed, supporting the notion that Wnt signaling controls adipogenesis (Vertino et al., 2005). Adipogenesis is promoted through activation of the CCAAT/enhancer binding proteins (C/EBPs) and peroxisome proliferator-activated receptors (PPARs) (Ross et al., 2000). To explore a role for the basal body/cilium in adipogenesis, BBS10 or BBS12 was suppressed in differentiating human preadipocytes in culture; perturbation of the basal body resulted in elevated levels of nuclear PPARγ and active GSK3β(Marion et al., 2009). In addition, adipocyte-derived dermal fibroblasts from patients carrying mutant BBS10 or BBS12 also displayed a significant increase in triglyceride content and higher secreted leptin levels in culture medium, suggesting that enhanced adipogenesis is a likely direct consequence upon loss of basal body proteins.
Similar to Wnt, Hedgehog (Hh) signaling is a conserved pathway that is required for embryonic development and the maintenance and regeneration of adult tissue. Upon binding of the ligand, Smoothened (Smo), a seven membrane spanning receptor is relieved from inhibition by Patched (Ptc) and processes Gli proteins into an activator form (GliA) in the cilium. Gli activator proteins translocate to the nucleus and transactivate downstream targets such as Axin2. Several components of the Hh pathway (Smo, Ptc, and Gli proteins) have been localized to the primary cilium in multiple cell types, including neurons and adipocytes (reviewed in (Oh and Katsanis, 2012). Similar to Wnt, Hh signaling functions in an anti-adipogenic manner, such that activation of the pathway leads to a reduction in PPARγ expression in adipogenic cells. While total suppression of Wnt signaling is necessary for differentiation of adipocytes, residual Hh responsiveness can be detected in mature adipocytes, suggesting that inhibition of Hh signaling is not a fate-determining step during adipogenesis (Pospisilik et al., 2010). To query whether the basal body/cilium is relevant to Hh signaling and adipogenesis, suppression studies of BBS12 were performed in cultured human adipocytes derived from human mesenchymal stem cells (Marion et al., 2012). Together with an increase in nuclear PPARγ during adipogenic differentiation, attenuation of Gli2 and Gli3 expression was observed, indicating that suppression of the basal body resulted in the repression of an anti-adipogenic program. Cognizant that these results may be due to cell line-derived artifacts, Bbs12−/− mice were generated; similar to in vitro studies, defects in insulin sensitivity and glucose absorption were observed (Marion et al., 2012). Together, these results suggest that ciliary mechanisms and Hh signaling are relevant to energy regulation in adipose tissue and contribute to obesity phenotypes observed in BBS. Of note, a role for Hh signaling in the homeostasis of adult hypothalamic neurons has not been elucidated.
As an anti-adipogenic signal, canonical Hh signaling inhibits adipogenesis in white adipose tissue by blocking the differentiation of white adipocytes, but not brown fat and muscle. However, a recent non-canonical Hh signaling pathway has been characterized to promote insulin-independent glucose uptake in brown adipose tissue and muscle (Teperino et al., 2012). Through other work demonstrating that Hh can acutely increase Ca2+ spike activity in neurons (Belgacem and Borodinsky, 2011), Smo-dependent activation of Ca2+ and AMP kinase (AMPK) was observed in human and mouse myocytes, leading to glucose uptake and reprogramming to a glycolytic state. Given that both canonical and noncanonical pathways are dependent on a functional cilium, Hh signaling effectors may hold therapeutic value in combating obesity and diabetes (Teperino et al., 2012).
Recently linked to the cilium is a degradative process known as autophagy (Pampliega et al., 2013; Tang et al., 2013). Activation of autophagy occurs under conditions of nutrient deprivation, as well as during development and differentiation (Jing and Lim, 2012). In light of a positive correlation between ciliogenesis and serum deprivation, tandem affinity purification was performed to identify potential ciliary interactors of LC3, a key effector of autophagy. Among several known cargo-adaptor proteins required for recruiting cargo to the autophagasome, centriolar satellite proteins such as PCM1, CEP131 and OFD1 were found to associate with LC3. During serum deprivation, OFD1 protein levels were reduced in wild-type cells, but not in autophagy-deficient Atg5−/− MEFs, suggesting that autophagy initiates ciliogenesis through degradation of OFD1 (Tang et al., 2013). Under normal nutrient conditions, OFD1 protein levels are unaffected; however, ciliogenesis is not favored through sequestration and degradation of IFT20 in autophagasomes (Pampliega et al., 2013). While these data suggest that the autophagy machinery localizes to the cilium and basal body, colocalization studies under serum-deprivation and compromised IFT (Ift88−/− cells) conditions verified that multiple autophagy-related proteins associated with the ciliary axoneme (ATG16L, AMBRA1, LC3, GABARAP and VPS15) and basal body (ATG16L, AMBRA1, LC3, GABARAP, VPS15, ATG14, VPS34, ATG7 and ATG5) (Pampliega et al., 2013).
Although unclear whether OFD1 degradation occurs at the hypothalamus and/or other sites of energy regulation, the role of autophagy during food intake is well-established. In the hypothalamus, inhibition of the autophagasome in orexigenic and anorexigenic neurons can regulate metabolism; mice lacking Atg7 in POMC neurons have higher post-weaning body weight, increased adiposity, and glucose intolerance (Coupe et al., 2012), while deletion of Atg7 in AgRP neurons results in significantly reduced body weight and total fat mass (Kaushik et al., 2011). In addition, mice lacking Atg7 in POMC neurons are not responsive to leptin and STAT3 activation is not observed (Coupe et al., 2012), suggesting that hypothalamic autophagy controls appetite and/or body weight. Similarly, adipose-specific deletion of Atg7 yielded mutant mice with 20% of the mass of white adipose tissue found in wild-type mice (Zhang et al., 2009). Given the localization of Atg7 to the basal body and the overlapping phenotypes between ciliary and autophagy mutants, we predict that mechanisms regulating autophagy and ciliogenesis will likely contribute to energy balance.
Alternate mechanisms of signal acquisition
A consistent theme that has emerged upon analyzing ciliary mutants is that disease phenotypes can be correlated with ciliary length changes. Upon exposure to aqueous cigarette smoke extract, autophagasome accumulation and autophagic activity in the lungs is observed in conjunction with shortening of the cilium (Lam et al., 2013). The severity of the cilia defect is a function of the concentration of cigarette smoke; higher cigarette smoke doses yield cilia that are 50% shorter, while lower doses result in cilia that are 20% shorter. Equivalently, significantly shorter neuronal cilia can be observed in the hypothalamus of diet-induced obese (DIO) mice and leptin/leptin-receptor deficient mice (Han et al., 2014). Changes in the ciliary length phenotypes in DIO mice are prominent for several reasons; 1) the phenotype is confined specifically to the ventromedial hypothalamus, with no changes in the CA3 region of the hippocampus; and 2) infusion of leptin in the third cerebroventricle rescues the short cilia phenotype. Given the changes in circulating leptin levels upon a lean and high-fat, high-sucrose diet for 14 weeks and that Bbs and Alms1 mutant mice develop leptin resistance prior to becoming obese, can leptin directly promote changes in ciliary length? Stimulation of either N1 hypothalamic neurons or primary hypothalamic cells with leptin for ~12 hours leads to a ~50% increase in ciliary length (Han et al., 2014). In addition, localization of a tagged leptin-receptor complex at the basal body suggested that leptin signaling machinery is associated with the cilium. Together the results argue that ciliary length in the hypothalamus can report leptin signaling strength during energy homeostasis.
Changes in ciliary length have also been appreciated in renal medullary cells from Bbs mice and bbs7 or bbs9-depleted paramecia; however, shorter cilia have not been reported in the hypothalamus of Bbs or Alms1 mutant mice. These data suggest that in addition to ciliary length and transport defects, other mechanisms must also account for the control of neuroendocrine function. Taking advantage of conservation in BBSome components in C.elegans and that disruption of bbs or ift proteins results in structural ciliary defects and diminished sensory behavioral responses seen in mammalian BBS and IFT mutants, Lee and colleagues asked whether alternate hypotheses could be explored in an organism with 60 ciliated sensory neurons (Lee et al., 2011). Like other species, C.elegans sense changes in the environment and secrete neuroendocrine hormones, including insulin-like peptides, in response to stress. Given the role of ciliated neurons in the secretion of hormones, ciliary defects should perturb this function. As predicted, multiple ift C.elegans mutant lines presented a dramatic reduction in insulin secretion. BBS proteins are also utilized to facilitate IFT in neurons; therefore, it was surprising to observe an increase in insulin secretion in mutant lines of the BBSome. To evaluate this conundrum, the authors suspected that the failure in insulin secretion must be independent of impaired IFT. Given the increase in insulin secretion and the observation of elevated secretions of dense-core vesicles, the BBSome complex may also regulate exocytosis. Further assays showed that a Rab27/rabphillin/CAPS exocytosis machinery functioned together with bbs proteins to promote vesicular transport. Interestingly, the altered body size, feeding and metabolic abnormalities in bbs mutants in C.elegans can be corrected by inhibiting the release of dense-core vesicles, suggesting that excessive neuroendocrine signaling activity may account for a fraction of BBS disease phenotypes (Lee et al., 2011).
Concluding remarks
The combination of genetic discoveries in humans, tissue-specific transgenic mouse models, and biochemical studies in cells and organisms has highlighted multiple roles of ciliary proteins in energy regulation and metabolism. These studies reinforce the role(s) of hypothalamic neurons and leptin signaling and also underscore the potential contribution of adipocyte differentiation in human disease phenotypes. These initial discoveries have now formed the platform to ask the next sets of questions, which are many. First, significant discrepancies remain to be resolved from existing data. Different ciliopathy mouse models exhibit obesogenic characteristics driven by apparently discrete components of signaling processes that may or may not be modulated by the cilium (the anatomical structure) or by hitherto unknown functions of ciliary proteins elsewhere in the neuronal soma. For example, cilia assembly in new neurons from the dentate gyrus occurs during a period that corresponds to glutamatergic synaptic activity (14-21 days after birth), highlighting the possibility that 1) proteins that are required for cilia formation are also required for synaptogenesis or 2) the cilium signals directly to the synapse (Kumamoto et al., 2012). In addition, the variable expressivity, severity, and temporal differences in ciliopathy mouse models of obesity, are not understood. It will be important to determine whether differences between mouse models are driven by discrete functions of ciliary proteins or genetic background; answering this question offer a perhaps unique opportunity to understand the variance of the phenotype in humans and to uncover new pathways and processes. An exemplar of variable expressivity and obesity can be highlighted in the correlation of dose-dependent body weight difference in humans and common variants in the first intron of the fat mass and obesity associated gene (FTO) (Frayling et al., 2007). SNPs associated with increased BMI are hypothesized to control the expression of the ciliary gene, RPGRIP1L. Mice heterozygous for RPGRIP1L are hyperphagic and have a higher body weight demonstrating that the regulation of adiposity can be embedded in the regulatory elements of genes near FTO (Stratigopoulos et al., 2014). In addition to common variants showing an effect on complex diseases, analysis of large exome sequence-based approaches have revealed that rare variants in BBS10 can also influence Type 2 diabetes (T2D) susceptibility (Lim et al., DOI: http://dx.doi.org/10.1016/j.ajhg.2014.09.015). Taken together, both common and rare variation in ciliopathy genes are likely to modulate obesity and T2D phenotypes. Finally, it remains unclear why obesity manifests in some but not all ciliopathies, even though the protein products of the mutated proteins co-localize with obesogenic molecules. This observation is most striking given that obesity is a core component of BBS and Alstrom syndromes, a secondary feature in Joubert Syndrome (JBTS) and of no clinical note in NPHP (Nephronophthisis) or PKD (Polycystic Kidney Disease).
The model of obesity leading to leptin and insulin resistance in BBS, as per recent studies, may not be the precursor to obesity but a consequential body response with respect to increased food intake. BBS proteins may function to simply localize SSTR3, MCHR1 or other receptors to the cilium thereby regulating food intake and feeding behaviors which can lead to obesity in BBS and Alstrom Syndrome. Although the role of BBS proteins have been studied with respect to adipogenesis, their role in energy expenditure in terms of mobilizing stored lipids is not known. Dietary deprivation of an essential amino acid leucine has been shown to increase lipolysis in brown adipose tissue by activating uncoupling protein (UCP-1) and results in higher thermogenesis (Cheng et al., 2010) suggesting possible studies addressing lipolysis in BBS mouse models. But, is the obesity in BBS a result of just stored fat which cannot be burnt? While this question needs to be addressed, the hyperphagic Kif3a knockout mouse model being corrected for its obesity by calorie restriction is a promising study that requires translation to a human ciliopathy such as BBS. The counter argument for this hypothesis is whether loss of Kif3a can be considered equal to loss of BBS. Nevertheless, this takes us back to the idea that “We are what we eat” and regulating the food intake to alleviate morbid obesity in BBS subjects in early childhood would, at least, reduce possible secondary effects such as leptin and insulin resistance.
Acknowledgements
This work was funded by NIH grants DK072301, DK075972-09, and HD042601. E.O. is a NARSAD Young Investigator and N.K. is a distinguished Jean and George Brumley Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ait-Lounis A, Baas D, Barras E, Benadiba C, Charollais A, Nlend Nlend R, Liegeois D, Meda P, Durand B, Reith W. Novel function of the ciliogenic transcription factor RFX3 in development of the endocrine pancreas. Diabetes. 2007;56:950–959. doi: 10.2337/db06-1187. [DOI] [PubMed] [Google Scholar]
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, et al. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- Arsov T, Silva DG, O'Bryan MK, Sainsbury A, Lee NJ, Kennedy C, Manji SS, Nelms K, Liu C, Vinuesa CG, et al. Fat aussie--a new Alstrom syndrome mouse showing a critical role for ALMS1 in obesity, diabetes, and spermatogenesis. Molecular endocrinology. 2006;20:1610–1622. doi: 10.1210/me.2005-0494. [DOI] [PubMed] [Google Scholar]
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annual review of genomics and human genetics. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Baldari CT, Rosenbaum J. Intraflagellar transport: it's not just for cilia anymore. Current opinion in cell biology. 2010;22:75–80. doi: 10.1016/j.ceb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgacem YH, Borodinsky LN. Sonic hedgehog signaling is decoded by calcium spike activity in the developing spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4482–4487. doi: 10.1073/pnas.1018217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzler J, Andrews ZB, Pracht C, Stohr S, Shepherd PR, Grattan DR, Tups A. Hypothalamic WNT signalling is impaired during obesity and reinstated by leptin treatment in male mice. Endocrinology. 2013;154:4737–4745. doi: 10.1210/en.2013-1746. [DOI] [PubMed] [Google Scholar]
- Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Pasek RC, Malarkey EB, Yazdi SM, McNair AD, Lewis WR, Nagy TR, Kesterson RA, Yoder BK. Leptin resistance is a secondary consequence of the obesity in ciliopathy mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7796–7801. doi: 10.1073/pnas.1210192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano DA, Sekine S, Hebrok M. Primary cilia deletion in pancreatic epithelial cells results in cyst formation and pancreatitis. Gastroenterology. 2006;131:1856–1869. doi: 10.1053/j.gastro.2006.10.050. [DOI] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nature reviews. Endocrinology. 2012;8:228–236. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S, Xiao F, Guo F. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes. 2010;59:17–25. doi: 10.2337/db09-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- Collin GB, Cyr E, Bronson R, Marshall JD, Gifford EJ, Hicks W, Murray SA, Zheng QY, Smith RS, Nishina PM, et al. Alms1-disrupted mice recapitulate human Alstrom syndrome. Human molecular genetics. 2005;14:2323–2333. doi: 10.1093/hmg/ddi235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin GB, Marshall JD, King BL, Milan G, Maffei P, Jagger DJ, Naggert JK. The Alstrom syndrome protein, ALMS1, interacts with alpha-actinin and components of the endosome recycling pathway. PloS one. 2012;7:e37925. doi: 10.1371/journal.pone.0037925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Xu X, Heimberg H, Mansouri A. Pancreatic beta-cells: from generation to regeneration. Seminars in cell & developmental biology. 2010;21:838–844. doi: 10.1016/j.semcdb.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupe B, Ishii Y, Dietrich MO, Komatsu M, Horvath TL, Bouret SG. Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell metabolism. 2012;15:247–255. doi: 10.1016/j.cmet.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki PG, Shah JV. The ciliary transition zone: from morphology and molecules to medicine. Trends in cell biology. 2012;22:201–210. doi: 10.1016/j.tcb.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Current biology : CB. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EE, Katsanis N. The ciliopathies: a transitional model into systems biology of human genetic disease. Current opinion in genetics & development. 2012;22:290–303. doi: 10.1016/j.gde.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaval B, Bright A, Lawson ND, Doxsey S. The cilia protein IFT88 is required for spindle orientation in mitosis. Nature cell biology. 2011;13:461–468. doi: 10.1038/ncb2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara K, Ogawa Y, Katsuura G, Numata Y, Masuzaki H, Satoh N, Tamaki M, Yoshioka T, Hayase M, Matsuoka N, et al. Involvement of agouti-related protein, an endogenous antagonist of hypothalamic melanocortin receptor, in leptin action. Diabetes. 1999;48:2028–2033. doi: 10.2337/diabetes.48.10.2028. [DOI] [PubMed] [Google Scholar]
- Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJ, Ong KK. Variability in the heritability of body mass index: a systematic review and meta-regression. Frontiers in endocrinology. 2012;3:29. doi: 10.3389/fendo.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst MB, Wunderlich CM, Hess S, Paehler M, Mesaros A, Koralov SB, Kleinridders A, Husch A, Munzberg H, Hampel B, et al. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11582–11593. doi: 10.1523/JNEUROSCI.5712-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart-Toland A, Mounzih K, Qiu J, Chehab FF. Effect of the genetic background on the reproduction of leptin-deficient obese mice. Endocrinology. 1999;140:732–738. doi: 10.1210/endo.140.2.6470. [DOI] [PubMed] [Google Scholar]
- Feuillan PP, Ng D, Han JC, Sapp JC, Wetsch K, Spaulding E, Zheng YC, Caruso RC, Brooks BP, Johnston JJ, et al. Patients with Bardet-Biedl syndrome have hyperleptinemia suggestive of leptin resistance. The Journal of clinical endocrinology and metabolism. 2011;96:E528–535. doi: 10.1210/jc.2010-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, Pazour GJ, Rosenbaum JL, Baldari CT. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nature cell biology. 2009;11:1332–1339. doi: 10.1038/ncb1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forti E, Aksanov O, Birk RZ. Temporal expression pattern of Bardet-Biedl syndrome genes in adipogenesis. The international journal of biochemistry & cell biology. 2007;39:1055–1062. doi: 10.1016/j.biocel.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusev A, Bhatia G, Zaitlen N, Vilhjalmsson BJ, Diogo D, Stahl EA, Gregersen PK, Worthington J, Klareskog L, Raychaudhuri S, et al. Quantifying missing heritability at known GWAS loci. PLoS genetics. 2013;9:e1003993. doi: 10.1371/journal.pgen.1003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature neuroscience. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- Han YM, Kang GM, Byun K, Ko HW, Kim J, Shin MS, Kim HK, Gil SY, Yu JH, Lee B, et al. Leptin-promoted cilia assembly is critical for normal energy balance. The Journal of clinical investigation. 2014;124:2193–2197. doi: 10.1172/JCI69395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydet D, Chen LX, Larter CZ, Inglis C, Silverman MA, Farrell GC, Leroux MR. A truncating mutation of Alms1 reduces the number of hypothalamic neuronal cilia in obese mice. Developmental neurobiology. 2013;73:1–13. doi: 10.1002/dneu.22031. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. The New England journal of medicine. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Rivest R, Richard D. Effects of leptin on corticotropin-releasing factor (CRF) synthesis and CRF neuron activation in the paraventricular hypothalamic nucleus of obese (ob/ob) mice. Endocrinology. 1998;139:1524–1532. doi: 10.1210/endo.139.4.5889. [DOI] [PubMed] [Google Scholar]
- Huang-Doran I, Semple RK. Knockdown of the Alstrom syndrome-associated gene Alms1 in 3T3-L1 preadipocytes impairs adipogenesis but has no effect on cell-autonomous insulin action. International journal of obesity. 2010;34:1554–1558. doi: 10.1038/ijo.2010.92. [DOI] [PubMed] [Google Scholar]
- Jing K, Lim K. Why is autophagy important in human diseases? Experimental & molecular medicine. 2012;44:69–72. doi: 10.3858/emm.2012.44.2.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HS, Kim YS, ZeRuth G, Beak JY, Gerrish K, Kilic G, Sosa-Pineda B, Jensen J, Pierreux CE, Lemaigre FP, et al. Transcription factor Glis3, a novel critical player in the regulation of pancreatic beta-cell development and insulin gene expression. Molecular and cellular biology. 2009;29:6366–6379. doi: 10.1128/MCB.01259-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ, Cuervo AM, Singh R. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell metabolism. 2011;14:173–183. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- Kumamoto N, Gu Y, Wang J, Janoschka S, Takemaru K, Levine J, Ge S. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nature neuroscience. 2012;15:399–405. S391. doi: 10.1038/nn.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF, Cervo M, Yao H, Chung AL, Mizumura K, et al. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. The Journal of clinical investigation. 2013;123:5212–5230. doi: 10.1172/JCI69636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Liu J, Wong D, Srinivasan S, Ashrafi K. Hyperactive neuroendocrine secretion causes size, feeding, and metabolic defects of C. elegans Bardet-Biedl syndrome mutants. PLoS biology. 2011;9:e1001219. doi: 10.1371/journal.pbio.1001219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrand A, Davis EE, Carvalho CM, Pehlivan D, Willer JR, Tsai IC, Ramanathan S, Zuppan C, Sabo A, Muzny D, et al. Recurrent CNVs and SNVs at the NPHP1 locus contribute pathogenic alleles to Bardet-Biedl syndrome. American journal of human genetics. 2014;94:745–754. doi: 10.1016/j.ajhg.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loktev AV, Jackson PK. Neuropeptide Y family receptors traffic via the Bardet-Biedl syndrome pathway to signal in neuronal primary cilia. Cell reports. 2013;5:1316–1329. doi: 10.1016/j.celrep.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Longo KA, Wright WS, Kang S, Gerin I, Chiang SH, Lucas PC, Opp MR, MacDougald OA. Wnt10b inhibits development of white and brown adipose tissues. The Journal of biological chemistry. 2004;279:35503–35509. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nature genetics. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion V, Mockel A, De Melo C, Obringer C, Claussmann A, Simon A, Messaddeq N, Durand M, Dupuis L, Loeffler JP, et al. BBS-induced ciliary defect enhances adipogenesis, causing paradoxical higher-insulin sensitivity, glucose usage, and decreased inflammatory response. Cell metabolism. 2012;16:363–377. doi: 10.1016/j.cmet.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Marion V, Stoetzel C, Schlicht D, Messaddeq N, Koch M, Flori E, Danse JM, Mandel JL, Dollfus H. Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1820–1825. doi: 10.1073/pnas.0812518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JD, Maffei P, Collin GB, Naggert JK. Alstrom syndrome: genetics and clinical overview. Current genomics. 2011;12:225–235. doi: 10.2174/138920211795677912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesaros A, Koralov SB, Rother E, Wunderlich FT, Ernst MB, Barsh GS, Rajewsky K, Bruning JC. Activation of Stat3 signaling in AgRP neurons promotes locomotor activity. Cell metabolism. 2008;7:236–248. doi: 10.1016/j.cmet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, Jackson PK. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes & development. 2010;24:2180–2193. doi: 10.1101/gad.1966210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muresan V, Lyass A, Schnapp BJ. The kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1027–1037. doi: 10.1523/JNEUROSCI.19-03-01027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- Oh EC, Katsanis N. Cilia in vertebrate development and disease. Development. 2012;139:443–448. doi: 10.1242/dev.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EC, Katsanis N. Context-dependent regulation of Wnt signaling through the primary cilium. Journal of the American Society of Nephrology : JASN. 2013;24:10–18. doi: 10.1681/ASN.2012050526. [DOI] [PubMed] [Google Scholar]
- Pampliega O, Orhon I, Patel B, Sridhar S, Diaz-Carretero A, Beau I, Codogno P, Satir BH, Satir P, Cuervo AM. Functional interaction between autophagy and ciliogenesis. Nature. 2013;502:194–200. doi: 10.1038/nature12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. The Journal of cell biology. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, Zhang X, Knauf C, Cani PD, Aumayr K, Todoric J, et al. Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell. 2010;140:148–160. doi: 10.1016/j.cell.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Przybylski RJ. Occurrence of centrioles during skeletal and cardiac myogenesis. The Journal of cell biology. 1971;49:214–221. doi: 10.1083/jcb.49.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. The Journal of clinical investigation. 2008;118:1458–1467. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TS. Olfactory Cilia in the Frog. The Journal of cell biology. 1965;25:209–230. doi: 10.1083/jcb.25.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Human molecular genetics. 2009;18:1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanyan Z, Kocharyan A, Pyrski M, Hubschle T, Watson AM, Schulz S, Meyerhof W. Leptin-target neurones of the rat hypothalamus express somatostatin receptors. Journal of neuroendocrinology. 2003;15:822–830. doi: 10.1046/j.1365-2826.2003.01077.x. [DOI] [PubMed] [Google Scholar]
- Stratigopoulos G, Martin Carli JF, O'Day DR, Wang L, Leduc CA, Lanzano P, Chung WK, Rosenbaum M, Egli D, Doherty DA, et al. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell metabolism. 2014;19:767–779. doi: 10.1016/j.cmet.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Haley J, Bulgakov OV, Cai X, McGinnis J, Li T. Tubby is required for trafficking G protein-coupled receptors to neuronal cilia. Cilia. 2012;1:21. doi: 10.1186/2046-2530-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Lin MG, Stowe TR, Chen S, Zhu M, Stearns T, Franco B, Zhong Q. Autophagy promotes primary ciliogenesis by removing OFD1 from centriolar satellites. Nature. 2013;502:254–257. doi: 10.1038/nature12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teperino R, Amann S, Bayer M, McGee SL, Loipetzberger A, Connor T, Jaeger C, Kammerer B, Winter L, Wiche G, et al. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414–426. doi: 10.1016/j.cell.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nature genetics. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr., Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nature genetics. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- Valente EM, Rosti RO, Gibbs E, Gleeson JG. Primary cilia in neurodevelopmental disorders. Nature reviews. Neurology. 2014;10:27–36. doi: 10.1038/nrneurol.2013.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO reports. 2012;13:1079–1086. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattikuti S, Guo J, Chow CC. Heritability and genetic correlations explained by common SNPs for metabolic syndrome traits. PLoS genetics. 2012;8:e1002637. doi: 10.1371/journal.pgen.1002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertino AM, Taylor-Jones JM, Longo KA, Bearden ED, Lane TF, McGehee RE, Jr., MacDougald OA, Peterson CA. Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Molecular biology of the cell. 2005;16:2039–2048. doi: 10.1091/mbc.E04-08-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ding X, Su S, Spector TD, Mangino M, Iliadou A, Snieder H. Heritability of insulin sensitivity and lipid profile depend on BMI: evidence for gene-obesity interaction. Diabetologia. 2009;52:2578–2584. doi: 10.1007/s00125-009-1524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Nusse R. Wnt proteins. Cold Spring Harbor perspectives in biology. 2012;4:a007864. doi: 10.1101/cshperspect.a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WS, Longo KA, Dolinsky VW, Gerin I, Kang S, Bennett CN, Chiang SH, Prestwich TC, Gress C, Burant CF, et al. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes. 2007;56:295–303. doi: 10.2337/db06-1339. [DOI] [PubMed] [Google Scholar]
- Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nature genetics. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- Yoshiba S, Hamada H. Roles of cilia, fluid flow, and Ca2+ signaling in breaking of left-right symmetry. Trends in genetics : TIG. 2014;30:10–17. doi: 10.1016/j.tig.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. The Journal of clinical investigation. 2009;119:428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Davenport JR, Croyle MJ, Haycraft CJ, Yoder BK. Disruption of IFT results in both exocrine and endocrine abnormalities in the pancreas of Tg737(orpk) mutant mice. Laboratory investigation; a journal of technical methods and pathology. 2005;85:45–64. doi: 10.1038/labinvest.3700207. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Nishimura D, Seo S, Vogel T, Morgan DA, Searby C, Bugge K, Stone EM, Rahmouni K, Sheffield VC. Bardet-Biedl syndrome 3 (Bbs3) knockout mouse model reveals common BBS-associated phenotypes and Bbs3 unique phenotypes. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20678–20683. doi: 10.1073/pnas.1113220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19860–19865. doi: 10.1073/pnas.0906048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]